Abstract
Purpose/Objective:
Hypofractionated radiotherapy (HRT) regimens for prostate cancer are emerging, but tolerance doses for late adverse events are scarce. The purpose of this study is to define dose-volume predictors for late gastrointestinal and genitourinary (GI and GU) toxicities after HRT in the multi-center NRG/RTOG 0415 low-risk prostate cancer trial (N=521).
Material/methods:
Treatment in the studied HRT arm was delivered as 70Gy at 2.5Gy/fraction with 3D-CRT/IMRT (N=108/413). At a median follow-up of 5.9 years, the crude late ≥Grade 2 GI and GU toxicities were 19% and 29%, respectively. For modeling, the complete HRT cohort was randomly split into training and validation (70% and 30%; preserved toxicity rates). Within training, dose-response modeling was based on dose-volume cut-points (EQD2Gy; bladder/rectum: α/β=6Gy/3Gy), age, acute ≥Grade 2 toxicity, and treatment technique using univariate and multivariate logistic regression on bootstrapping (UVA and MVA). Candidate predictors were determined at p≤0.05, and the selected MVA models were explored on validation where model generalizability was judged if the area under the receiver-operating curve in validation (AUCvalidation) was within AUCtraining±SD with p≤0.05, and with an Hosmer-Lemeshow p-value (pHL)>0.05.
Results:
Three candidate predictors were suggested for late GI toxicity: the minimum dose to the hottest 5% rectal volume (D5%[Gy]), the absolute rectal volume <35Gy, and acute GI toxicity (AUC=0.59-0.63; p=0.02-0.04). The two generalizable MVA models, i.e.. D5%[Gy] with or without acute GI toxicity (AUCvalidation=0.64. 0.65; p=0.01, 0.03; pHL=0.45-0.56), suggest that reducing late GI toxicity from 20% to 10% would require reducing D5%[Gy] from ≤65Gy to ≤62Gy (logistic function argument: 17+(0.24D5%[Gy])). Acute GU toxicity showed only a trend to predict late GU toxicity (AUCtraining=0.57; p=0.07).
Conclusion:
Uate GI toxicity, following moderate HRT for low-risk prostate cancer, increases with higher doses to small rectal volumes. This work provides quantitative evidence that limiting small rectal dose ‘hotspots’ in clinical practice of such HRT regimens is likely to further reduce the associated rates of GI toxicity.
Keywords: prostate, cancer, radiotherapy, hypofractionation, adverse event, toxicity, gastrointestinal, genitourinary, bladder, rectum
Introduction
Hypofractionated radiotherapy (HRT) leads to shortened treatment times in comparison to conventional fractionation, and is likely to reduce the mean cost per patient up to 65% compared to conventionally fractionated RT (CFRT) [1]. In four recent phase III randomized controlled trials, i.e. the PROFIT [2], the CHHiP [3], the HYPRO [4], and the RTOG 0415 [5] trials, comparing moderate HRT (2.5-3 4Gy/fraction; physical prescribed dose: 60-70Gy) with CFRT (1-8-2.0Gy/fraction; physical prescribed dose: 74-78Gy) for localized prostate cancer, treatment outcome efficacy of HRT was demonstrated with similar biochemical recurrence-free survival rates to those observed after CFRT (77-91% vs. 77-88%) [2–5]. However, in all four trials, the rate of late adverse events namely moderate to severe late gastrointestinal (GI) and genitourinary (GU) toxicity tended to be higher in the HRT compared to the CFRT arm [2, 3, 5, 6]. In NRG Oncology’s RTOG 0415 trial, both the ≥Grade 2 late GI and GU toxicity rates were significantly higher for HRT compared to CFRT (22% vs. 20% (p=0.005); 26% vs. 21% (p=0.009)) [5].
A higher incidence of late adverse events may translate into increased expenses to manage these complications and may consequently offset the cost effectiveness of HRT regimens [1]. Thus, reducing the rate and severity of late adverse events is likely to further increase the usefulness of HRT regimens. To succeed in this, normal tissue tolerance doses applicable to HRT regimens will be required. Since there is currently no such evidence-based guidance, this study investigated dose-volume predictors of late GI and GU adverse events (hereafter referred to as late GI and GU toxicity) following HRT for prostate cancer in the multicenter NRG Oncology/RTOG 0415 trial [5].
Material/methods
Of the 545 recruited patients within the HRT arm of the trial, 521 patients were eligible for this study since they had retrievable dose-volume data and complete follow-up on late GI and GU toxicity. The median follow-up time for these patients was 5.9 (range: 0.4-8.5) years. Further information on the trial design has been presented previously [5].
Treatment planning and organ definitions
All 521 patients received daily image-guided moderate HRT to 70Gy in 28 fractions (2.5Gy/day) delivered either with three-dimensional conformal RT (3D-CRT; N=108) or with intensity-modulated RT (IMRT; N=413) between July 2006 and March 2010. The clinical target volume (CTV) encompassed the prostate gland, and the planning target volume (PTV) was defined as the CTV with a 3D isotropic 4-10mm expansion added. The maximum PTV dose was typically within 7% of the prescription dose (deviations of ≥7% but <10% were considered minor acceptable variations). At the time of the computed tomography simulation, extreme distension of the bladder and rectum were to be avoided. The bladder was delineated from the base to the dome, and the rectum from the anus (at the ischial tuberosities) to the rectosigmoid flexure (a total cranio-caudal length of ~15cm); both as solid structures.
Late GI and GU toxicity
Acute (within three months after HRT completion) and late (more than three months after HRT completion) GI and GU toxicity were prospectively recorded using the clinician-reported National Cancer Institute Common Terminology Criteria for Adverse Events v.3.0 [7]. The endpoints of interest for the purpose of this study were late GI and GU toxicity, also represented by distinct symptoms (GI: Colitis, Constipation, Diarrhea, Fecal incontinence, Hemorrhoids, and Proctitis (including rectal bleeding); GU: Bladder obstruction, Bladder spasm, Cystitis, Pollakiuria, Renal GU Other, Urethral obstruction, Urethral stricture, Urinary incontinence, Urinary retention, and Urinary stenosis; Table S1). The overall goal was to assess dose-response relationships for maximum-recorded late GI and GU toxicities within the follow-up time scheme available at the start of this analysis (i.e., within a population median of 5.9 years after complete HRT), and to achieve this we focus on symptoms with a rate of ≥10% for moderate to severe (≥Grade 2) toxicity.
Estimation of tolerance doses for late GI and GU toxicity
Harmonization and extraction of dose-volume histogram data
Before dose-response modeling, all doses were converted into equivalent doses in 2-Gy fractions (EQD2Gy) to account for fractionation effects [8] assuming α/β=6Gy for the bladder [9], and α/β=3Gy for the rectum ((6)[EQD2Gy]; (3)[EQD2Gy]) [10]. From the EQD2Gy-converted dose-volume histograms (DVHs), the relative and the absolute volumes receiving ≥xGy (VxGy[%], VxGy[cc]), the absolute volumes receiving <xGy (Vx<Gy[cc]), and the minimum dose to the hottest x% volume (Dx%[Gy]) were extracted. The VxGy[%], VxGy[cc], and Vx<Gy[cc], were extracted in 5Gy intervals (within 5-75Gy), and Dx%[Gy] in 5% intervals (within 5-95%). The maximum, the mean, and the minimum bladder and rectal doses (Min[Gy], Mean[Gy], and Max[Gy]), and their volumes were also extracted. Thus a total of 68 dose-volume cut-points per structure were extracted, and the nomenclature of these follows that of the AAPM Task Group 263 report [11].
Dose-response modeling
To establish new predictive models for HRT and following the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis, TRIPOD, statement [12], internal and external validation was performed. More specifically, the prediction model followed a type 2a TRIPOD model (random split-sample development and validation). The complete HRT cohort was randomly divided into 70% training data and 30% validation data (N=365 and 156) but preserving the late GI and GU toxicity rates between these divisions. The training data were used for model building; validation data were used to validate the final models identified in training.
Within training, dose-response modeling was based on logistic regression investigating the above-mentioned 68 dose-volume cut-points (continuous), as well as age (continuous), acute ≥Grade 2 toxicity (Grade 0-1/≥Grade 2: 0/1), and treatment technique (IMRT/3DCRT: 0/1) as potential predictors for late GI and GU toxicities. Internal validation was addressed using bootstrapping with 1000 sample populations. Candidate univariate predictors were defined if the associated overall bootstrap samples averaged logistic regression p-value was <0.05, and if the associated Spearman’s rank correlation coefficient (|Rs|) was <0.80 with any other selected variable.
The candidate predictors were subject to multivariate logistic regression utilizing bootstrapping with 1000 sample populations, and ultimately candidate multivariate models (p≤0.05 also of all inherent predictors) selected in ≥10% of the 1000 bootstrap models were explored on validation. A stepwise selection was used in the multivariate analysis with the objective of minimizing the Akaike Information Criterion.
The logistic regression coefficients for each predictor in the candidate multivariate models were applied to the corresponding predictors in the validation cohort, i.e., without re-fitting, or bootstrapping. A candidate multivariate model derived from training was considered final if it was generalizable in validation, i.e., if the area under the receiver-operating curve in validation (AUCvalidation) was within the mean ± standard deviation (SD) of the AUC in training (AUCtraining) across all bootstrap samples for corresponding models, and if the p-value of the Hosmer-Lemeshow test (pHL) [13] calculated in quintiles was >0.05. Performance metrics (AUC, p-values, and pHL) are reported as the mean ± SD across the 1000 bootstrap populations in training, while as one value/metric in validation. All analyses were undertaken in MATUAB.v.R2016a.
Results
General late GI and GU toxicity, as well as Proctitis and Polliakiuria qualified for analyses since these four endpoints presented with a symptom rate of ≥10% for the studied ≥Grade 2 cutoff (rate: 19%, 28%, 14%, 18%; Table S1). The remaining symptoms presented with considerably lower rates (GI: 1-2%; GU: <1-6%).
Predictive models: late GI toxicity
Candidate predictors
In training, a total of five candidate predictors were identified with associated AUCtraining in the range 0.56±0.03 to 0.63±0.04 (p=0.02-0.04±0.06-0.09; Table 1). The three candidate predictors V30<Gy[cc], V35<Gy[cc], and V40<Gy[cc] emphasized rectal volumes spared to intermediate dose levels, while the fourth candidate predictor D5%[Gy] emphasized small rectal volumes irradiated to high doses. In addition, acute GI toxicity was a candidate predictor. Since V30<Gy[cc], V35<Gy[cc], and V40<Gy[cc] were strongly correlated to one another (|Rs|=0.99). only V35<Gy[cc], which presented with a lower p-value than V30<Gy[cc] and V40<Gy[cc], was considered a final candidate predictor together with D5%[Gy] and acute GI toxicity. The pHL of all three predictors indicated good agreement between the observed and predicted rate of late GI toxicity (pHL=0.51-0.55±0.03-0.05).
Table 1.
An overview of the results from the dose-response modeling procedure for late GI toxicity (top) and Proctitis (bottom). Note: Training: AUC, p and pHL given as the mean (standard deviation) across the bootstrap samples. Abbreviations: AUC: Area under the receiver-operating characteristics curve; β0: Intercept; β1-3: Regression coefficients. Dx%[Gy]: The minimum dose to the hottest x% volume; MVA: Multivariate analysis; pHL: Hosmer-Lemeshow p-value; UVA: Univariate analysis; VxGy[%], and VxGy[cc]: The relative and the absolute volumes receiving ≥xGy; Vx<Gy[cc]: the absolute volumes receiving <xGy
| Late GI toxicity | Data | Candidate predictors | Model frequency | AUC | p | pHL | Final UVA/MVA | β0 | β1-3 |
|---|---|---|---|---|---|---|---|---|---|
| UVA I | Training | D5%[Gy] | 0.63 (0.04) | 0.02 (0.06) | 0.55 (0.05) | Yes | −17 | 0.24 | |
| II | V30<Gy[cc] | 0.59 (0.04) | 0.04 (0.09) | 0.51 (0.03) | −0.76 | −0.017 | |||
| III | V35<Gy[cc] | 0.59 (0.04) | 0.03 (0.07) | 0.51 (0.03) | Yes | −0.69 | −0.017 | ||
| IV | V40<Gy[cc] | 0.59 (0.04) | 0.04 (0.08) | 0.51 (0.03) | −0.68 | −0.016 | |||
| V | Acute GI toxicity | 0.56 (0.03) | 0.04 (0.10) | 0.52 (0.02) | Yes | −1.5 | 1.1 | ||
| MVA I | 1. D5%[Gy] 2. V35<Gy[cc] 3. Acute GI toxicity |
21% | 0.66 (0.04) | 0.0003 (0.002) | 0.54 (0.06) | −12 | 0.18 −0.013 1.05 |
||
| II | 1. D5%[Gy] 2. Acute GI toxicity |
28% | 0.65 (0.04) | 0.002 (0.01) | 0.56 (0.07) | −16 | 0.23 1.04 |
||
| III | 1. V35<Gy[cc] 2. Acute GI toxicity |
26% | 0.63 (0.04) | 0.003 (0.02) | 0.52 (0.03) | −0.85 | −0.017 1.09 |
||
| IV | 1. D5%[Gy] | 11% | 0.63 (0.04) | 0.02 (0.06) | 0.55 (0.05) | −17 | 0.24 | ||
| MVA | Validation | MVA I | 0.61 | 0.05 | 0.46 | ||||
| MVA II | 0.64 | 0.03 | 0.52 | Yes | |||||
| MVA III | 0.57 | 0.23 | 0.51 | ||||||
| MVA IV | 0.65 | 0.01 | 0.45 | Yes | |||||
| Proctitis | |||||||||
| UVA | Training | D5%[Gy] | 0.64 (0.04) | 0.02 (0.05) | 0.60 (0.07) | Yes | −24 | 0.34 | |
| Validation | 0.62 | 0.07 | 0.82 | Yes |
Final models
From the final univariate candidate predictors, four candidate multivariate models were suggested with a model frequency of 11-28% (p=0.0003±0.002-0.02±0.02; Table 1):
D5%[Gy], V35<Gy[cc], acute GI toxicity (AUCtraining=0.66±0.04)
D5%[Gy], acute GI toxicity (AUCtraining=0.65±0.04)
V35<Gy[cc], acute GI toxicity (AUCtraining=0.63±0.04)
D5%[Gy] (AUCtraining=0.63±0.04)
The observed and the predicted rate of late GI toxicity agreed well for all four models since pHL was >0.05 (pHL=0.52±0.03-0.56±0.07).
Of the two candidate multivariate models, models II and IV, which both included D5%[Gy] but with (II) or without (IV) acute GI toxicity, were generalizable in validation: AUCvalidation=0.64 (AUCtraining=0.65±0.04) and AUCvalidation=0.65 (AUCtraining=0.65±0.04). The associated normal tissue complication probability (NTCP) equations using the training regression coefficients for these two models were:
-
II.
NTCP = 1/1+exp−(−16+(0.23D5%[Gy])+(1.04Acute GI toxicity))
-
IV.
NTCP = 1/1+exp−(−17+(0.24D5%[Gy]))
For model IV, varying α/β from 3 Gy to either 1Gy, or 5 Gy did not alter the agreement between the observed and the predicted late GI toxicity, but replacing D5%[Gy] with either D1%[Gy] or D10%[Gy] did (Figure S1). The D5%[Gy] at the observed 20% late GI toxicity rate in training was 65Gy (Figure 1). A reduction in late GI toxicity to 15%, requires D5%[Gy]≤64Gy, whereas a two-fold decrease to 10% would require D5%[Gy]≤62. The corresponding thresholds for patients also experiencing acute GI toxicity were D5%[Gy]≤59, D5%[Gy]≤58, and D5%[Gy]≤55, respectively.
Figure 1.
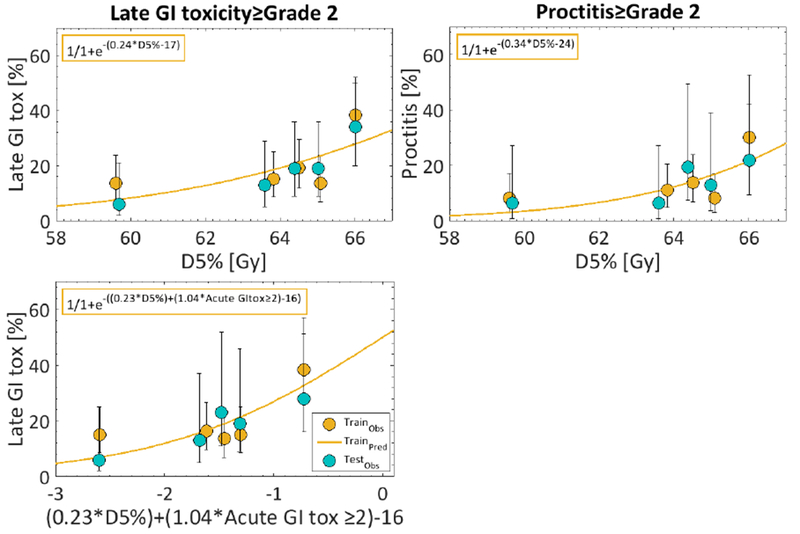
Dose-response curves based on the final two models for late GI toxicity (upper left: Model IV: D5%[Gy]; lower left: Model II. D5%[Gy] and acute GI toxicity) and Proctitis: (upper right: D5%[Gy]) separated between the training cohort (orange: line=prediction; dots=data) and the validation cohort (blue). Note: The corresponding equations deduced from dose-response modeling in training are inserted in the upper left corner.
Predictive models: Proctitis
Candidate predictors and final models
Only D5%[Gy] was a candidate predictor for Proctitis, and, therefore, a multivariate analysis was not conducted. In training, D5%[Gy] performed similarly for Proctitis as for late GI toxicity (AUCtraining=0.64±0.04; p=0.02±0.05; pHL=0.60±0.07), and had a similar size effect in the validation data (AUCvalidation=0.62), but only approached significance (p=0.07). At the observed 14% Proctitis rate in training, D5%[Gy] was 64Gy (Figure 1), whereas a reduction to, e.g., a 10% rate according to this model (equation: 1/1+exp−(−24+(0.34D5%[Gy]))) would require D5%[Gy]≤63Gy.
Predictive modeling: late GU toxicity and Polliakiuria
For late GU toxicity and Polliakiuria, no candidate predictors were identified in training, but acute GU toxicity showed a predictive trend for both late GU toxicity endpoints (GU toxicity: AUCtraining=0.57±0.03; ptraining=0.07±0.14; Pollakiuria: AUCtraining=0.59±0.03; ptraining=0.06±0.14).
Discussion
The goal of the present study was to define tolerance doses applicable to late toxicities after moderate hypofractionated radiotherapy (HRT) for prostate cancer. This was demonstrated for patients treated in the HRT arm on the NRG Oncology/RTOG 0415 [5]. Two robust dose-response models were successfully generated for late GI toxicity, and in general reducing the extent of rectal ‘dose hotspots’, i.e., keeping the minimum dose to the hottest 5% of the rectal volume, D5%[Gy] to below an (3) [EQD2Gy] of 62Gy, and yet respecting the remaining dose-volume constraints to other normal tissues as well as the prescribed tumor dose, is likely to reduce the rate of ≥Grade 2 late GI toxicity from the observed 20% to 10%. In addition, a similar dose-response relationship was established between D5%[Gy] and Proctitis. Adhering to a D5%[Gy] limit of 62Gy is, according to this model, likely to generate a two-fold reduction from the observed 14% to a 7% Proctitis rate.
Although the observed rate of late GU toxicity was higher than that of late GI toxicity (29% vs. 19%), no dose-response relationship was established for late GU toxicity or any symptom of this domain. Repeating the analyses using a considerably lower α/β value, i.e. 0.8Gy, [18], did neither result in any identified dose-response relationship for late GU toxicity or Pollakiuria. Thus, these results suggest that mitigation of late GU toxicity likely requires identification of the associated critical structure(s) [9,19] rather than focusing on the bladder.
While the majority of patients that developed late GI toxicity in the studied cohort had been treated using IMRT (N=82/101), these patients to a larger extent fulfilled the high-dose planning constraints compared to the fraction that had been treated using 3DCRT (V69Gy[%]≤25, V74Gy[%]≤15: 94% vs. 84%, 78% vs. 74%; Table S2). Unlike two recent studies [14, 15] that demonstrated a technique-specific dose-response relationship of late rectal bleeding [14] and ≥Grade 2 late GI toxicity [15] after CFRT with the 3DCRT-based dose-response relationship overestimating that of IMRT, we did not elucidate any relationship between treatment technique and late GI toxicity. A more evenly balanced proportion between patients treated with IMRT and 3DCRT than provided within the current cohort could shed further light on the potential existence of such a relationship also after HRT. Of note, the late GI toxicity rate is an order of magnitude higher than that observed after high dose CRT regimens for the same disease and stage, e.g., 19% vs. 2% in [20]. The latter rate was enabled combining IMRT with a high-dose rectal constraint, i.e. [EQD2Gy] of 75.6Gy≤30% [21]. The majority of patients in the current cohort were similarly planned using IMRT and a high dose constraint was also in place for the rectum ([EQD2Gy] of 74Gy≤15%; Table S2). However, adherence to this constraint was only 77% among the patients that developed late GI toxicity and the aggregated adherence was 84%. Thus, to further reduce the rate of late GI toxicity for patients treated according to the current HRT schedule and associated moderate HRT regimens we suggest implementation of the suggested D5%[Gy] ≤62Gy, but also advocate the use of rectal spacers in order to more easily achieve sparing of the rectum to very high doses [22].
In the prior study by Thor et al [14] a particular emphasis of the two final multivariate dose-response models derived after 3DCRT in an inter-institutional cohort was on rectal dose sparing (Min[Gy], and V55<Gy[cc]). Although IMRT was more frequently used than 3DCRT in the current cohort (79% vs. 21%), and the endpoint deviated from that studied by Thor et al [14], a similar trend of a dose-sparing relationship was observed with rectal V35<Gy[cc] being a candidate predictor for late GI toxicity. However, the two candidate models in which V35<Gy[cc] was included were borderline generalizable within the holdout validation cohort, and these models were, therefore, not deemed final. The only robust dose-volume cut-point included in our two final models for late GI toxicity, i.e., D5%[Gy] highlighted instead that a larger amount of rectal dose ‘hotspots’ explained late GI toxicity. A similar dose hotspot relationship with D5%[Gy] was further also confirmed for Proctitis. In the other cohort studied by Thor et al, which included IMRT treated patients only, a rectal dose-sparing relationship was also observed (V5Gy[cc]) [14]. In contrast to that single-institutional finding, the dose-volume cut-points used in this analysis were deduced from a cohort treated at several institutions, which minimizes the probability of this being a spurious finding primarily related to the prescription dose level [10, 14]. In a post-modeling exercise, the translation of this finding into a high best-fit volume dependence parameter value a within the Lyman-Kutcher-Burman model (LKB) [16, 17] was also explored, i.e., whether the highest rectal doses would also trigger the likelihood of toxicity according to the LKB formalism. This exercise was performed for late GI toxicity in the complete data using a Maximum Likelihood grid search estimation over 1000 bootstrapped sample populations. The best-fit a was estimated to be 30 (95% percentile bootstrap CI: 9-60), which is noticeably higher than a=11 (95%CI: 7-25) as proposed in the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) report devoted to GI toxicity [10].
In the QUANTEC report, eight covariates previously found to increase the risk of late GI toxicity were outlined: Advanced age, androgen deprivation therapy (ADT), diabetes, hemorrhoids, inflammatory bowel disease, prior abdominal surgery, rectal volume, and severe acute rectal toxicity [10]. The influence of ADT on late GI toxicity was assumed to be negligible given that this type of therapy was not allowed per protocol (other than in the setting of salvage therapy for prostate cancer recurrence). Of the remaining seven covariates outlined in QUANTEC, this study investigated whether age, rectal volume, and (≥Grade 2) acute GI toxicity, but also if RT technique, predisposed for late GI toxicity or Proctitis. Neither age, nor rectal volume was a candidate predictor (Training: AUC=0.53-0.60; p=0.14-0.47). However, presence of acute GI toxicity was a candidate predictor for late GI toxicity but only borderline for Proctitis (Training: AUC=0.56, 0.59, p=0.04, 0.06). Also for late GI toxicity, acute GI toxicity was included in one of the two final models together with D5%[Gy]. In the presence of acute GI toxicity and for the same rate of late GI toxicity, a more conservative D5%[Gy] threshold is warranted compared to in its absence, e.g., to manage late GI toxicity (cf. Results; Predictive models: late GI toxicity; Final models). Clinicians seeking to apply this information in their current practice are advised as follows: Regardless of modality (3DCRT or IMRT) the higher the rectal (3)[EQD2Gy] D5%[Gy], the greater the risk of late GI toxicity, and D5%[Gy] ≤65, ≤64, and ≤62 corresponds to a late GI toxicity rate of ~20%, 15%, and 10%, respectively. These fractionation-corrected D5%[Gy] levels should be converted into the fractionation scheme currently deployed such that the rectal D5%[Gy] can be routinely evaluated and minimized. If possible, and without deteriorating delivery of the prescription dose, an ideal goal would be to reduce D5%[Gy] to ≤55 taking also into account the increased likelihood of developing late GI toxicity in the presence of acute GI toxicity. To this end, the influence of diabetes, hemorrhoids, inflammatory bowel disease, and/or prior abdominal surgery on these suggested D5%[Gy] guidelines remains uncertain, but efforts to further untie this are encouraged.
For late GI toxicity, the best AUC obtained of 0.65 for the model including both D5%[Gy] and acute GI toxicity suggests that improvements are likely to come from other sources than rectal dose and the covariates investigated here but probably also by focusing on distinct symptoms that matter to patients. Also, mitigation of both the acute and late GI toxicity rates is likely to result from use of careful image-guided RT as previously proposed also for CFRT of prostate cancer, [10] together with recent advancements in further separating the rectum from the prostate [22].
Conclusion
This study provides a quantitative model of risk for late GI toxicity and proctitis with high doses to small rectal volumes. Late GI toxicity and proctitis are functions of rectal D5%[Gy], which should be kept to 62 Gy or lower if feasible in clinical practice of moderate HRT regimens.
Supplementary Material
Highlights.
High doses to small rectal volumes (D5%[Gy]) predict both ≥Grade 2 late gastro-intestinal (GI) toxicity and ≥Grade 2 proctitis after hypofractionated radiotherapy
Striving for a D5%[Gy] to below an equivalent dose in 2 Gy fractions of 62 Gy is likely to reduce the rate of ≥Grade 2 late GI toxicity from the observed 20% to 10% and a two-fold reduction also of the Proctitis rate (from 14% to 7%)
Acknowledgments/Conflicts of interest
Both JOD and MT were supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. In addition, DB was supported by a NCI sponsored NRG Oncology grant during the conduct of this study, BK was supported by a grant from Janssen scientific Affairs, RP by grant NCI U10 CA180822, and HS by a grant from ACR-NRG Oncology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: Comparison of toxicity. J Clin Oncol 2014; 32: 1195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Catton CN, Lukka H, Gu CS, et al. Randomized trial of hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017; 35:1884–90 [DOI] [PubMed] [Google Scholar]
- [3].Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomized, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016; 17:1047–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localized prostate cancer (HYPRO): final efficacy results from a randomized, nmulti-centre, open-label, phase 3 trial. Lancet Oncol 2016; 17:1061–9 [DOI] [PubMed] [Google Scholar]
- [5].Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fraction schedules in patients with low-risk prostate cancer. J Clin Oncol 2016; 34:2325–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol 2016; 17:464–74 [DOI] [PubMed] [Google Scholar]
- [7].Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- [8].Bentzen SM, Dörr W, Gahbauer R, et al. Bioeffect modeling and equieffective dose concepts in radiation – oncology, quantity and units. Int J Radiat Oncol Biol Phys 2012; 105:266–8 [DOI] [PubMed] [Google Scholar]
- [9].Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, and Shipley WU. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl):116–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Michalski JM, Gay H, Jackson A, Tucker SL, and Deasy JO. Radiation dose-volume effects in radiationinduced rectal injury. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl):123–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mayo CS, Moran JM, Bosch W, et al. American Association of Physicists in Medicine Task Group 263: Standardizing Nomenclatures in Radiation Oncology. Int J Radiat Oncol Biol Phys 2018; 100:1057–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moons KGM, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and Elaboration. Ann Intern Med 2015; 163:1–73 [DOI] [PubMed] [Google Scholar]
- [13].Hosmer DW and Lemeshow S. A goodness-of-fit test for the multiple logistic regression model. Commun in Stats 1980; 10:1043–69 [Google Scholar]
- [14].Thor M, Jackson A, Zelefsky MJ, et al. Inter-institutional analysis demonstrates the importance of lower than previously anticipated dose regions to prevent late rectal bleeding following prostate radiotherapy. Radiother Oncol 2018; March 9: [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Troeller A, Yan D, Marina O, et al. Comparison and limitations of DVH-based NTCP models derived from 3D-CRT and IMRT data for prediction of gastrointestinal toxicities in prostate cancer patients using propensity score matched pair analysis. Int J Radiat Oncol Biol Phys 2015; 91:435–43 [DOI] [PubMed] [Google Scholar]
- [16].Lyman JT. Complication probability as assessed from dose–volume histograms. Radiat Res 1985. (Suppl, 8):13–9 [PubMed] [Google Scholar]
- [17].Kutcher GJ, Burman C, Brewster L, Goitein M, and Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys 1991; 21:137–146 [DOI] [PubMed] [Google Scholar]
- [18].Cozzarini C, Rancati T, Palorini F, et al. Patient-reported urinary incontinence after radiotherapy for prostate cancer: Quantifying the dose-effect. Radiother Oncol 2017; 125:101–6 [DOI] [PubMed] [Google Scholar]
- [19].Ghadjar P, Zelefsky MJ, Spratt DE, et al. Impact of dose to the bladder trigone on long-term urinary function after high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2014; 88:339–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J radiat Oncol Biol Phys 2012; 84:125–9 [DOI] [PubMed] [Google Scholar]
- [21].Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, and Zelefsky M. Long-term survival and toxicity n patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2013; 85:686–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karsh LI, Gross ET, Pieczonka CM, et al. Absorbable hydrogel spacer use in prostate radiotherapy: A comprehensive review of phase 3 clinical trial published data. Urology 2017; November 23: [Epuh ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


