Abstract
Late-life depression (LLD) is associated with cognitive impairments and reduced gray matter volume (GMV); however the mechanisms underlying this association are not well understood. The goal of this study was to characterize changes in depression severity, cognitive function, and brain structure associated with pharmacologic antidepressant treatment for LLD. We administered a detailed neurocognitive battery and conducted structural magnetic resonance imaging (MRI) on 26 individuals with LLD, pre-/post- a 12-week treatment trial with venlafaxine. After calculating changes in cognitive performance, GMV, and depression severity, we calculated Pearson’s correlations, performed permutation testing, and false discovery rate correction. We found that loss of GMV over 12 weeks in the superior orbital frontal gyrus was associated with less improvement in depression severity and that increased GMV in the same was associated with greater improvement in depression severity. We detected no associations between changes in cognitive performance and improvements in either depressive symptoms or changes in GMV.
Keywords: late-life depression, MRI, cognition
Introduction
Late-life depression (LLD), defined as major depression in individuals over age 65, affects 5–8% of adults 65 and older (Buchtemann et al., 2012; Panza et al., 2010), is associated with functional disability, medical and psychiatric hospitalizations followed by institutionalization, worsened medical comorbidities, and increases in all-cause mortality. LLD also doubles the risk of developing dementia (Diniz et al., 2013), and is linked with cerebral dysfunction in several frontal and limbic regions (Alvarez and Emory, 2006; Bouckaert et al., 2016; Drevets, 2000; Mackin et al., 2014; Vasic et al., 2008), and investigation of these associations may lead to improved understanding and care. Previous work has indicated that greater cognitive performance correlates with lower depression severity, however this relationship is complex and indirect (Arvanitakis et al., 2016; Butters et al., 2011; Mulsant et al., 2006). Other evidence indicates an association between greater gray matter volume (GMV) and greater cognitive performance (Papenberg et al., 2016; Shimoda et al., 2015; Smagula et al., 2016).
Imaging techniques, including functional MRI (fMRI), have identified an association between depression severity in LLD and structural volumes as well as functional brain activation (Diniz et al., 2015; Khalaf et al., 2015). Some older adults treated for depression show better performance on cognitive measures, which in turn has been associated with greater gray matter volume (GMV) (Diniz et al., 2015; Manard et al., 2016). Voxel-based morphometry studies have consistently observed lower GMV in frontal and limbic regions associated with poorer performance on executive function tasks (Li et al., 2010; Rao et al., 2007). Other studies have shown greater gray matter volume following successful electroconvulsive therapy (Bouckaert et al., 2016).
Another consistent finding is low hippocampal volumes in individuals with depression, which is supported by the glucocorticoid hypothesis in depression (Andreescu et al., 2008; Butters et al., 2008; Gerritsen et al., 2011; Hickie et al., 2005; Janssen et al., 2007; Lloyd et al., 2004; Sawyer et al., 2012). It states that depression is neurotoxic to the brain and memory capacity may be affected through an overactive hypothalamic-pituitary-adrenal (HPA) activation, which can lead to chronic glucocorticoid exposure (the hippocampus being acutely sensitive to this effect). It is thought that this effect is also more prevalent in late-onset depression compared to early onset depression (Andreescu et al., 2008; Hickie et al., 2005; Lloyd et al., 2004).
We aimed to investigate associations between intervention-related changes in GMV, depression severity, and cognition. Understanding these longitudinal relationships may provide additional insight into the neurophysiological processes underlying the relationship between depression treatment response variability and the risk of neurocognitive decline in late life.
Methods
Study Design and Participants
All participants (N=26) were > 60 years old and met criteria for an episode of major depression that was diagnosed according to DSM-IV criteria and scored at least 15 on the Montgomery-Asberg Depression rating scale (MADRS) (Montgomery and Asberg, 1979). The methods of the study have been described previously (Karim et al., 2016). All participants had a 12-week, open-label trial of venlafaxine, with MRI scans prior to and after treatment. The University of Pittsburgh Institutional Review Board approved this study and all participants provided written informed consent prior to scanning. Years lived with depression was defined as current age minus age of first lifetime episode of major depression. Participants on antidepressant medication underwent a washout period of 2 weeks prior to starting treatment (6 weeks if on fluoxetine).
Exclusion Criteria
Participants were excluded if they met any of the following criteria: history of mania or psychosis, alcohol or substance abuse within the last 3 months, dementia, stroke, neurodegenerative disease (e.g., Parkinson’s Disease, multiple sclerosis), or unstable medical conditions which could complicate treatment response (e.g., uncontrolled severe hypertension). Five MRI scans were performed following informed consent, however only two were used in this analysis: baseline and end of the trial (12 weeks). A total of 37 participants signed consent but some were excluded due to venlafaxine side effects (N=2), non-adherence to protocol (N=1), an inaccurate diagnosis of major depressive disorder (N=1), or did not complete both scans and/or neurocognitive assessments (N=7). Participants (N=26) were classified as responders if they had a change in MADRS greater than or equal to 50 percent.
Dosage Information and Group Classification
Venlafaxine was started at 37.5mg and was titrated in 37.5mg increments every 3 days, to a target of 150mg/day. At approximately 6 weeks, non-responders had their dose increased in 37.5–75mg increments to a target dose of 300mg/day.
Neurocognitive Assessments
Along with the Mini-Mental State Examination (MMSE), neurocognitive performance was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)(Randolph et al., 1998) as well as selected subtests of the Delis-Kaplan Executive Function System (DKEFS) (Delis, 2001) at baseline and after 12 weeks of treatment with venlafaxine. Participants were excluded if they had an MMSE less < 25.
The RBANS measures performance and aptitude in attention, language, visuospatial functioning, and delayed memory. All raw scores are standardized to index scores based on age. The subtests from the RBANS included in this analysis were: (1) Coding task to measure sustained attention and information speed; (2) List and Story recall to measure delayed recall of verbal information; (3) Figure recall to measure delayed recall of visuospatial information.
Three subtests were used from the D-KEFS to measure set shifting (Trail Making), response inhibition (Color-Word Interference test), and combined inhibition and set shifting (Color-Word Interference test: inhibition component). Raw scores of this assessment were standardized based on their respective tests’ age-based norms.
MRI data acquisition
MRI data was collected (at baseline and 12 weeks) using a 3T Siemens Trio TIM scanner with a 12-channel head coil. An axial whole-brain high-resolution T1-weighted MPRAGE sequence was collected (repetition time=2300 ms, inversion time=900 ms, flip angle=9°) with a field of view 256×224 and 176 slices.
Region of Interest (ROI) selection
ROIs were selected due to evidence supporting associations between grey matter atrophy across all frontal regions and the limbic system as well as limbic system-associated regions (Vasic et al., 2008; Wu et al., 2006).
The Automated Anatomical Labeling (AAL) template was used to define twelve ROI’s: bilateral amygdala, hippocampus, parahippocampus, and inferior, middle, and superior frontal gyrus (each separated into operculum, triangular, and orbital aspects). These were based on previous findings from VBM and AAL studies that suggest a relationship between worse atrophy in frontal and limbic regions and greater depression severity (Amico et al., 2011; Li et al., 2010; Rao et al., 2007).
Calculating ROI Volumes
We used the automated labeling pipeline (ALP), which has been described in detail (Wu et al., 2006). After the structural image was skull-stripped automatically with FSL’s Brain Extraction Tool using the default parameters, trained students in the lab manually corrected it in ITK-SNAP (Insight Toolkit (Yoo, 2004)). This method starts with a grid-based piecewise linear registration and then uses a demons (deformable image registration algorithm) registration algorithm as a fine-tuning procedure for a voxel-level spatial deformation (registration library in ITK (Yoo, 2004)). The fully deformable registration allows for a high degree of spatial deformation, which seems to give it a particular advantage over other standard registration packages.
Following this coregistration, we computed the size of each ROI and divided this by the total size of the brain (intracranial volume, ICV), which accounts for differences in total size. The brain was manually (by trained students in ITK-SNAP) skull-stripped (which generates a mask of gray/white matter and cerebrospinal fluid) and the total volume of this mask was the ICV. To measure changes in and associations between depression severity, GMV, and cognitive performance, we computed change scores by calculating percent change from baseline [(baseline - post treatment)/baseline*100]. Positive changes in MADRS and in cognition indicated improvement while in GMV positive change indicated increased volume. This was done for all seven cognitive variables, twelve ROI’s, and MADRS.
Statistical Analysis
This was a hypothesis generating (exploratory) data analysis in which we measured changes in and associations between depression severity, GMV, and cognition. Descriptive statistics are presented in Table 1 (as median and interquartile range). To test differences between responders and non-responders Wilcoxon’s rank sum was used for continuous measures Fisher’s exact test for categorical or binary measures. Pearson’s correlation was used to test the association between our variables of interest (103 possible associations). Due to the small sample size, permutation testing (5000 iterations) was performed to generate a distribution for each association and to compute a p-value. To control for multiple comparisons we used false discovery rate (FDR) correction with α< 0.05 (Benjamini and Hochberg, 1995). Bootstrapping (5000 iterations) was used to estimate 95% confidence intervals, which were not corrected for multiple comparisons. We anticipated that many correlations would not survive FDR correction but wished to provide information about which larger associations could be of interest in more adequately powered studies.
Table 1.
Descriptive statistics for this sample. Age, Education, and Years lived with depression are measured in years (W=Wilcoxon’s rank sum). Years lived with depression was defined as current age minus age of first lifetime episode of major depression. Median and interquartile range (IQR) is reported when Wilcoxon’s rank sum test is done. Frequencies are reported when using Fisher’s exact p. MMSE – Mini-mental state examination; MADRS – Montgomery-Asberg Depression Rating Scale, MMSE – Mini-mental state examination.
| Responders [N=15] | Non-Responders [N=11] | W, Fisher’s Exact Test | p-value | |
|---|---|---|---|---|
| Age | 65 (5) | 67 (5) | W=185 | 0.349 |
| Sex (F) | 13 | 8 | Fisher’s exact p | 0.62 |
| Race (CC/AA) | 11/4 | 11/0 | Fisher’s exact p | 0.113 |
| Education | 15 (3) | 15 (2) | W=200 | 0.916 |
| Years lived with Depression | 29 (20) [N=11] | 33 (17) | W=123 | 0.818 |
| (Single/Recurrent) Depression | 2/11 [N=13] | 4/7 | Fisher’s exact p | 0.357 |
| MMSE | 29 (1) | 28 (1) | W=118 | 0.094 |
| Baseline MADRS | 26 (7) | 29 (5) | W=179 | 0.222 |
| End of trial MADRS | 7 (8) | 16 (7) | W=118 | 0.010* |
| End of trial Venla | 144 (121) [N=11] | 181 (110) [N=7] | W=16 | 0.433 |
| End of trial Desvenla | 253 (118) [N=11] | 405 (152) [N=7] | W=10.5 | 0.133 |
| Baseline Coding (Processing Speed) | 10.3 (5.9) | 8.7 (3.8) | W=218 | 0.2832 |
| Baseline List Recall (Delayed Verbal Memory) | 10.0 (3.3) | 11.4 (2.7) | W=188 | 0.4589 |
| Baseline Story Recall (Delayed Verbal Memory) | 10.3 (5.2) | 10.3 (2.9) | W=190 | 0.5575 |
| Baseline Figure Recall (Delayed Visuospatial Memory) | 8.9 (5.1) | 8.8 (3.0) | W=205 | 0.7821 |
| Baseline Trail Making (Set Shifting) | 10 (4.5) | 10.5 (1.5) | W=187 | 0.4382 |
| Baseline Color-Word Interference-Inhibition (Response Inhibition) | 10.3 (5.0) | 12.0 (4.5) | W=185 | 0.367 |
| Baseline Color-Word Interference-Inhibition/Switching (Response Inhibition and Switching) | 9.0 (4.5) | 10.0 (1.5) | W=191 | 0.5998 |
Results
GMV Changes and Improvement in Depression Severity
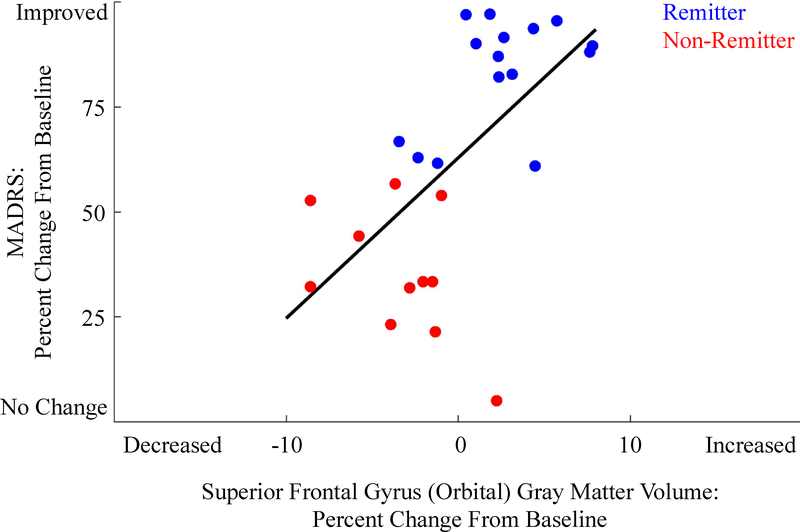
After correcting for multiple comparisons (via FDR), only one association was significant. We found that a decrease of GMV (maximum decrease of 0.74cm3) in the superior orbital frontal gyrus (SFG Orbital, average volume of 8.1cm3 with [min, max]=[6.02cm3, 9.69cm3]) was associated with less improvement in depression severity r(25)=0.636, p<0.001 and a 95% confidence interval (CIunc) equal to [0.35,0.80] (see Fig 1). Increased GMV (maximum increase of 0.63cm3) in this region was associated with high improvement in depression symptoms. Change in GMV was not associated with serum venla/desvenla (or dosage) or years lived with depression (age minus age of first depressive episode), however the changes in superior frontal gyrus (SFG) were associated with years lived with depression r(21)=−0.52, p<0.05.
Figure 1.
The association between changes in superior frontal gyrus (orbital) GMV and changes in MADRS (depression severity). Note that all participants improved (some less than others) – none worsened during treatment. Also, there are both increases and decreases in GMV. Responders are labeled in blue, while non-responders are labeled in red. Note: No tests comparing responders and non-responders were conducted.
Effects Requiring Further Study
Considering the relevance of this dataset for informing future longitudinal research, and the small sample size we report the strength of these associations as measured by Pearson’s correlation even though they did not reach the statistical significance after controlling for multiple comparisons, in order to foster further testing in adequately powered studies. The correlation and respective 95% CIunc are presented for all the investigated regions. Only variables that had CIunc that did not include zero are reported.
Change in Amygdala GMV was associated with changes in depression severity r(25)=0.465 [0.04,0.76], which seemed to also be associated with years lived with depression r(21)=−0.51, p<0.05. Even after considering uncorrected associations, we found that changes in depression severity were not directly associated with changes in neurocognitive test performance. Change in inferior frontal gyrus (triangular) GMV was positively associated with improvement in figure recall (visuospatial and constructional competence) r(25)=0.366 [0.01,0.64]. Middle frontal gyrus GMV was negatively associated with color-word interference test (inhibition) r(25)=0.442 [−0.74,−0.06], which was also associated with years lived with depression r(21)=−0.45, p<0.05. Inferior frontal gyrus (orbital) GMV was negatively associated with improvement in difference in set-shifting (adjusted for motor speed) r(25)=0.421 [−0.76,−0.02].
Discussion
We found that the change in superior frontal gyrus GMV was positively associated with improvement in depression severity (as measured by MADRS). While all participants improved in MADRS, those who had the smallest improvement also had the greatest atrophy in this region. We did not detect a significant association between improvement in neurocognitive performance with either improvement in depressive symptoms or changes in GMV.
Previous studies have shown altered orbitofrontal frontal cortex connectivity in major depressive disorder (Tadayonnejad et al., 2014). Greater superior frontal volumes (Marano et al., 2015) as well as greater (Ballmaier et al., 2004; Lai et al., 2000) and lower (Lai et al., 2000; Taylor et al., 2007) volumes in orbitofrontal regions have also been associated with late life depression. We also found that those with the largest improvement in depression severity had increased GMV in this region. This is consistent with previous studies that have found associations between depression severity and volumetric changes in superior orbital frontal areas (Hou et al., 2016). Lower GMV in this region may be interpreted as persistent phenomena where continued depressive symptoms slightly reduce GMV while improvement of those symptoms may increase those volumes, which may be more consistent with findings that the orbitofrontal regions have lower volumes in depressed individuals.
Some associations (while not surviving multiple comparisons correction) showed effects that require further study to determine their clinical significance. We found that the amygdala gray matter volume seemed to have a similar association with depression severity. Improvement in depression severity was not significantly associated with improvement in cognitive test performance, even after considering uncorrected associations. This is supported by previous findings that identify depression as not being directly related with cognitive performance (Gallassi et al., 2001; Lichtenberg et al., 1995). This is not surprising given our previous findings that cognitive impairment in LLD is trait-like and persists beyond the depressive episode (Koenig et al., 2015).
While cognitive scores were not directly associated with changes in depression severity, there were some associations with GMV. Specifically, we found that increased GMV in the inferior frontal gyrus (triangular) was associated with better performance on figure recall (visuospatial memory). Increased middle frontal gyrus GMV was associated with worse inhibition. A similar association was found between the inferior frontal gyrus (orbital) and difference in set shifting. These results require further confirmatory longitudinal studies.
Years lived with depression (age minus age of first depressive episode) was significantly associated with changes in amygdala volume, middle frontal gyrus (orbital), and superior frontal gyrus, but was not associated with change in SFG (orbital) GMV. This further supports the neurotoxic effects of depression on the development and plasticity of the brain, where a greater number of years lived with depression seem to be a factor in determining changes in GMV. However, we found that SFG (orbital) volume changes were associated with changes in depression severity in the trial and not to years lived with depression (although SFG volume was associated with years lived with depression), which suggests that there exists some neurobiological mechanism that drives these changes. A possible mechanism is an increase in glial density in the OFC that may occur after successful pharmacotherapy, which continues on its intended track in those that experience a less successful treatment course. Past studies have indicated differences in glial density (Rajkowska et al., 1999) and it is possible that glial density increases following pharmacotherapy (though that is currently speculative). The OFC is a critical region that is involved in emotion regulation (Goldin et al., 2008) and structural changes in this region (either through aging related changes in volume or increased white matter lesions) may both predispose individuals to depression (low cognitive control of emotions) as well as affect their responsiveness to pharmacotherapy.
These data contribute to the growing body of research that supports the relationship between LLD severity, GMV atrophy, and cognitive impairments. Previous findings have established a relationship between depression severity and GMV across all cortices and limbic structures (Alvarez and Emory, 2006; Bouckaert et al., 2016; Drevets, 2000; Vasic et al., 2008). The literature has shown that while there is a slight improvement in cognition in late life coinciding with improvement in depression severity, although this relationship is more complex and is indirect (Arvanitakis et al., 2016; Butters et al., 2011; Mackin et al., 2014; Mulsant et al., 2006). However, there is evidence that directly correlates higher GMV and improved cognitive performance (Papenberg et al., 2016; Shimoda et al., 2015; Smagula et al., 2016) and our previous work suggests that depression is associated with accelerated molecular brain aging and persistent cognitive impairment (Diniz et al., 2017; Diniz et al., 2015).
The current study is longitudinal and analyses are all bidirectional correlation analyses, thus causal relationships are not specifically tested. The present results should be viewed in light of several limitations. This study has a relatively small sample size (N=26), thus further investigation is required in a larger sample to determine the clinical significance of these associations. This study was conducted in a late-life sample and so these results cannot be generalized to mid-life depression. There is no control group, so we cannot compare these treatment-related changes in a clinical sample to a non-depressed group. Past studies have shown changes in GMV in the hippocampus following acute exercise interventions (Erickson et al., 2011), in the visual and frontal cortex following juggling (Draganski et al., 2004) (even after only 7 days of training (Driemeyer et al., 2008)), and even in Heschl’s gyrus following musical training (Hyde et al., 2009), however it is possible that some of this variance is likely accounted for by artifact, motion across time, and even the toolbox used to analyze the data. While we have previously shown that reproducibility within repeated measures designs is higher (Tudorascu et al., 2016) and much of the variability between studies is likely due to different toolboxes (or different acquisitions), considering the large number of studies that report such changes, it is unlikely that all the variance is due solely to artifacts or motion and that there is an underlying neuroplastic mechanism in the brain that drives such changes.
There are several such mechanisms that may increase GMV: tissue volume changes could be due to synaptic changes (though this may be an unlikely mechanism in late-life), glial changes (which are thought to be the most dominant mechanism), and/or increases in dendritic length. Each of these changes could increase or decrease observed GMV, however several other mechanisms involve changes in fluid volume, where past studies have shown changes in pulsatility and other tissue properties that may affect volume (Desmidt et al., 2017).
Using pre- and post- treatment data, this project attempted to provide an integrated view of the relationships among changes in GMV, cognition, and depressive symptoms during open-trial pharmacotherapy of older adults with major depression. Further understanding of these processes may provide additional insight into the risk for dementia posed by depression, the benefits of successful treatment with antidepressants for brain structure and function, and may elucidate cases in which depression is a prodromal expression of dementia, rather than a true risk factor.
Acknowledgments
We would like to thank all participants for their cooperation during this study. This work was funded by NIH grants: R01 MH076079, R01 MH083660, R01 AG033575, R34 MH101371, P30 MH090333, and P50 AG005133, and the Charles F. Reynolds III and Ellen G. Detlefsen Endowed Professorship in Geriatric Psychiatry (Howard J. Aizenstein).
Footnotes
Each of the co-authors had substantial contributions to the design, analysis, interpretation, and participated in manuscript drafting or revision. All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez JA, Emory E, 2006. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 16(1), 17–42. [DOI] [PubMed] [Google Scholar]
- Amico F, Stauber J, Koutsouleris N, Frodl T, 2011. Anterior cingulate cortex gray matter abnormalities in adults with attention deficit hyperactivity disorder: a voxel-based morphometry study. Psychiatry Res 191(1), 31–35. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, Reynolds CF 3rd, Aizenstein H, 2008. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology 33(11), 2566–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA, 2016. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct 221(4), 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A, 2004. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry 161(1), 99–108. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological), 289–300. [Google Scholar]
- Bouckaert F, De Winter FL, Emsell L, Dols A, Rhebergen D, Wampers M, Sunaert S, Stek M, Sienaert P, Vandenbulcke M, 2016. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J Psychiatry Neurosci 41(2), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtemann D, Luppa M, Bramesfeld A, Riedel-Heller S, 2012. Incidence of late-life depression: a systematic review. J Affect Disord 142(1–3), 172–179. [DOI] [PubMed] [Google Scholar]
- Butters MA, Bhalla RK, Andreescu C, Wetherell JL, Mantella R, Begley AE, Lenze EJ, 2011. Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. Br J Psychiatry 199(3), 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, 2001. Delis-Kaplan executive function scale (D-KEFS). San Antonio: The Psychological Corporation. [Google Scholar]
- Desmidt T, Brizard B, Dujardin PA, Ternifi R, Remenieras JP, Patat F, Andersson F, Cottier JP, Vierron E, Gissot V, Kim K, Aizenstein H, El-Hage W, Camus V, 2017. Brain Tissue Pulsatility is Increased in Mid-Life Depression: A Comparative Study using Ultrasound Tissue Pulsatility Imaging. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd, 2013. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 202(5), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Reynolds CF 3rd, Sibille E, Lin CW, Tseng G, Lotrich F, Aizenstein HJ, Butters MA, 2017. Enhanced Molecular Aging in Late-Life Depression: the Senescent-Associated Secretory Phenotype. Am J Geriatr Psychiatry 25(1), 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, Becker JT, Lopez OL, Lotze MT, Klunk WE, Reynolds CF, Butters MA, 2015. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry 20(5), 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A, 2004. Neuroplasticity: changes in grey matter induced by training. Nature 427(6972), 311–312. [DOI] [PubMed] [Google Scholar]
- Drevets WC, 2000. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 126, 413–431. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A, 2008. Changes in gray matter induced by learning--revisited. PLoS One 3(7), e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF, 2011. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108(7), 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallassi R, Morreale A, Pagni P, 2001. The relationship between depression and cognition. Arch Gerontol Geriatr Suppl 7, 163–171. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI, 2011. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes--the SMART Medea study. Biol Psychiatry 70(4), 373–380. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ, 2008. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 63(6), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G, 2005. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry 186, 197–202. [DOI] [PubMed] [Google Scholar]
- Hou Z, Wang Z, Jiang W, Yin Y, Yue Y, Zhang Y, Song X, Yuan Y, 2016. Divergent topological architecture of the default mode network as a pretreatment predictor of early antidepressant response in major depressive disorder. Sci Rep 6, 39243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G, 2009. Musical training shapes structural brain development. J Neurosci 29(10), 3019–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J, Hulshoff Pol HE, de Leeuw FE, Schnack HG, Lampe IK, Kok RM, Kahn RS, Heeren TJ, 2007. Hippocampal volume and subcortical white matter lesions in late life depression: comparison of early and late onset depression. J Neurol Neurosurg Psychiatry 78(6), 638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, Andreescu C, Tudorascu D, Smagula SF, Butters MA, Karp JF, Reynolds C, Aizenstein HJ, 2016. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf A, Edelman K, Tudorascu D, Andreescu C, Reynolds CF, Aizenstein H, 2015. White Matter Hyperintensity Accumulation During Treatment of Late-Life Depression. Neuropsychopharmacology 40(13), 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig AM, DeLozier IJ, Zmuda MD, Marron MM, Begley AE, Anderson SJ, Reynolds CF 3rd, Arnold SE, Becker JT, Butters MA, 2015. Neuropsychological functioning in the acute and remitted States of late-life depression. J Alzheimers Dis 45(1), 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR, 2000. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry 48(10), 971–975. [DOI] [PubMed] [Google Scholar]
- Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, Lin WC, Su TP, 2010. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage 50(1), 347–356. [DOI] [PubMed] [Google Scholar]
- Lichtenberg PA, Ross T, Millis SR, Manning CA, 1995. The relationship between depression and cognition in older adults: a cross-validation study. J Gerontol B Psychol Sci Soc Sci 50(1), P25–P32. [DOI] [PubMed] [Google Scholar]
- Lloyd AJ, Ferrier IN, Barber R, Gholkar A, Young AH, O’Brien JT, 2004. Hippocampal volume change in depression: late- and early-onset illness compared. Br J Psychiatry 184, 488–495. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Nelson JC, Delucchi KL, Raue PJ, Satre DD, Kiosses DN, Alexopoulos GS, Arean PA, 2014. Association of age at depression onset with cognitive functioning in individuals with late-life depression and executive dysfunction. Am J Geriatr Psychiatry 22(12), 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manard M, Bahri MA, Salmon E, Collette F, 2016. Relationship between grey matter integrity and executive abilities in aging. Brain Res 1642, 562–580. [DOI] [PubMed] [Google Scholar]
- Marano CM, Workman CI, Lyman CH, Munro CA, Kraut MA, Smith GS, 2015. Structural imaging in late-life depression: association with mood and cognitive responses to antidepressant treatment. Am J Geriatr Psychiatry 23(1), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Houck PR, Gildengers AG, Andreescu C, Dew MA, Pollock BG, Miller MD, Stack JA, Mazumdar S, Reynolds CF 3rd, 2006. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol 26(2), 113–120. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V, 2010. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry 18(2), 98–116. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Ferencz B, Mangialasche F, Mecocci P, Cecchetti R, Kalpouzos G, Fratiglioni L, Backman L, 2016. Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Hum Brain Mapp 37(10), 3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA, 1999. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45(9), 1085–1098. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN, 1998. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of clinical and experimental neuropsychology 20(3), 310–319. [DOI] [PubMed] [Google Scholar]
- Rao H, Gillihan SJ, Wang J, Korczykowski M, Sankoorikal GM, Kaercher KA, Brodkin ES, Detre JA, Farah MJ, 2007. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry 62(6), 600–606. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC, 2012. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging & mental health 16(6), 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Kimura M, Yokota M, Okubo Y, 2015. Comparison of regional gray matter volume abnormalities in Alzheimers disease and late life depression with hippocampal atrophy using VSRAD analysis: a voxel-based morphometry study. Psychiatry Res 232(1), 71–75. [DOI] [PubMed] [Google Scholar]
- Smagula SF, Lotrich FE, Aizenstein HJ, Diniz BS, Krystek J, Wu GF, Mulsant BH, Butters MA, Reynolds CF 3rd, Lenze EJ, 2016. Immunological biomarkers associated with brain structure and executive function in late-life depression: exploratory pilot study. Int J Geriatr Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Yang S, Kumar A, Ajilore O, 2014. Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS One 9(4), e96033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan KR, 2007. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med 37(12), 1763–1773. [DOI] [PubMed] [Google Scholar]
- Tudorascu DL, Karim HT, Maronge JM, Alhilali L, Fakhran S, Aizenstein HJ, Muschelli J, Crainiceanu CM, 2016. Reproducibility and Bias in Healthy Brain Segmentation: Comparison of Two Popular Neuroimaging Platforms. Front Neurosci 10, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasic N, Walter H, Hose A, Wolf RC, 2008. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord 109(1–2), 107–116. [DOI] [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF 3rd, Aizenstein HJ, 2006. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 148(2–3), 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo T, 2004. Insight into Images: Principles and Practice for Segmentation, Registration, and Image Analysis (Wellesey, MA: AK Peters; ). [Google Scholar]