Abstract
Many vegetarians report that meat is unpleasant, but little else is known about their affective responses to meat and non-meat foods. Here we explored affective responses to food images in vegetarians and omnivores and tested the hypothesis that vegetarians have global differences in affective processing (e.g., increased disgust sensitivity). We presented pictures of different food items and recorded participants’ affective experience while we recorded peripheral physiology. We found that vegetarians’ self-reported experience of meat meal images was less pleasant than omnivores’, but that other food images were equally pleasant across the two groups. Moreover, vegetarians and omnivores had strikingly similar physiological responses to all food images – including meat meals. We interpret these results from a psychological constructionist perspective, which posits that individuals conceptualize changes in their bodily states in ways that match their beliefs, such that increased sympathetic nervous system activity may be conceptualized as an experience of excitement about a delicious meat meal for omnivores but as an experience of displeasure for a vegetarian who believes meat is cruel, wasteful, impure, or unhealthy. This interpretation is consistent with emerging neuroscience evidence that the brain constructs experience by predicting and making meaning of internal sensations based on past experience and knowledge.
Keywords: Psychophysiology, Affect, Food, Vegetarian, Meat
1. Introduction
Humans eat to obtain calories and nutrients, but they also eat for pleasure (Lowe & Butryn, 2007). There is enormous variation in what people eat and how people experience the very same food. For omnivores, meat is generally considered a delicious food that is often served on special occasions (Fiddes, 1991), whereas vegetarians experience meat very differently (for review see Ruby, 2012), often reporting that they experience meat negatively (Amato & Partridge, 1989; Barnes-Holmes, Murtagh, Barnes-Holmes, & Stewart, 2010; De Houwer & De Bruycker, 2007; Rozin, Markwith, & Stoess, 1997; Stockburger, Renner, Weike, Hamm, & Schupp, 2009). Many people who convert to vegetarianism report that the hedonic value of meat shifts over time such that although meat is pleasant before becoming a vegetarian, it later becomes unpleasant (Amato & Partridge, 1989). This ‘hedonic shift’ (Rozin et al., 1997) may help vegetarians to maintain their diets since desire for the taste of meat is one of the most common reasons people abandon a vegetarian diet (Barr & Chapman, 2002; Haverstock & Forgays, 2012). Beyond the observation that vegetarians experience meat negatively, however, little else is known about the affective and emotional responses of vegetarians to meat or other foods.
We test two alternative hypotheses about vegetarians’ affective responses to food. The specificity hypothesis suggests that affective differences between vegetarians and omnivores are specific to meat stimuli. That is, vegetarians experience meat as negative, but non-meat foods and other stimuli are experienced just as positively or negatively as they are for omnivores. The alternative, the generality hypothesis, suggests that vegetarians have general differences in affective processing (Fessler, Arguello, Mekdara, & Macias, 2003). For instance, Fessler et al. (2003) noted the possibility that meat may be more disgusting to vegetarians because they are more sensitive to stimuli typically experienced as disgusting (i.e., higher trait disgust; Haidt, Mccauley, & Rozin, 1994), although the authors found no support for this hypothesis using survey measures of disgust sensitivity and self-reported amount of meat eating (among people who were mostly omnivores, Fessler et al., 2003).
Like Fessler et al. (2003), other previous studies of vegetarians’ affective responses also have relied almost exclusively on self-reports (Rozin et al., 1997), and those few studies that have used research methods that go beyond self-reports have not investigated affective and emotional responding to meat and other foods (see, e.g., Barnes-Holmes et al., 2010; De Houwer & De Bruycker, 2007; Stockburger et al., 2009). Critically, this means little is known about the bodily responses that may accompany (or even drive) vegetarians’ affective experience of meat and other foods. Indeed, emerging neuroscience evidence posits a central role for the body in conscious experience and perception, specifically the idea that affect arises from metabolic energy regulation (i.e., allostasis; Sterling, 2012; Sterling & Laughlin, 2015) and the resulting internal sensations from the body (i.e., interoception; Craig, 2015) (Barrett, 2017; Barrett & Simmons, 2015; Chanes & Barrett, 2016; Clark, 2013). Anatomical, physiological, and metabolic evidence (Chanes & Barrett, 2016; Kleckner et al., 2017) indicates that we experience the world as we predict it to be (i.e., consistent with our internal model of the body in the world) with sensory inputs either confirming or being used to adjust that internal model. However, to our knowledge, peripheral physiological measures have never been used to study the bodily and affective responses of vegetarian and omnivores to food or images of food.
In the present study, participants reported how appetizing they found a variety of food images, including meat and vegetarian meals, rotten foods, and sweet foods (e.g., a cupcake) and also reported their affective and emotional feelings while viewing images of these foods. In addition, we recorded two commonly used measures of autonomic nervous system (ANS) activity: (1) electrodermal activity (EDA), a measure of sympathetic nervous system (SNS) activation of the eccrine sweat glands which is often positively associated with feelings of activation or arousal (see, e.g., Bradley, Codispoti, Cuthbert, & Lang, 2001; Lang, Greenwald, Bradley, & Hamm, 1993), and heart period (the duration in ms between consecutive heart beats), which has been shown to increase (i.e., decrease in heart rate) in response to viewing affectively negative pictures (Bradley et al., 2001; Codispoti, Bradley, & Lang, 2001; Lang et al., 1993). We also used facial electromyography to measure activation over the corrugator supercilii facial muscle region, which under specific experimental conditions can be associated with the experience of negative affect (Bradley et al., 2001; Lang et al., 1993; for review see Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000), and activation over the levator labii facial muscle region, which some researchers have suggested to be associated with the experience of disgust (Vrana, 1993), both to food (Hoefling et al., 2009) and to moral violations (Chapman, Kim, Susskind, & Anderson, 2009). However, others have convincingly argued that bodily activity is more strongly associated with general affective experience (i.e., feelings of positivity/negativity and activation/de-activation) than specifically with the experience of discrete emotional states like disgust (see Cameron, Lindquist, & Gray, 2015 for a discussion). Thus, we propose that activation over the levator labii muscle region may reflect unpleasant affect associated with bodily activity rather than being specifically associated with disgust.
The primary aim of this study was to explore the affective responses of vegetarians and omnivores using self-reported affect, ANS measures, and facial muscle activity to test whether these measures provided greater support for the specificity hypothesis, which posits that vegetarians experience viewing images of meat meals as negative but respond to images of other stimuli like omnivores, or the generality hypothesis, which posits that vegetarians have more general, global differences in affective processing.
We also explored two auxiliary hypotheses by measuring how vegetarians and omnivores responded to pictures of animals on farms. First, the inclusion of non-food stimuli that are closely related to meat provides a broader test of the generality and specificity hypotheses. In particular, the generality hypothesis would predict that vegetarians would perceive greater animal suffering and experience more negative emotions when viewing images of animals on farms. A strong version of specificity hypothesis would suggest that both vegetarians and omnivores would have similar responses to viewing images of animals on farms. However another possibility is that because farm animals are so related to meat, vegetarians might experience viewing images of animals on farm differently (compared to omnivores). This is an interesting possibility since vegetarians commonly report that they avoid ingesting meat due to their concern about animal suffering (Amato & Partridge, 1989; Fox & Ward, 2008a; Rozin et al., 1997), and vegetarians also report perceiving that animals experience a greater range of emotions than omnivores (Bilewicz, Imhoff, & Drogosz, 2011). Second, previous work shows that omnivores engage in motivated denial of mind such that they report perceiving less animal suffering when they have recently eaten meat compared to when they have eaten non-meat meals (Bastian, Loughnan, Haslam, & Radke, 2012; Loughnan, Haslam, & Bastian, 2010). We investigated whether omnivores’ experiences of animal suffering would be influenced by whether participants first rated the appeal of images of meat meals. Specifically, we hypothesized that omnivores would report less perceived animal suffering if they first viewed and rated images of meat meals.
2. Methods
2.1. Participants
Eighty-six participants were recruited from Northeastern University and the greater Boston community through flyers. One participant was excluded prior to analyses due to failure to comply with experimental instructions. The final sample consisted of 85 participants: 40 vegetarians (75% female; age M = 21.43; SD = 3.21) and 45 omnivores (67% female; age M = 20.32; SD = 3.56; see Table 1 for demographic data). There are no consensus criteria for being considered a vegetarian (see Ruby, 2012 for discussion): some vegetarians avoid all animal products (vegans), some eat fish (pescetarian), and others eat meat when convenient or in social situations where avoiding meat might cause social conflict. For the purpose of this study, vegetarians were self-identified (some ate fish, but all avoided red meat and chicken). Six vegetarians also identified as vegan (consumed no animal products). Eligible participants were native English speakers without skin allergies, sensitive skin, chronic medical conditions, mental illness, asthma, or a history of cardiovascular illness or stroke. Eligible participants also had not taken medications to treat ADHD, insomnia, anxiety, high blood pressure, rheumatoid arthritis, epilepsy/seizures, cold/flu, or fever/allergies (i.e., those medications with autonomic effects) within the 72 h before the study session, and were asked to refrain from consuming caffeine, tobacco, diet pills, sleeping pills, and alcohol for 12 h prior to the experiment. To ensure food stimuli would be maximally evocative, all participants were also asked to refrain from eating for four hours before the study. Subjects received $5 per half hour of participation. The study took approximately 3 h to complete.
Table 1.
Participant demographic information. A Fisher’s exact test was used to test if the proportion of female participants differed between the two groups. BMI was tested with an independent samples t-test. BMI = Body Mass Index.
| Vegetarian | Omnivore | p value | |||
|---|---|---|---|---|---|
| n | 40 | 45 | |||
| % female | 75% | 67% | .27 | ||
| Mean | SD | Mean | SD | ||
| Age (years) | 21.43 | 3.21 | 20.32 | 3.56 | .14 |
| BMI | 22.98 | 6.96 | 23.01 | 3.78 | .98 |
2.2. Questionnaires
Participants completed a set of questionnaires that asked about demographic and health information. Participants reported their age, gender, height, weight, and dominant hand on the demographic questionnaire. The health questionnaire included questions about history of cardiovascular illness, asthma and skin allergies, chronic medical problems, family medical history, and any current medications. Participants also reported their current level of stress, number of hours they slept the previous night, how many hours per week they typically spent exercising, and their average daily consumption of alcohol, tobacco, sleeping pills and diet pills. Finally, participants reported their consumption of such products in the last 12 h to ensure compliance with eligibility criteria. In addition, participants completed a set of individual difference questionnaires at the end of the experimental session including the Individual Differences in Anthropomorphism Questionnaire (IDAQ; Waytz, Cacioppo, & Epley, 2010), and the Disgust Scale-Revised (DS-R; Olatunji et al., 2007).
2.3. Picture task
During the primary experimental task, participants viewed and rated pictures of different foods and animals while autonomic physiological measures and facial muscle activity was recorded (described below). Participants viewed 5 blocks of images: cooked meat (meat meals), vegetarian meals (vegetable meals), rotten food, sweet food, and pictures of animals on farms (for sample stimuli, see Table 2). The order in which blocks were presented was: meat meals1, animals on farms, vegetable meals1, rotten foods2, and sweet foods2. Blocks with the same superscript were randomly ordered (e.g., some participants saw: rotten foods then sweet foods, while others saw: sweet foods then rotten foods). Five pictures came from the International Affective Picture System (IAPS; Lang et al., 2008) and the rest were collected online. Pictures of the same type were presented in blocks of 12 pictures. Each picture was shown for 6 s (as in Lang et al., 1993) with a jittered ITI duration randomly drawn from a normal distribution, M = 6 s, SD = 2 s. After each picture was shown, participants made a single judgment using a continuous slider scale. For food pictures, participants were asked: ‘how appetizing is this?’ (from 0 = ‘not appetizing’ to 1 = ‘very appetizing’). For pictures of animals on farms, participants were asked: ‘how much is this animal suffering?’ (from 0 = ‘not at all’ to 1 = ‘very much’). Participants also completed a block of trials at the end of the experimental session that were not analyzed in the current investigation. During the entire task, participants were instructed to remain as still as possible, but they were given the opportunity to move if needed after each trial.
Table 2.
Picture blocks and ratings. Blocks are listed in order of presentation. Blocks with the same superscript were randomly ordered with each other. Each block contained 12 pictures shown in random order for 6 s each. After each picture, participants were asked to make a judgment about that picture. After each block, participants reported their affect and emotions during that block.
| Picture type | Example picture | Ratings |
|---|---|---|
| Meat meals1 |  |
How appetizing is this? |
| Animals on farms |  |
How much is this animal suffering? |
| Vegetable meals1 |  |
How appetizing is this? |
| Vanilla baseline Sweet foods2 |  |
How appetizing is this? |
| Rotten foods2 |  |
How appetizing is this? |
After viewing each block of 12 pictures, participants were asked to report how they felt during the previous block of pictures. First they reported how they felt on the dimensions of valence and arousal using a 100-point slider scale. Above the scale was a manikin that visually depicted valence and arousal (the 9-figure self-assessment manikin; Bradley & Lang, 1994; valence scores varied from −1 = negative to 1 = positive; arousal varied from 0 = deactivated to 1 = activated). Participants also reported the degree to which they felt: disgusted, guilty, angry, sad, happy, and hungry (from 0 = ‘not at all’ to 1 = ‘extremely’). These ratings were made in the order listed here using a 100-point continuous slider scale. Due to a software error, ratings of hunger were not recorded for the first 23 participants.
After the first three blocks, participants completed a 2–3 min ‘vanilla’ baseline task (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992). The goal of this baseline task was to have participants complete a simple cognitive task, so they would not continue to think about stimuli from prior blocks and could more easily return to their basal physiological state. For this task, participants were presented with a series of 12 colored squares (duration varied, randomly drawn from a normal distribution, M = 12 s, SD = 2 s) and were asked to count the number of red squares that appeared over the entire block.
2.4. Procedure
After providing written informed consent, participants’ eligibility was confirmed by the experimenters. Next, electrodes were applied for recording electrodermal activity (EDA), the electrocardiogram (ECG), and facial electromyography (fEMG) over the corrugator supercilii and levator labii facial muscle regions. Next, participants filled out the health and demographic questionnaires. Participants then completed a 3-min resting baseline. Next they received instructions for the picture task, which they completed as described above. After the picture task, the electrodes were removed. Finally, participants completed the individual differences questionnaires before being debriefed and remunerated.
2.4.1. Physiological measurement
All physiological measures were sampled at 1000 Hz using BioLab v. 3.0.13 (Mindware Technologies LTD; Gahanna, OH). Electrodermal activity was recorded from the thenar and hypothenar eminences of the palm on the participant’s non-dominant hand using disposable, prefilled (0.5% chloride salt) Ag/AgCl (11 mm inner diameter) electrodes from Biopac (Goleta, CA). In cases where the paste was insufficient, we added a small amount of isotonic electrode paste (Biopac; Goleta, CA) to the electrodes. The electrocardiogram was recorded by placing pregelled ConMed (Westborough, MA) Cleartrace Ag/AgCl electrodes in a modified lead II configuration on the collarbone and torso. Facial muscle activity was recorded by placing reusable Ag/AgCl electrodes (Mindware Technologies LTD; Gahanna, OH) filled with high conductivity gel (Signa Gel from BioMedical Instruments; Warren, MI) over the corrugator supercilii and levator labii muscle regions on the left side of the participant’s face (as recommended by Fridlund & Cacioppo, 1986). For one participant, sensors were placed on the right side of the face due to a facial piercing that prevented proper site preparation and placement on the left side of the face. For all participants, a reference electrode was placed behind the ear on the mastoid process (on the same side as the other electrodes). Before placement, each site was cleaned with alcohol and exfoliated using an abrasive gel (Lemon prep; Mindware Technologies LTD). To ensure proper signal conductivity, facial skin was abraded until conductance was below 5 KOhms when possible (M = 3.08, SD = 2.41). Preparation was terminated if participants reported discomfort. Due to equipment malfunctions, some physiological data was not available for some participants, so the number of observations varies for some comparisons (see Online Supplemental materials for additional details on data analysis).
2.4.2. Physiological data processing
For analyses, continuous ANS and fEMG signals for each trial were extracted resulting in 1 s of pre-stimulus data and 6 s of post-stimulus onset data. Raw EMG data were filtered using a 90 Hz high-pass filter. Because raw fEMG signals vary around zero, we calculated the absolute value of facial muscle activity. Data were then binned into 0.5-s intervals for each trial. For each signal, the 1-s mean pre-stimulus value was subtracted from each of the post-stimulus 0.5 s bins to create poststimulus change scores (as in Codispoti et al., 2001). This allowed for the visualization of responses over time during picture viewing. In studies using similar paradigms (e.g., Bradley, Moulder, & Lang, 2005; Codispoti et al., 2001), there are two prominent response phases in the heart period response during picture viewing: an early and late phase. For statistical analysis for all variables, we computed means for these two phases: 0–3 s (early phase) and 3–6 s (late phase) post-stimulus onset. To analyze cardiac data, each trial was visually inspected by trained research assistants, and automatic R-spike detection was verified using MindWare analysis software (HRV version 3.0.22; Gahanna, OH). In-house MATLAB (MathWorks, Natick, MA) scripts were used to process electrodermal activity and fEMG (corrugator and levator) data. Skin conductance level was used as our measure of electrodermal activity.
2.5. Data analysis
For statistical analysis, Greenhouse-Geisser corrections were used where appropriate. To most efficiently use available data, missing data was only dropped for specific analyses (pairwise deletion; see supplemental analysis notes for additional details). To test our hypotheses, we were primarily interested in whether a vegetarian vs. omnivore diet influenced the dependent variables. Therefore, in the analysis we focus on main effects and interactions involving diet, and on specific hypotheses based on prior literature. We do not report all comparisons, although all means and standard deviations are presented in tables within the Online Supplemental materials (Table S1–S3). The complete dataset and analysis notes are also available in the Online Supplemental materials.
3. Results
We first present the analyses of the affective ratings of the images to test whether there is stronger support for the specificity or generality hypotheses. Next, we assessed participant’s ratings of their own affect and emotion. Third, we assessed participants’ peripheral physiological responses. Lastly, we examined whether there were expected individual differences in anthropomorphism or disgust sensitivity in vegetarians vs. omnivores, and whether there was evidence of motivated denial of mind.
3.1. Affective ratings of images
To test whether vegetarians and omnivores experienced all pictures differently (generality hypothesis) or experienced only meat pictures differently (specificity hypothesis), we conducted a repeated-measures ANOVA on ratings of the images with diet (vegetarian vs. omnivore) as a between-subjects factor and picture type (meat meals, vegetable meals, rotten foods, sweet foods, animals on farms) as a within-subjects factor. Ratings were influenced by participant’s diet and type of picture, as demonstrated by a main effect of diet, F(1,82) = 42.23, p < .001, = 0.340, and picture type, F(3.47,284.10) = 444.74, p < .001, = 0.844. Main effects were qualified by a significant interaction between diet and picture type, F(3.47,284.10) = 67.86, p < .001, = 0.453. To understand the interaction, we directly compared how the different pictures were rated by vegetarians and omnivores with a series of planned, independent samples t-tests. Vegetarians reported that meat meals were significantly less appetizing, t(82) = −13.40, p < .001, = 0.686 (Fig. 1; see Table S1 for means and standard errors of the mean [SEMs], and 95% confidence intervals [ CIs ]). However there was no difference in how appetizing the two groups rated the other foods: vegetable meals, t(82) = 1.42, p = .159, rotten foods, t(82) = 0.59, p = .558, sweet foods, t(82) = −1.57, p = .121. Both groups reported that animals were experiencing substantial amounts of suffering (Fig. 1), and there was no difference between the groups, t(82) = 0.86, p = .395.
Fig. 1.
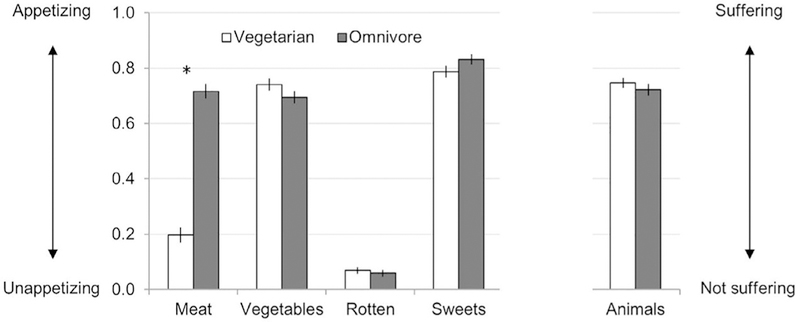
Picture ratings. Error bars represent standard errors. * represent p < .05 for t-test comparing vegetarians to omnivores. Ratings were made using 100-point slider scales. For food pictures, participants were asked: ‘how appetizing is this?’ (from 0 = ‘not appetizing’ to 1 = ‘very appetizing’). For pictures of animals on farms, participants were asked: ‘how much is this animal suffering?’ (from 0 = ‘not at all’ to 1 = ‘very much’).
3.2. Participant’s self-reported affective and emotional experience
To test whether vegetarians’ and omnivores’ self-reported affective states differed after viewing all pictures (generality hypothesis) or only after viewing pictures of meat meals (specificity hypothesis), we ran a repeated-measures MANOVA with diet as the between participant factor, picture type (meat meals, vegetable meals, rotten foods, sweet foods, animals on farms) as the repeated measure, and participants’ own affective and emotional ratings (valence, arousal, disgusted, guilty, angry, sad, and happy) as the dependent variables. Ratings of hunger were analyzed separately since the first 23 participants were missing hunger ratings. This analysis revealed a significant interaction between diet and picture type, F(28, 1162.41) = 4.158, p < .001. To understand this interaction, we conducted a series of MANOVAs, one for each type of picture, with diet as the between participant factor, and affective and emotional ratings (valence, arousal, disgusted, guilty, angry, sad, and happy) as the dependent variables. Again ratings of hunger were analyzed separately since the first 23 participants were missing hunger ratings. We ran one MANOVA for each block of pictures (meat meals, vegetable meals, rotten foods, sweet foods, and animals on farms). There was no effect of diet for vegetable meals, F(7,76) = 0.56, p = .786, rotten foods, F(7,76) = 1.576, p = .155, or sweet foods, F (7,76) = 0.249, p = .971, meaning that vegetarians and omnivores reported similar affective and emotional states when viewing vegetable meals, rotten foods, and sweet foods (see Fig. 2 & Table S2). However, diet did influence how participants reported feeling when viewing meat meals, F(7,76) = 9.75, p < .001, = 0.473, and when viewing animals on farms, F(7,76) = 2.35, p = .031, = 0.178. To understand these effects, we ran follow-up independent samples t-tests that directly compared vegetarians to omnivores on each variable. When viewing meat meals, vegetarians reported feeling more disgusted, t(82) = 5.47, p < .001, and sad, t(82) = 2.49, p < .016, and less pleasant, t (82) = −5.35, p < .001, happy, t(82) = −5.51, p < .001, and hungry, t(59) = −6.87, p < .001, compared to omnivores (Fig. 2, see Table S2 for means and CIs). When viewing animals on farms, vegetarians felt more disgusted, t(82) = 2.08, p < .042, less hungry, t (59) = −2.11, p < .04, and less pleasant, t(82) = −1.99, p = .050, but these differences were quite small compared to the differences observed for meat meals (see Table S2 for means and CIs). Interestingly, both groups felt similar levels of arousal, guilt, anger, sadness, and happiness when viewing animals on farms (see Fig. 2, Table S2).
Fig. 2.
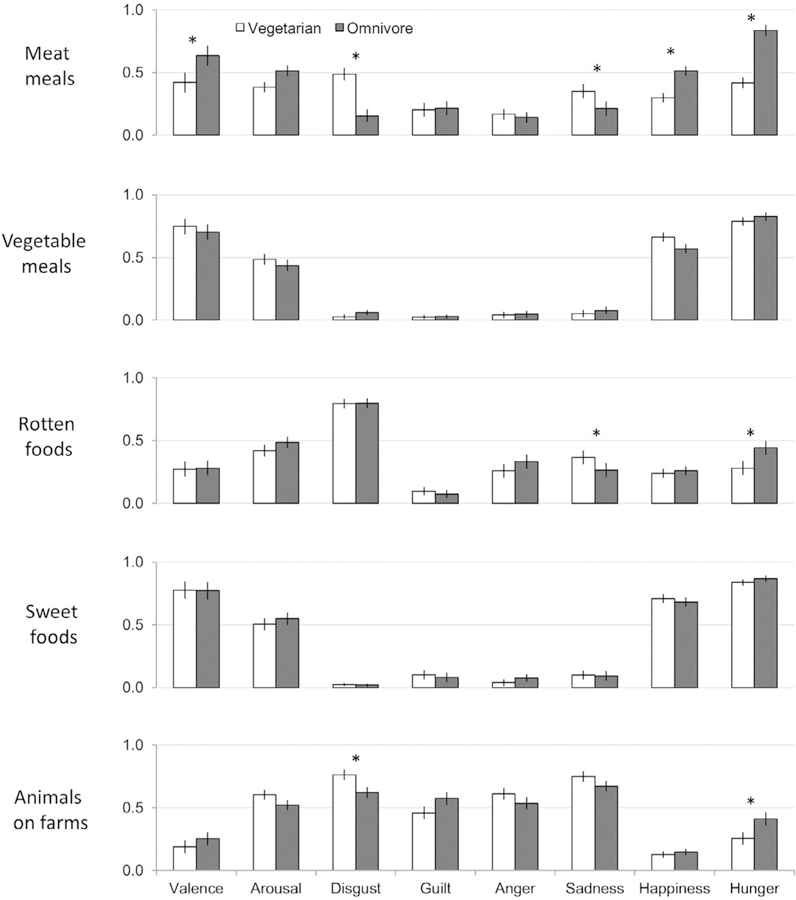
Affect and emotion ratings. Error bars represent standard errors. * represent p < .05 for t-test comparing vegetarians to omnivores. Ratings were made using 100-point slider scales. The original valence ratings on a −1 to +1 scale were transformed to be on a 0–1 scale to match the other ratings for depiction in this figure only. Valence varied from 0=negative to 1=positive; arousal varied from 0=deactivated to 1=activated. Participants also reported the degree to which they felt: disgusted, guilty, angry, sad, happy, and hungry (from 0=‘not at all’ to 1=‘extremely’).
3.3. Peripheral physiological responses
To test whether vegetarians’ and omnivores’ physiological responses differed in response to all pictures (generality hypothesis) or differed only in response to meat meals (specificity hypothesis) we first visualized responses in 0.5-s bins in Fig. 3 (as in Bradley et al., 2005 ; Codispoti et al., 2001). To test our hypotheses, we conducted a series of repeated measures ANOVAs (one for each physiological measure) with diet (vegetarian vs. omnivore) as a between-participant variable and picture type (meat meals, vegetable meals, rotten foods, sweet foods, and animals on farms) and response bin (early = 0–3 s vs. late = 3–6 s) as repeated-measures variables. Response bins were determined based prior literature that also used these same early and late time bins (Bradley et al., 2005; Codispoti et al., 2001). Change scores (from baseline) for each physiological measure served as the dependent variable in each analysis. Again, because we were primarily interested in the effects of diet, we focus on main effects and interactions that involve diet and comparisons where the literature makes clear predictions, so all comparisons are not presented. All means and measures of variance are reported in Online Supplemental Materials (Table S3).
Fig. 3.
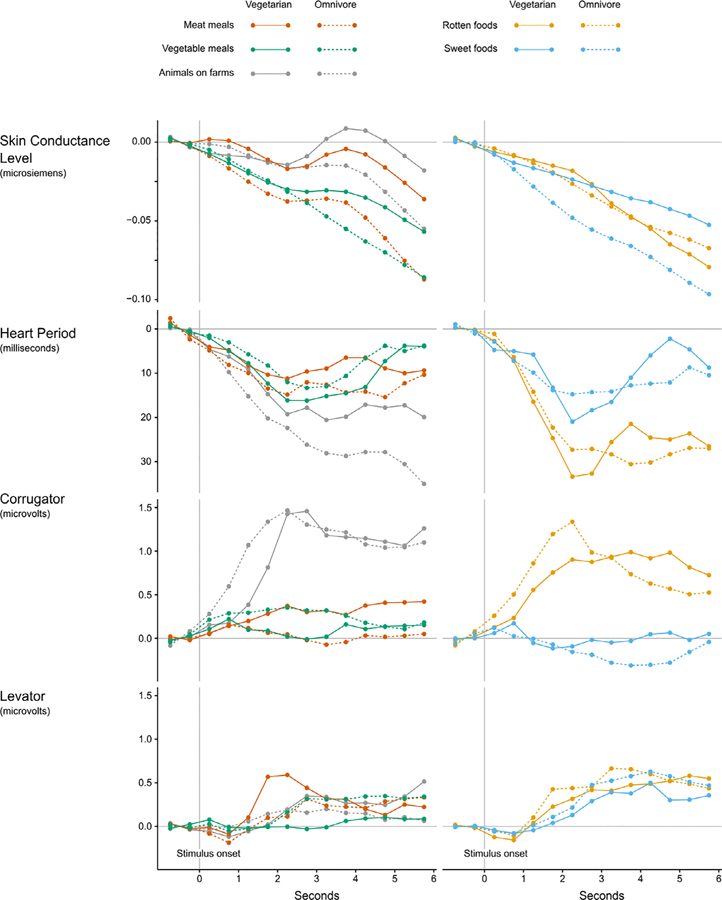
Physiological responses over time. Stimulus onset was at 0 s, offset at 6 s.
3.3.1. Electrodermal activity
For changes in skin conductance level, there was an interaction between diet and response bin, F(1,235.57) = 4.26, p = .043, = 0.055, but no main effect of diet (p = .076). To examine the interaction, we next completed two separate 2 (diet) by 5 (picture type) repeated-measures ANOVAs, one for each response bin. For the early bin (0–3 s), there was no effect of diet (p = .238) nor any interaction between diet and picture type (p = .439). For the later bin (3–6 s), there was a trend toward an effect of diet, F(1,73) = 3.88, p = .053, = 0.050, but no interaction between diet and picture type (p = .45). The finding of a trend-level effect of diet on skin conductance level can be seen in Fig. 3: vegetarians had greater changes in skin conductance level in the later time bin than omnivores across all picture types (except rotten foods). To follow up on this finding, we tested whether vegetarians and omnivores had different basal skin conductance levels during a three minute resting baseline period before the picture task started. Mean skin conductance levels were not significantly different between vegetarians and omnivores during this resting baseline period, t(77) = 0.011, p = .991.
3.3.2. Heart period
For heart period, there was no main effect of diet (p = .518) and diet did not interact with picture type (p = .626) or response bin (p = .144), so we did not conduct extensive follow-up tests. However, since previous work has shown the heart period is influenced by the valence of an image (heart rate deceleration in response to negative pictures; Bradley et al., 2001; Codispoti et al., 2001; Lang et al., 1993) we tested whether positive and negative foods influenced cardiac response. We focused on the later time bin since this was the bin in which the differences between stimuli were maximally different (see Fig. 3), although the patterns are similar for the early time bin. Consistent with previous findings showing prolonged heart period in response to negative pictures (Bradley et al., 2001; Lang et al., 1993), there was a greater prolongation of heart period (i.e., greater HR deceleration) for rotten compared to sweet food pictures, t(77) = 3.80, p < .001, = 0.158, across all participants.
3.3.3. Facial EMG
For fEMG activity over the corrugator supercilii muscle region, there was no main effect of diet (p = .902), and diet did not interact with picture type (p = .535), or time bin (p = .110), so again extensive follow-up tests were not completed. Previous work has shown increased corrugator activity in response to negative pictures (Bradley et al., 2001; Lang et al., 1993; for review see Cacioppo et al., 2000), so as with heart period, we tested whether picture type would influence corrugator activity (focusing again on the late bin, although again, the early bin shows the same pattern, see Fig. 3). Consistent with the previous research showing increased corrugator activity to negative pictures, we found greater corrugator activity when viewing rotten food pictures compared to sweet foods, t(74) = 4.05, p < .001, = 0.181, across all participants.
For facial EMG activity over the levator labii muscle region, there was no main effect of diet (p = .849), and diet did not interact with picture type (p = .530), or time bin (p = .122), so we did not conduct follow-up tests. Since previous researchers have claimed levator labii muscle region activity is associated with disgust (Chapman et al., 2009; Vrana, 1993), we tested whether rotten foods would elicit enhanced levator labii activity compared to sweet foods across all participants. We found no significant difference in levator labii activity for rotten compared to sweet foods during the later bin, t(74) = 0.447, p = .656, and visually, there were no clear patterns in levator labii muscle activity (see Fig. 3).
3.4. Individual difference measures
To test whether vegetarians and omnivores differed in trait level anthropomorphism (Waytz et al., 2010) or disgust sensitivity (Olatunji et al., 2007), we compared the two groups using independent samples ttests. As shown in Table 3, there were no group-level differences in anthropomorphism, t(72) = 0.647, p = .31, or trait disgust sensitivity, t(72) = 0.054, p = .957.
Table 3.
Participant trait information. Differences between vegetarians and omnivores were tested with independent samples t-tests. IDAQsum = Individual Differences in Anthropomorphism scale (higher numbers represent more anthropomorphism; possible range 0–150; Waytz et al., 2010). DS-R =Disgust Scale-Revised (higher numbers represent higher trait disgust; possible range 0–25; Olatunji et al., 2007).
| Vegetarian (n = 40) | Omnivore (n = 45) | p value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| IDAQsum | 53.76 | 22.70 | 48.06 | 25.27 | .31 |
| DS-R | 14.74 | 3.90 | 14.79 | 4.77 | .96 |
3.5. Motivated denial of mind
To test our secondary hypothesis concerning whether seeing meat meals prior to animals influenced the perceived suffering of animals (referred to as motivated denial of mind; Bastian et al., 2012; Loughnan et al., 2010) we compared participants who viewed meat meals first to those who viewed vegetable meals first prior to making ratings of animal suffering. We used a repeated measures ANOVA with order (meat meals first or vegetable meals first) and diet (vegetarian vs. omnivore) as between participant factors, picture type (meat meals, vegetable meals, rotten foods, sweet foods, animals on farms) as the repeated measure, and participants’ affective ratings of the images were the dependent variable. There was no main effect of order, F (1,80) = 0.022, p = .883, and order did not interact with any of the other factors. In particular, contrary to our predictions based on previous research (Bastian et al., 2012; Loughnan et al., 2010), omnivores’ ratings of perceived suffering of animals was not influenced by first seeing meat meals (M = 0.72, SD = 0.15) compared to first seeing vegetable meals (M = 0.73, SD = 0.15), t(43) = 0.163, p = .871.
4. Discussion
Our behavioral findings generally supported the specificity hypothesis: vegetarians and omnivores had similar affective responses to non-meat stimuli. We found that vegetarians and omnivores were similar in how they evaluated the appeal of non-meat food items (i.e., vegetable meals, rotten foods, sweet foods) and the suffering of animals, and they differed only in the rated appeal of meat meals. As predicted, vegetarians rated meat meals as less appetizing than omnivores (see also Amato & Partridge, 1989; Barnes-Holmes et al., 2010; De Houwer & De Bruycker, 2007; Rozin et al., 1997; Stockburger et al., 2009). Also consistent with the specificity hypothesis, vegetarians and omnivores reported feeling similar affective and emotional states when viewing non-meat foods, and were most different in their self-reported affective and emotional experiences when viewing meat meals. In particular, vegetarians reported feeling more disgusted and sad, and less pleasant, happy, and hungry when viewing meat meals compared to omnivores. Moreover, although vegetarians reported more negative emotions when viewing animals on farms compared to omnivores, these differences were quite small compared to the differences observed when viewing meat meals. This pattern of self-reported results suggests that vegetarians did not experience all images as hedonically less pleasant compared to omnivores, nor were they generally more affectively reactive to both positive and negative stimuli compared to omnivores (i.e., they did not report sweets were more appetizing and rotten foods were more unappetizing compared to omnivores). Finally, also consistent with the specificity hypothesis, we found similar levels of self-reported disgust sensitivity in vegetarians and omnivores (see also Fessler et al., 2003).
Despite robust differences in self-reported experience of meat meals, however, vegetarians and omnivores did not differ in terms of their cardiac and facial EMG responses to the meat meals (or any other stimuli). Both groups had similar cardiac responses (i.e., heart period) and facial muscle activity over both corrugator supercilii and levator labii muscle regions across all picture conditions, including to images of meat meals and animals on farms. In addition, although vegetarians (compared to omnivores) exhibited generally elevated skin conductance levels (pre-stimulus corrected amplitude changes) in response to images of meat meals, this difference was not specific to meat meals; rather, vegetarians also exhibited elevated skin conductance levels in response to vegetable meals, sweet foods, and images of animals on farms. This latter finding suggests that the experimental context may have led to greater overall skin conductance reactivity for vegetarians than for omnivores, perhaps because they were aware that they would be viewing images of meat meals and other stimuli related to vegetarianism.
Importantly, the lack of group differences in heart period and facial EMG responses to food and animal images is not due to a general lack of reactivity to the images utilized in our experiment since we found several of the expected patterns of physiological responding across the different types of pictures for the sample as a whole. We observed increased heart period (i.e., slower heart rates) and increased corrugator supercilii activity in response to rotten compared to sweet foods, although we found no clear pattern of activation over the levator labii muscle region across picture conditions despite prior suggestions that levator labii activation occurs during feelings of disgust (Chapman et al., 2009; Hoefling et al., 2009; Vrana, 1993). Additionally, both groups had robust electrodermal responses when viewing pictures of animals on farms (presented visually in Fig. 3), suggesting that looking at animals and considering whether they experience suffering is evocative for both vegetarians and omnivores. Heart period and corrugator supercilii muscle activity also increased when viewing animals on farms (Fig. 3), both of which have been previously associated with experiencing unpleasant affect in a picture-viewing paradigm (Bradley et al., 2001; Codispoti et al., 2001; Lang et al., 1993). Therefore, these results are consistent with the reported feelings of greater negative affect and the occurrence of feeling negative emotions (disgust, guilt, anger, and sadness) by both vegetarians and omnivores in the present study when viewing animals on farms.
The contrasting results from self-report versus physiological responses to meat highlights the need to collect multiple sources of data when attempting to characterize affective and emotional responses. Multiple data sources are critical because recent meta-analytic evidence demonstrates that there are no consistent cross-study autonomic signatures for a given emotion (Siegel et al., 2018). Indeed, the pattern of self-report and peripheral physiological results here is anticipated by emerging neuroscience evidence and theorizing concerning how the brain constructs experience (see, for example, Barrett, 2017; Barrett & Simmons, 2015; Clark, 2013; Denève & Jardri, 2016; Friston, 2010). According to these perspectives, we experience the world as we predict it to be (i.e., consistent with our internal model of our body in the world), with sensory input typically either confirming or adjusting that internal model1. Thus, vegetarians may experience meat as unpleasant because that is consistent with their internal model (i.e., their existing belief that meat is cruel, wasteful, impure, or unhealthy), and sensory input from the body (e.g., increased SNS activity) is incorporated into experience as confirming that internal model (i.e., it is experienced as affectively negative). Conversely, omnivores who predict enjoying meat meals based on their past experiences might interpret the same sensory input (i.e., increased SNS activity) as confirming their internal model (i.e., it is experienced as affectively positive, consistent with their viewing meat meals as hedonically pleasant). In both cases, individuals’ internal models (i.e., their predictions based on past experience) can drive their experience of the meat meals. Consistent with this interpretation, many people who convert to vegetarianism report that meat becomes less hedonically pleasant over time (Amato & Partridge, 1989; Rozin et al., 1997). One possible explanation for this ‘hedonic shift’ (Rozin et al., 1997) is that vegetarians’ experience of meat changes as they update their internal model of the world based on their new experiences and beliefs as vegetarians.
Predictions (in the form of beliefs) also have metabolic consequences. For instance, cephalic phase responses prepare the body for incoming food by secreting digestive enzymes that aid in metabolism (Power & Schulkin, 2008). As famously noted by Pavlov (1902), the body is making predictions (Clark, 2013) about incoming nutrients and preparing accordingly. In one experiment, when people believed they were consuming a high calorie ‘indulgent’ milkshake, they had increased physiological satiation (measured by a greater decrease in the appetite hormone, ghrelin) compared to those consuming a ‘sensible’ milkshake – even though the milkshakes were identical (Crum, Corbin, Brownell, & Salovey, 2011). Predictions made while chewing food also influences gastric motor activity such that unappetizing food results in dysrhythmic patterns of gastric myoelectric activity whereas appetizing food leads to the more typical rhythmic patterns associated with ingestion (Stern, Jokerst, Levine, & Koch, 2001). These findings suggest that predictions change how the body prepares for processing incoming food and these predictions enhance metabolic efficiency (e.g., Sterling, 2004, 2012). An open question for future research is how vegetarians’ bodies would prepare to metabolize meat: would a vegetarian’s brain predict that meat is a non-food item or would the brain ‘betray’ their dietary commitments by martialing digestive resources anyway?
Participants’ expectations in our study may have contributed to both subjective and physiological responding. For instance, participants knew they would not be consuming the foods pictured, which may have dampened their physiological responses to images of food. Recent evidence suggests that physiological responses to food pictures may be larger if people expect to consume the food they are viewing (Verastegui-Tena, Schulte-Holierhoek, van Trijp, & Piqueras-Fiszman, 2017). More pronounced differences might emerge between vegetarians and omnivores if they anticipated having to consume the foods shown. Future research should therefore extend this work from simply viewing images to viewing or interacting with more complex, multisensory stimuli and real foods that participants anticipate consuming. In addition, as part of the informed consent, participants were told the types of images they would be shown and that we would be assessing their affective responses to the images. It is possible these expectations either dampened or heightened affective responding during the study by orienting participants to particular values or social norms. For instance, knowing images of meat and animals would be presented could have generated arousal in vegetarians. This is consistent with our primary physiological finding that vegetarians evidenced larger increases in peripheral physiological arousal as measured by greater electrodermal change during all picture presentations over the experimental session compared to omnivores. Additionally, many vegetarians view their diet as a moral choice (Rozin et al., 1997) and a source of personal identity (Fox & Ward, 2008b). Because of this, vegetarians may also feel social pressure to make their affective reactions conform to others who share their identity (e.g., vegetarians may report meat is disgusting because they believe other vegetarians will report meat is disgusting). Moving forward, understanding how expectations may shape vegetarians’ and omnivores’ responses to food across multiple levels (e.g., selfreported, physiology, behavior) will be critical to building a more complete understanding of vegetarianism.
Additionally, future research might look at different motivations and cultural contexts for avoiding meat (for review see Ruby, 2012). Our study included people who converted to vegetarianism for animal welfare-related reasons and people who grew up avoiding meat as a cultural or religious tradition. These two groups might have very different internal models concerning meat, and thus very different experiences of and responses to meat and animals on farms. A future study with a sufficient sample size of both types of vegetarians could examine whether there is important heterogeneity among vegetarians’ affective, emotional, and physiological responses to meat.
Somewhat surprisingly, we found that vegetarians and omnivores reported similar levels of perceived animal suffering for animals on farms, and we found similar levels of self-reported anthropomorphism among vegetarians and omnivores. This suggests that vegetarians and omnivores may be similar in their tendency to perceive minds in nonhuman animals in self-reports. This contrasts with previous studies which found evidence that omnivores perceive animals as experiencing fewer emotions (Bilewicz et al., 2011) or deny animals’ experience of suffering as a way to reduce cognitive dissonance (Bastian et al., 2012 ; Loughnan et al., 2010). Also contrary to predictions, we found no evidence of motivated denial of mind among omnivores: the order in which images of meat meals and animals on farms were presented did not influence perceptions of animal suffering by omnivores when viewing animals on farms. However, previous studies that found motivated denial of mind required participants to actively choose to eat meat (Bastian et al., 2012; Loughnan et al., 2010), which may have resulted in stronger feelings of dissonance (conflict between choosing to eat meat and causing suffering to animals) and motivation to escape it. Another possible explanation for the lack of differences between vegetarians and omnivores is that our study focused on food, and this study framing may have impacted participants’ tendencies to see the meat as food and/or impacted the tendency to anthropomorphize. Future research should test the specific conditions under which vegetarians and omnivores perceive animal suffering and animal minds differently or similarly. Finally, because this study was exploratory (the first study to measure physiology in vegetarians and omnivores), future pre-registered confirmatory studies are needed (Nosek & Lakens, 2014; Wagenmakers, Wetzels, Borsboom, van der Maas, & Kievit, 2012).
4.1. Conclusion
In sum, we found no evidence of general affective differences between vegetarians and omnivores in self-reports of affective and emotional experience—rather the two groups differed in their affect only when viewing images of meat meals—consistent with the specificity hypothesis. Vegetarians and omnivores, however, had very similar patterns of cardiac and facial EMG responding across all image types, including to meat images. One possibility is that vegetarians conceptualize their bodily responses to be consistent with their diet and belief system, which may lead to more self-reported negative affect to meat meals and animals on farms, and we speculate that this may protect them from giving in to the temptation of meat. Indeed, desire for the taste of meat is one of the most common reasons given by vegetarians for returning to meat eating (Barr & Chapman, 2002; Haverstock & Forgays, 2012). Understanding the role of affective responding in the decision to eat meat (or not) may have important implications for health and well-being. In the United States alone, eating meat leads to an estimated $28.6–61.4 billion per year in healthcare costs (as of 1992; Barnard, Nicholson, & Howard, 1995), and eating too much red meat, in particular, increases mortality rates (Pan et al., 2012) and increases the risk of diseases with a large societal impact such as diabetes (Pan, Sun, & Bernstein, 2011; Song, Manson, Buring, & Liu, 2004). Despite this, many people continue to eat meat—in large part because they find it hedonically pleasant.
Supplementary Material
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.foodqual.2018.06.008.
In addition, in some circumstances, the internal model is so strong that even disconfirmatory sensory evidence is ignored in favor of the internal model.
References
- Amato P, & Partridge S (1989). The new vegetarians: Promoting health and protecting life New York and London: Plenum Press. [Google Scholar]
- Barnard ND, Nicholson A, & Howard JL (1995). The medical costs attributable to meat consumption. Preventive Medicine, 24(6), 646–655. [DOI] [PubMed] [Google Scholar]
- Barnes-Holmes D, Murtagh L, Barnes-Holmes Y, & Stewart I (2010). Using the implicit association test and the implicit relational assessment procedure to measure attitudes toward meat and vegetables in vegetarians and meat-eaters. The Psychological Record, 60(2), 287–305. [Google Scholar]
- Barr SI, & Chapman GE (2002). Perceptions and practices of self-defined current vegetarian, former vegetarian, and nonvegetarian women. Journal of the AmericanDietetic Association, 102(3), 354–360. 10.1016/S0002-8223(02)90083-0. [DOI] [PubMed] [Google Scholar]
- Barrett LF (2017). How emotions are made: The secret life of the brain New York, NY: Houghton Mifflin Harcourt. [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16(7), 419–429. 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B, Loughnan S, Haslam N, & Radke HRM (2012). Don’t mind meat? The denial of mind to animals used for human consumption. Personality & Social Psychology Bulletin, 38(2), 247–256. 10.1177/0146167211424291. [DOI] [PubMed] [Google Scholar]
- Bilewicz M, Imhoff R, & Drogosz M (2011). The humanity of what we eat:Conceptions of human uniqueness among vegetarians and omnivores. European Journal of Social Psychology, 41(2), 201–209. 10.1002/ejsp.766. [DOI] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, & Lang PJ (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1(3), 276–298. 10.1037//1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(I), 49–59. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Moulder B, & Lang PJ (2005). When good things go bad: The reflex physiology of defense. Psychological Science, 16(6), 468–473. 10.1111/j.0956-7976.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. Vol. 36 In Smith JC, Bradley MM, Scott RP, & Lang PJ (Eds.). Handbook of emotions (pp. 173–191). . [Google Scholar]
- Cameron CD, Lindquist K, & Gray K (2015). A constructionist review of morality and emotions: No evidence for speci fic links between moral content and discrete emotions. Personality and Social Psychology Review, 1–24. 10.1177/1088868314566683. [DOI] [PubMed]
- Chanes L, & Barrett LF (2016). Redefining the role of limbic areas in cortical processing. Trends in Cognitive Sciences, 20(2), 96–106. 10.1016/j.tics.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA, Kim DA, Susskind JM, & Anderson AK (2009). In bad taste: Evidence for the oral origins of moral disgust. Science, 323(5918), 1222–1226. 10.1126/science.1165565. [DOI] [PubMed] [Google Scholar]
- Clark A (2013). Whatever next? Predictive brains, situated agents, and the future of cognitive science. The Behavioral and Brain Sciences, 36(3), 181–204. 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Bradley MM, & Lang PJ (2001). Affective reactions to briefly presented pictures. Psychophysiology, 38(3), 474–478. [PubMed] [Google Scholar]
- Craig A (2015). How do you feel? An interoceptive moment with your neurobiological self Princeton University Press. [Google Scholar]
- Crum AJ, Corbin WR, Brownell KD, & Salovey P (2011). Mind over milkshakes: Mindsets, not just nutrients, determine ghrelin response. Health Psychology, 6, 1–6. 10.1037/a0023467. [DOI] [PubMed] [Google Scholar]
- De Houwer J, & De Bruycker E (2007). Implicit attitudes towards meat and vegetables in vegetarians and nonvegetarians. International Journal of Psychology, 42(3), 158–165. 10.1080/00207590601067060. [DOI] [Google Scholar]
- Denève S, & Jardri R (2016). Circular inference: Mistaken belief, misplaced trust. Current Opinion in Behavioral Sciences, 11, 40–48. 10.1016/j.cobeha.2016.04.001. [DOI] [Google Scholar]
- Fessler D, Arguello AP, Mekdara JM, & Macias R (2003). Disgust sensitivity and meat consumption: A test of an emotivist account of moral vegetarianism. Appetite, 41(1), 31–41. 10.1016/S0195-6663(03)00037-0. [DOI] [PubMed] [Google Scholar]
- Fiddes N (1991). Meat: A natural symbol New York and London: Routledge. [Google Scholar]
- Fox N, & Ward K (2008a). Health, ethics and environment: A qualitative study of vegetarian motivations. Appetite, 50(2–3), 422–429. 10.1016/j.appet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Fox N, & Ward KJ (2008b). You are what you eat? Vegetarianism, health and identity. Social Science & Medicine, 66(12), 2585–2595. 10.1016/j.socscimed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, & Cacioppo JT (1986). Guidelines for human electromyographic research. Psychophysiology, 23(5), 567–589. [DOI] [PubMed] [Google Scholar]
- Friston K (2010). The free-energy principle: A unified brain theory? Nature Reviews Neuroscience, 11(2), 127–138. 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Haidt J, Mccauley C, & Rozin P (1994). Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual Differences, 16(5), 701–713. 10.1016/0191-8869(94)90212-7. [DOI] [Google Scholar]
- Haverstock K, & Forgays DK (2012). To eat or not to eat. A comparison of current and former animal product limiters. Appetite, 58(3), 1030–1036. 10.1016/j.appet.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Hoefling A, Likowski KU, Deutsch R, Häfner M, Seibt B, Mühlberger A, … Strack F (2009). When hunger finds no fault with moldy corn: Food deprivation reduces food-related disgust. Emotion, 9(1), 50–58. 10.1037/a0014449. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, … Feldman Barrett L (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nature Human Behaviour, 1(5), 69 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert B (2008). International Affective Picture System(IAPS): Digitized photographs, instruction manual and affective ratings. Technical Report A-6
- Lang PJ, Greenwald MK, Bradley MM, & Hamm AO (1993). Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology, 30(3), 261–273. [DOI] [PubMed] [Google Scholar]
- Loughnan S, Haslam N, & Bastian B (2010). The role of meat consumption in the denial of moral status and mind to meat animals. Appetite, 55(1), 156–159. 10.1016/j.appet.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Lowe MR, & Butryn ML (2007). Hedonic hunger: A new dimension of appetite? Physiology and Behavior, 91(4), 432–439. 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Nosek BA, & Lakens D (2014). Registered reports: A method to increase the credibility of published results. Social Psychology, 45(3), 137–141. 10.1027/1864-9335/a000192. [DOI] [Google Scholar]
- Olatunji BO, Williams NL, Tolin DF, Abramowitz JS, Sawchuk CN, Lohr JM, & Elwood LS (2007). The Disgust Scale: Item analysis, factor structure, and suggestions for refinement. Psychological Assessment, 19(3), 281–297. 10.1037/1040-3590.19.3.281. [DOI] [PubMed] [Google Scholar]
- Pan A, Sun Q, & Bernstein A (2011). Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. The American, 1–9. 10.3945/ajcn.111.018978.INTRODUCTION. [DOI] [PMC free article] [PubMed]
- Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, … Hu FB (2012). Red meat consumption and mortality. Archives of Internal Medicine, 172(7), 555–563. 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP (1902). The work of the digestive glands (Tompson WH, Trans.)London: Charles Griffin Co Ltd. [Google Scholar]
- Power M, & Schulkin J (2008). Anticipatory physiological regulation in feeding biology: Cephalic phase responses. Appetite, 50, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Markwith M, & Stoess C (1997). Moralization and becoming a vegetarian:The transformation of preferences into values and the recruitment of disgust. Psychological Science, 8(2), 67–73. [Google Scholar]
- Ruby MB (2012). Vegetarianism. A blossoming field of study. Appetite, 58(1), 141–150. 10.1016/j.appet.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Siegel EH, Sands MK, Van den Noortgate W, Condon P, Chang Y, Dy J, … Barrett LF (2018). Emotion fingerprints or emotion populations? A meta-analytic investigation of autonomic features of emotion categories. Psychological Bulletin, 144(4), 343–393. 10.1037/bul0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Manson JE, Buring JE, & Liu S (2004). A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The women’s health study. Diabetes Care, 27(9), 2108–2115. 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- Sterling P (2004). Principles of allostasis: optimal design, predictive regulation, pathophysiology, and rational therapeutics. In Schulkin J (Ed.). Allostasis, homeostasis, and the costs of physiological adaptation Cambridge, UK: Cambridge University Press. [Google Scholar]
- Sterling P (2012). Allostasis: A model of predictive regulation. Physiology and Behavior, 106(1), 5–15. 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sterling P, & Laughlin S (2015). Principles of neural design Cambridge, MA: MIT Press. [Google Scholar]
- Stern R, Jokerst M, Levine M, & Koch K (2001). The stomach’s response to unappetizing food: Cephalic vagal effects on gastric myoelectric activity. Neurogastroenterology and Motility, 13, 151–154. [DOI] [PubMed] [Google Scholar]
- Stockburger J, Renner B, Weike AI, Hamm AO, & Schupp HT (2009).Vegetarianism and food perception. Selective visual attention to meat pictures. Appetite, 52(2), 513–516. 10.1016/j.appet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Verastegui-Tena L, Schulte-Holierhoek A, van Trijp H, & Piqueras-Fiszman B (2017). Beyond expectations: The responses of the autonomic nervous system to visual food cues. Physiology and Behavior, 179(March), 478–486. 10.1016/j.physbeh.2017.07.025. [DOI] [PubMed] [Google Scholar]
- Vrana SR (1993). The psychophysiology of disgust: Differentiating negative emotional contexts with facial EMG. Psychophysiology, 30(3), 279–286. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Wetzels R, Borsboom D, van der Maas HLJ, & Kievit RA(2012). An agenda for purely confirmatory research. Perspectives on Psychological Science, 7(6), 632–638. 10.1177/1745691612463078. [DOI] [PubMed] [Google Scholar]
- Waytz A, Cacioppo JT, & Epley N (2010). Who sees human? The stability and importance of individual differences in anthropomorphism. Perspectives on Psychological Science, 5(3), 219–232. 10.1177/1745691610369336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


