Abstract
Purpose:
This study evaluated the potential role of immune cells and molecules in the pathogenesis and clinical course of PanNET.
Experimental Design:
Surgically resected PanNETs (N=104) were immunohistochemically analyzed for Ki67 index, mitotic rate, macrophage, CD4+ cell, and CD8+ T cell infiltration, as well as HLA class I, PD-L1, and B7-H3 expression. Results were correlated with clinicopathological characteristics as well as with disease-free (DFS) and disease-specific (DSS) survival.
Results:
The median age of the 57 WHO grade 1 and 47 WHO grade 2 patients was 55 years. High intratumoral CD8+ T cell infiltration correlated with prolonged DFS (P=0.05), especially when the number of tumor-associated macrophages (TAMs) was low. In contrast, high peritumoral CD4+ cell and TAM infiltration were associated with a worse DFS and DSS. PD-L1 and B7-H3 were expressed in 53 and 78% PanNETs, respectively. HLA class I expression was defective in about 70% PanNETs. HLA-A expression correlated with favorable DSS in PD-L1-negative tumors (P=0.02). TAM infiltration (P=0.02), WHO grade (P=0.04), T stage (P=0.01), and lymph node positivity (P=0.04) were independent predictors of DFS. TAM infiltration (P=0.026) and T stage (P=0.012) continued to be predictors of DFS in WHO grade 1 PanNET patients. TAM infiltration was the sole independent predictor of DSS for WHO grade 1 and 2 patients (P=0.02). Therefore, this biomarker may contribute to identifying WHO grade 1 patients with poor prognosis.
Conclusions:
TAM infiltration appears to be the most informative prognostic biomarker in PanNET. It may represent a useful immunotherapeutic target in PanNET patients.
Keywords: Pancreatic Neuroendocrine Tumor (PanNET), Tumor Associated Macrophage (TAM), Immune Response, Human Leukocyte Antigen (HLA), Immune checkpoint
Introduction
Pancreatic neuroendocrine tumors (PanNETs) represent approximately 1–2% of all pancreatic malignancies (1). PanNETs are subdivided into functional (F-PanNETs) and non-functional (NF-PanNETs) based on their ability to elicit clinical symptoms due to inappropriate neuropeptide hormone secretion (2). The incidence of NF-PanNETs has been increasing steadily over the past two decades, accounting for nearly 60–80% of newly diagnosed PanNETs (2,3). This increase can be attributed, at least in part, to the improved and more frequent use of imaging in clinical practice (4). Similar to pancreatic ductal adenocarcinoma (PDAC), surgical resection of a PanNET continues to be the only curative therapy. Currently, three staging systems are used to categorize PanNETs. The most frequently used staging system is the WHO classification which divides tumors into 3 grades based on proliferation markers and morphology (5,6). The WHO classification provides a good algorithm for patient stratification. However, patients with low grade PanNETs continue to have a disparate disease course (7). This highly variable outcome in low grade PanNETs makes treatment and follow up difficult. These findings emphasize the need of novel prognostic biomarkers to improve the stratification of PanNET patients.
The impressive clinical responses to checkpoint inhibitor-based immunotherapy observed in many types of cancer have rekindled interest (8,9) in the role of immunosurveillance in the pathogenesis and clinical course of cancer. Immunotherapeutic strategies are being developed for many types of cancer. Nevertheless, to the best of our knowledge, only limited and at times conflicting information is available regarding the role of the immune microenvironment in the pathogenesis and clinical course of PanNET (10,11). Furthermore, six out of the seven published studies have been performed with a sample size which may not be sufficient to draw reliable conclusions (10–16). Other (15,16) studies have combined PanNET with other types of neuroendocrine tumors, therefore, questioning the applicability of the conclusions presented to PanNET. The limitations of the currently available information have a negative impact on the rational design of strategies which enhance the ability of a patient’s immune system to control his/her tumor and/or to counteract escape mechanisms utilized by tumor cells to avoid their destruction by the host’s immune system. In the present study, we have analyzed the immune microenvironment which may play a role in the interactions of PanNET cells with host’s immune system. They include i) measuring a patient’s immune response to his/her own PanNET by characterizing the extent of tumor-associated macrophages (TAMs), CD4+ cells and CD8+ T cells; ii) HLA class I molecules, which play a crucial role in the recognition of PanNET cells by host’s immune system by presenting tumor antigen (TA)-derived peptides to cognate CD8+ T cells; iii) HLA class II antigens which mediate the interactions of tumor cells with the host’s CD4+ cells; and iv) PD-L1 and B7-H3, which are both members of the B7 ligand family. PD-L1 has a negative impact on the anti-tumor activity of cognate T cells by triggering the release of inhibitory signals following its interaction with its receptor PD-1, which is expressed on T cells and NK cells (17). On the other hand, B7-H3 has been reported to be a ‘double-edged sword’. It inhibits the anti-tumor activity of cognate T cells and is associated with a poor prognosis in some malignancies such as lung, colorectal, prostate and ovarian cancers (8,9,18–21), but is associated with a good prognosis in other malignancies such as gastric and pancreatic cancer (22,23). The results of the aforementioned immunological evaluations were correlated with clinicopathologic parameters as well as disease-free (DFS) and disease-specific (DSS) survival to determine their clinical relevance.
Materials and Methods
Patients and tissues
PanNET specimens were obtained from 104 patients who underwent surgical resection between 1995 and 2012 at the Massachusetts General Hospital. PanNETs were confirmed histopathologically by a gastrointestinal pathologist (VD) according to the WHO classification system. Clinicopathologic data collected included patient gender, age, race, age-adjusted Charlson Age Comorbidity Index (CACI) (24), tumor size, WHO grade, lymph node positivity, postoperative complications according to the Clavien-Dindo classification, as well as recurrence and survival data. Tumors were formalin fixed and paraffin embedded according to standard procedures. Mitotic counts and Ki67 labeling index (Ki67LI) were enumerated as previously described (25). Adjuvant therapy data were not included in the analyses given that adjuvant therapy was administered to all except one (N=25) of the patients with disease recurrence (N=26), but to none of the patients who were recurrence-free at the time of last follow-up (N=78). Furthermore, the majority (N=14) of patients with recurrence were treated with an octreotide-based regimen (Supplementary Table. S1). The present study was conducted in accordance with the guidelines set by the Declaration of Helsinki. It was approved by the Institutional Review Board (IRB). Written informed consent was obtained from all the patients enrolled in this study.
Antibodies
The mouse mAb HCA2 recognizes β2m-free HLA-A (excluding -A24), -B7301, and -G heavy chains (26); mAb HC-10 recognizes β2m-free HLA-A3, -A10, -A28, -A29, -A30, -A31, -A32, -A33, and all β2m-free HLA-B (excluding -B5702, -B5804, and -B73) and -HLA-C heavy chains (26,27); mAb NAMB-1 recognizes both free and HLA class I heavy chain-associated human β2m (28); and mAb LGII-612.14 recognizes HLA-DR, DQ, and DP β chains (29). mAbs were purified from ascitic fluid by affinity chromatography on Protein G. Purity and activity of mAb preparations were monitored by SDS-PAGE and by reactivity with the corresponding antigens in binding assays and Western blot analysis. Mouse anti-human CD8 (M7103, clone C8/144B) and CD68 (M0876, clone PG-M1) mAbs are from Dako (Carpinteria, CA), rabbit anti-human CD4 (ab133616, clone EPR6855), FoxP3 (ab99963, clone SP97), CD163 (ab182422, clone EPR19518) mAbs and DDX58 (RIG-I, ab45428) polyclonal antibody are from Abcam (Cambridge, MA), rabbit anti-human PD-L1 (13684, clone E1L3N) and Phospho-Stat1(Ser727) (8826, clone D3B7) mAbs are from Cell Signaling (Danvers, MA), and goat anti-human B7-H3 antibody (AF1027, polyclonal) is from R&D Systems (Minneapolis, MN).
Immunohistochemical staining and scoring
Tissue sections (5 mm) from formalin-fixed, paraffin-embedded PanNET samples were used as substrates in immunohistochemical (IHC) reactions. IHC staining was performed with all the antibodies as described (30), except for the PD-L1-specific mAb. In the latter case, FFPE sections were deparaffinized and subjected to antigen retrieval using the Leica Bond protocol (Leica Microsystems Inc., Buffalo Grove, Ill) with proprietary Retrieval ER2 (ethylene diamine tetraacetic acid solution, pH 9.0). The PD-L1-specific mAb was used at the 1:150 dilution; the signal was detected by the Polymer Refine Kit (Leica Microsystems Inc.) on a Leica Bond Rx autostainer. Macrophages were identified by CD68- and CD163-specific mAbs in a double staining technique.
Tumor cell staining with HLA class I-specific and HLA class II-specific mAb was scored as positive, heterogeneous, and negative when >75%, 25%−75%, and <25%, respectively, of PanNET cells were stained (31). Staining obtained with CD4-, CD8-, CD68-, CD163- and FoxP3-specific mAbs was scored by counting the number of stained tumor-infiltrating immune cells in four high-power fields (HPF, x400) of maximal concentration of cells, around the tumor (peritumoral) and within the tumor (intratumoral). Subsequently, the mean number of cells per HPF was calculated. PD-L1 staining was scored as positive when ≥5% of PanNET cells were stained (32,33), irrespective of the location of the staining on the cell membrane or in the cytoplasm. PD-L1 staining was also assessed in stromal fibroblasts and pericytes. Staining obtained with the B7-H3-, RIG-I- and pSTAT1-specific antibodies was scored as negative when no staining was detected, and positive when staining was present. Scoring of B7-H3 staining also took into account its distribution on the PanNET cell membrane, in PanNET cell cytoplasm and in stromal cells (fibroblasts and pericytes). Scoring was performed by two investigators (LC and TM) independently and was verified by a pathologist (VD). The consensus was reached when the difference between the results obtained by the two investigators was less than 20%. The investigators who scored the stained tissue sections had no knowledge of patients’ characteristics. Numbers of tumor-infiltrating CD4+ cells, CD8+ and FoxP3+ T cells, as well as TAM (CD68+CD163+) were analyzed in tertiles of equal sample size (low: 0–33rd percentile; median: 34th-66th percentile; high: 67th-100th percentile).
Statistical analysis
Continuous variables are presented as medians (interquartile range [IQR]). The inter-rater reliability for the assessment of the IHC staining was analyzed using Cohen’s κ. The correlation between continuous variables was assessed with Spearman’s rho. Comparison of categorical variables among groups was performed using the Fisher’s exact test. Comparison of continuous variables among groups was performed using the Mann-Whitney U test or the Kruskal-Wallis method, as appropriate. Differences in the number of different types of tumor infiltrating cells were analyzed using the Wilcoxon’s signed rank test. Hierarchical cluster analyses were performed based on the immunological characteristics analyzed (HLA-A, HLA-B/C, β2m and PD-L1 expression by PanNET cells; number of peritumoral or intratumoral CD4+ cells, CD8+ T cells and total TAMs). This analysis was performed with an agglomeration schedule. The cluster distance was expressed as the square euclidean distance. A dendrogram was plotted using Ward’s method. A heatmap with cluster characteristics was created.
DFS and DSS were calculated from the date of operation to the date of recurrence or disease-specific death, respectively (event), or to the date of the last follow-up visit (censored). Survival curves were plotted using the Kaplan-Meier method. Differences in DFS and DSS between groups were analyzed by the log-rank test. Multivariate survival analyses were performed using the backward conditional Cox method. P<0.05 was considered to be statistically significant. All the tests used were two-tailed. Statistical analyses were performed with the IBM SPSS Statistics software for Windows, Version 20.0 (IBM Corp., Armonk, NY).
Results
Clinicopathologic features
The median age of the 104 resected PanNET patients was 55 years (IQR 47–63); 51 (49%) patients were female (Table 1). The majority (95, 91%) of PanNETs were non-functional with a median size of 2.8cm (range = 0.7–16.0). Fifty-seven (55%) tumors were WHO grade 1 and the remaining 47 (45%) were grade 2. Eighteen (17%) patients had positive lymph nodes. The 90-day mortality was 0% and twenty (19%) had a postoperative complication of Clavien-Dindo grade III or greater. The median DFS and DSS could not be calculated because more than 50% of patients were alive or disease-free at the end of the follow-up period. Both parameters were not reached after a median follow-up of 69.6 months (IQR 41.2–109.3). As a result, the mean DFS and DSS values were used. The mean DSS from the time of operation to death was 190 months (95%CI: 181–198).
Table 1.
Clinicopathological characteristics of 104 PanNET patients who underwent to surgery at MGH between 1995 and 2012.
| Median (IQR) | Number (%) | |
|---|---|---|
| Gender | ||
| Male | 53 (51) | |
| Female | 51 (49) | |
| Age at surgery, year 55 (47–63) | ||
| Race | ||
| Caucasian | 90 (86) | |
| African American | 6 (6) | |
| Hispanic | 4 (4) | |
| Asian | 3 (3) | |
| Other | 1 (1) | |
| CACI 3 (1–5) | ||
| Functional | ||
| Yes | 9 (9) | |
| No | 95 (91) | |
| Tumor size, cm 2.8 (2.0–4.5) | ||
| WHO grade | ||
| 1 | 57 (55) | |
| 2 | 47 (45) | |
| Mitotic count /10HPF 0.2 (0.0–2.0) | ||
| Ki67 labeling index, % 2.0 (1.2–3.2) | ||
| T stage | ||
| 1 | 27 (26) | |
| 2 | 59 (57) | |
| 3 | 16 (15) | |
| 4 | 2 (2) | |
| Lymph node positivity | ||
| Yes | 18 (17) | |
| No | 86 (83) | |
| Clavien-Dindo grade (90 days) | ||
| 0-II | 84 (81) | |
| III-V | 20 (19) | |
IQR: interquartile range; CACI: Charlson Age Comorbidity Index; HPF: high-power field
Tumor infiltrating lymphocytes and TAMs in the tumor microenvironment
Immune cell infiltrates were present in all tumor samples (100%). Representative staining patterns with CD4-, CD8-, CD68- and CD163- specific mAbs are shown in Fig. 1A. The median number of peri-tumoral CD8+ T cells/HPF (23, IQR 12–50) was higher than that of intra-tumoral CD8+ T cells counted per HPF (3, IQR 1–6). The median number of peri- and intra-tumoral CD4+ cells/HPF were 31 (IQR 17–55) and 2 (IQR 1–6), respectively (Supplementary Table. S2). FoxP3+ cells were rarely detected in the PanNETs tested. Therefore, no analysis could be performed. Both peritumoral CD8+ T cells (P<0.001) and CD4+ cells (P<0.001) were significantly more numerous than their intratumoral counterparts.
Figure 1.
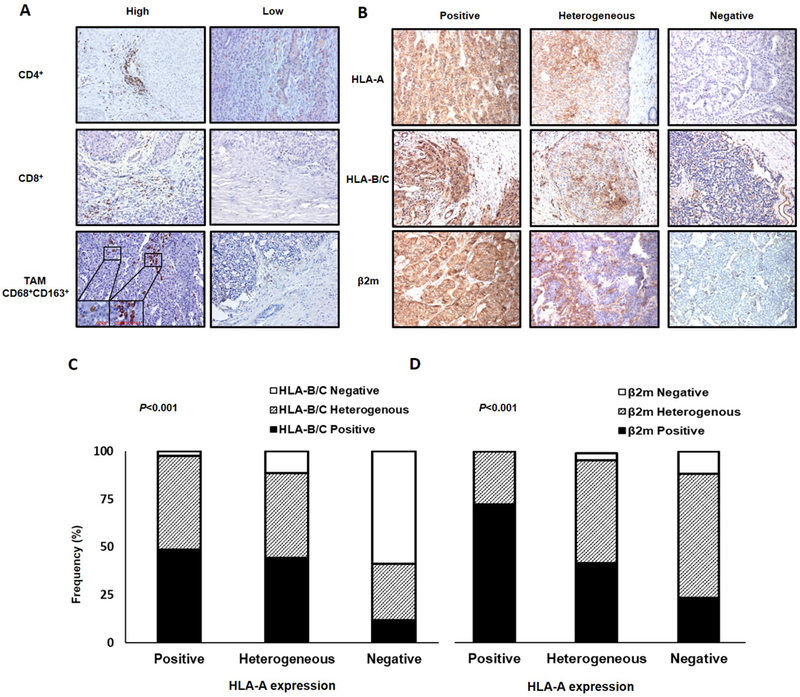
Immune cell infiltration and HLA class I expression in PanNET tumors. A, PanNET tissue sections were IHC stained with rabbit anti-CD4, mouse anti-CD8, mouse anti-CD68, and rabbit anti-CD163 mAbs. Different levels of CD4+ cell, CD8+ T cell, and TAM (CD68+CD163+) infiltration (X200 original magnification, insets X800 original magnification) are shown. B, PanNET tissue sections were IHC stained with mouse anti-HLA-A mAb HCA2, anti-HLA-B/C mAb HC-10, and anti-β2m mAb NAMB-1. Representative patterns of positive, heterogeneous, and negative HLA-A, HLA-B/C and β2m staining (X200 original magnification) are shown. C, HLA-A expression is significantly associated with HLA-B/C expression (P<0.001). D, HLA-A expression is significantly associateted with β2m expression (P<0.001). P values derived from Fisher exact test.
Intra-tumoral TAMs were rarely detected in the PanNETs tested. As a result, the total number of TAMs (peri-tumoral and intra-tumoral) was used. The median number of TAMs per HPF was 2 (IQR 1–10) (Supplementary Table. S2). The number of TAMs was correlated to that of peritumoral CD4+ cells (Spearman’s rho=0.26, 95%CI: 0.042–0.45, P=0.01), although it was significantly lower than that of both peritumoral CD4+ cells (P<0.001) and CD8+ T cells (P<0.001). The inter-rater reliability for the assessment of tumor infiltrating cells was very high (Cohen’s κ=0.81, P<0.001).
HLA class I and HLA class II expression
HLA-A, HLA-B/C and β2m expression was defective (negative or heterogeneous) in 75 (72%), 68 (65%) and 58 (56%) of the 104 PanNETs tested, respectively. Specifically, HLA-A, HLA-B/C and β2m expression was scored as negative in 34 (33%), 27 (26%), and 4 (4%) cases, and as heterogeneous in 41 (39%), 41 (39%) and 54 (52%) cases, respectively (Supplementary Table. S3). Representative staining patterns of PanNET lesions for HLA class I subunits are shown in Fig. 1B.
HLA-A and HLA-B/C expression was strongly associated with each other (P<0.001, Fig. 1C). In fact, HLA-A and HLA-B/C expression patterns were concordant in 63 (61%) tumors; of those, 20 (32%), 23 (36%) and 20 (32%) tumors were positive, heterogeneous and negative, respectively. HLA-A (P<0.001, Fig. 1D), but not HLA-B/C (P=0.06) expression was significantly associated with β2m expression.
HLA class II antigens were not detected in PanNET cells in any of the tumor samples analyzed. In contrast, blood vessel endothelial cells which were used as a positive control were stained by the HLA class II-specific mAb LGII-612.14 used.
PD-L1 and B7-H3 expression
Representative examples of PD-L1 and B7-H3 staining in PanNets are shown in Fig. 2A; the results are summarized in Supplementary Table S4. PD-L1 staining was mostly restricted to PanNET cells, and was expressed in 55 (53%) cases. In 40 (73%) of them, PD-L1 was expressed only in the PanNET cell cytoplasm, while in 4 (7%) only on the PanNET cell membrane, and in the remaining 11 (20%) on both the PanNET cell membrane and in the cytoplasm. Stromal PD-L1 expression was observed in 25 (24%) PanNETs and was positively associated with PanNET cell PD-L1 expression (P=0.001). Only 6 (24%) cases demonstrated stromal PD-L1 expression without a corresponding PanNET cell expression.
Figure 2.
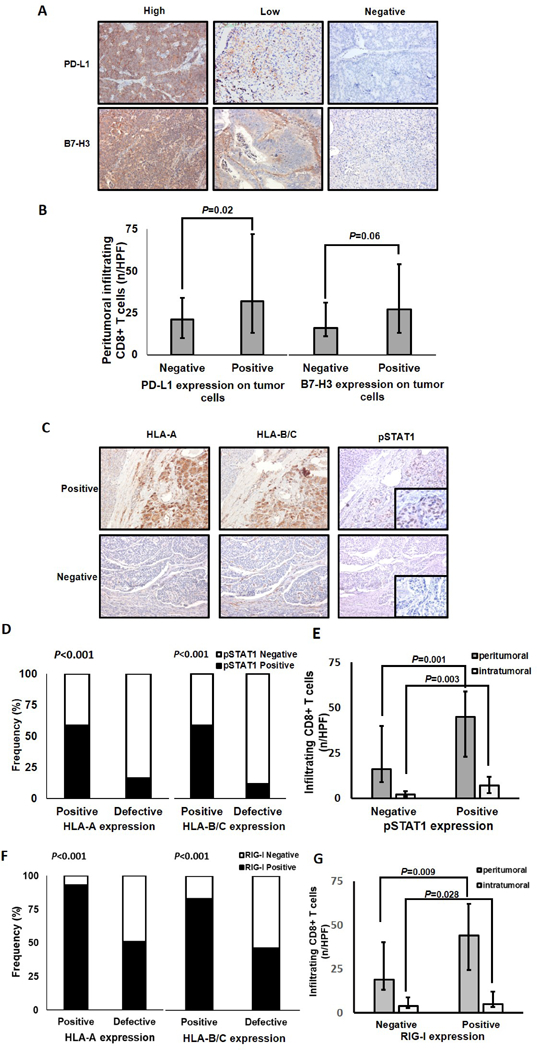
Association of PD-L1, B7-H3, phospho-STAT1, and RIG-I expression with the extent of peritumoral CD8+T-cell infiltration and with HLA class I expression in PanNETs. A, PanNET tissue sections were IHC stained with rabbit anti-PD-L1 mAb and goat anti-B7-H3 pAb. Representative patterns of highly positive embrane/cytoplasmic, scattered positive membrane/cytoplasmic and negative PD-L1 staining (X200 original magnification) are shown. Representative patterns of highly positive membrane/cytoplasmic, low membrane/cytoplasmic and negative B7-H3 staining (X200 original magnification) are shown. B, Peritumoral CD8+ T-cell number is significantly associated with PD-L1 expression (P=0.02), but not with cytoplasmic and/or membranous B7-H3 expression (P=0.06). C, PanNET tissue sections were IHC stained with mouse anti-HLA-A mAb HCA2, mouse anti-HLA-B/C mAb HC-10, rabbit anti-phosphoStat1 (pSTAT1) mAb, and rabbit anti-RIG-I pAb. The positive HLA-A and HLA-B/C expression in PanNETs with positive pSTAT1 expression and the low/negative HLA-A and HLA-B/C expression in PanNETs with negative phosphoStat1 expression (X200 original magnification, insets X400 original magnification) are shown. D, Positive HLA-A and HLA-B/C expression is significantly associated with positive pSTAT1 expression (P<0.001). E, Peritumoral and intratumoral CD8+ T-cell number is significantly associated with pSTAT1 expression (P=0.001 and P=0.003). F, HLA-A and HLA-B/C expression is significantly associated with RIG-I expression (P<0.001). G, Peritumoral and intratumoral CD8+ T-cell number is significantly associated with positive RIG-I expression (P=0.009 and P=0.028). Bars, median values. Error bars, interquartile range. P values derived from Mann–Whitney U tests.
B7-H3 was expressed by PanNET cells in 81 (78%) tumors. In 54 (67%) of them, B7-H3 was expressed only in the PanNET cell cytoplasm, in 5 (6%) only on the PanNET cell membrane, and in the remaining 22 (27%) on both the PanNET cell membrane and in the cytoplasm. B7-H3 was expressed in stromal cells of 76 (73%) tumors, with no relationship to its cytoplasmic or cell membrane expression by PanNET cells.
There was no association between PD-L1 and B7-H3 expression. Neither PD-L1 nor B7-H3 expression was associated with HLA-A or HLA-B/C expression; however, membranous B7-H3 expression (with or without cytoplasmic expression) was significantly associated with β2m expression (P=0.04). The mechanism(s) underlying this association is(are) not known; the only feature shared by these two molecules, that we are aware of, is the location of the two encoding genes in the chromosome 15 long arm (34,35).
Phospho-STAT1 (pSTAT1) activation and RIG-I expression
Representative staining examples of PanNET lesions for pSTAT1 expression are shown in Fig. 2C. Phospho-STAT1 was expressed in only 30 (29%) PanNETs, with a significant direct association with HLA-A and HLA-B/C expression (P<0.001 for both, Fig. 2D).
Representative staining examples of PanNET lesions for RIG-I expression are shown in Supplementary Fig. S1. RIG-I was expressed in 65 (62.5%) PanNETs with a significant association with HLA-A, HLA-B/C (P<0.001 for both, Fig. 2F) and pSTAT1 (P<0.006) expression.
Association of tumor infiltration extent by T cells with HLA class I, checkpoint molecule, pSTAT1 and RIG-I expression
Positive HLA-A (35 vs 19 cells/HPF, P=0.01; 5 vs 2 cells/HPF, P=0.002, respectively, Supplementary Fig. S2A and S2B), HLA-B/C (45 vs 16 cells/HPF, P<0.001; 7 vs 2 cells/ HPF, P<0.001, respectively, Supplementary Fig. S2A and S2B), pSTAT1 expression (45 vs 18 cells/ HPF, P=0.001; 6 vs 3 cells/ HPF, P=0.003, Fig. 2E) and RIG-I expression (42 vs 20 cells/ HPF, P=0.009; 5 vs 4 cells/ HPF, P=0.028, respectively, Fig. 2G) were associated with a higher number of peritumoral and intratumoral CD8+ T cells. Noteworthy is that positive PD-L1 expression on tumor cells (32 vs 21 cells/HPF, P=0.02) was associated with a higher number of peritumoral CD8+ T cells (Fig. 2B). Positive cytoplasmic and/or membranous B7-H3 expression was associated with a higher number of peritumoral CD8+ T cells (27 vs 16 cells/HPF, P=0.06, Fig. 2B). However, the latter association did not reach statistical significance.
Clustering of tumors based on immune characteristics
Clustering analysis of the tested PanNETs based on their immunohistochemical characteristics divided the patients into 3 groups. Cluster 1 tumors appear to be immunologically inactive (“cold”) with defective HLA expression and low immune cell infiltration, while cluster 3 tumors appear to be more ”hot” with positive HLA expression and dense cell infiltration. Cluster 2 tumors appear to be immunologically “lukewarm” with intermediate immunologic activity. The extent of TAM infiltration was not significantly different among the 3 identified clusters. (Data not shown)
Association of the immune profile of PanNETs with their clinicopathologic characteristics
Patients with positive lymph nodes had a high number of peritumoral CD4+ cells in their primary PanNETs (43 vs 29 cells/HPF, P=0.04). Furthermore, PanNETs with a high Ki67LI had a high number of TAMs (Spearman’s rho=0.30, 95%CI: 0.11–0.48, P=0.003). Similarly, a mitotic count of 2 or more mitoses/10 HPF was associated with PD-L1 expression on PanNET cells (P=0.04) and B7-H3 expression in stromal cells(P=0.03) (Table. S5).
Association of clinicopathologic characteristics of PanNETs and tumor infiltration extent by immune cells with patients’ survival
The median follow-up was 69.6 months (IQR 41.2–109.3) and the mean DFS from the time of operation to death was 142 months (95%CI: 124–160).
Patients with WHO grade 2 (98 vs 163 months, P=0.04), T stage>2 (44 vs 159 months, P<0.001), positive lymph nodes (47 vs 156 months, P<0.001), mitotic rate>2 mitoses/10HPF (73 vs 159 months, P=0.01), and Ki67 labelling index ≥3% (103 vs 149, P=0.01) had a shorter DFS. Additionally, a high number of peritumoral CD4+ cells (≥45/HPF) (high: 113 months vs medium and low: 123 months, P=0.02, Fig. 3A) or of TAMs (≥6/HPF) (high: 113 months vs medium and low: 121 months, P<0.05, Fig. 3B) were associated with shorter DFS. The latter association was enhanced when the number of intratumoral CD8+ T cells was low (P<0.05). Conversely, a high number of intratumoral CD8+ T cells (≥5/HPF) was associated with a longer DFS (high: 170 vs medium and low: 104 months, P=0.05, Fig. 3C) (Table 2). The latter association was enhanced when the number of TAMs was low (P<0.05).
Figure 3.
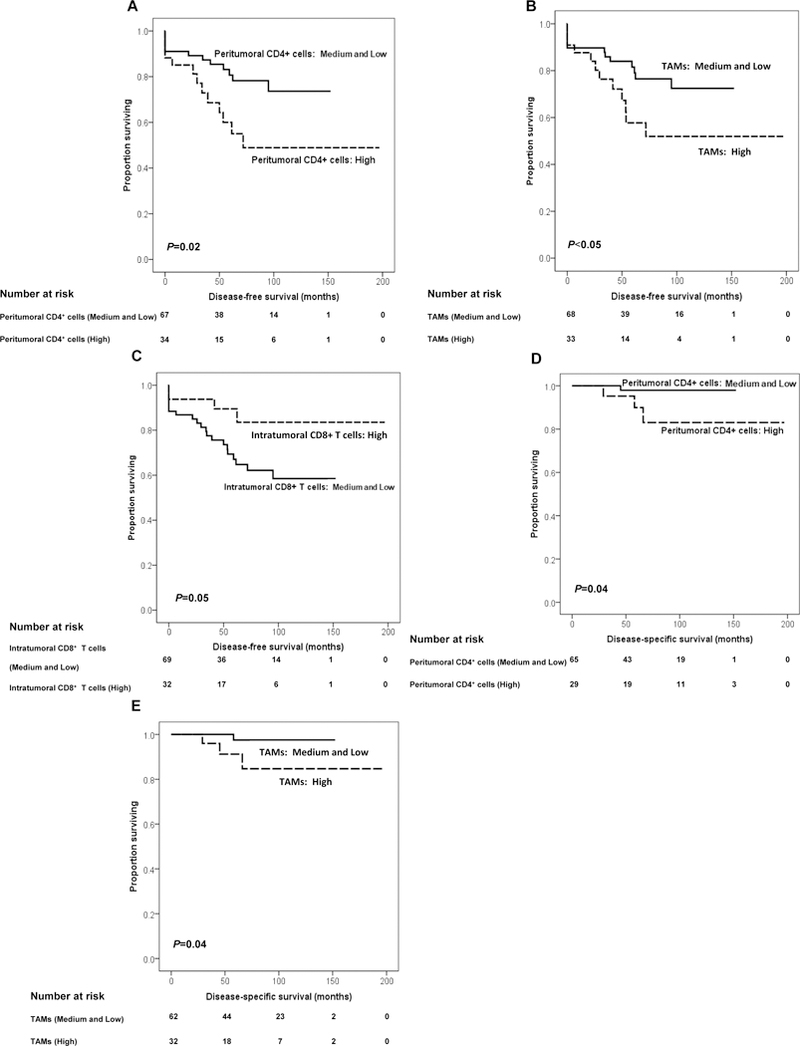
Association of the extent of tumor infiltration by immune cells with DFS and DSS in patients with PanNET. DFS based on peritumoral CD4+ cell, TAM, and intratumoral CD8+ T-cell number is shown. Prognosis is depicted with Kaplan–Meier curves: (A) for peritumoral CD4+ cell number: high versus medium and low (mean survival: high: 113 vs. medium and low: 123 months, P=0.02), (B) for TAM number: high versus medium and low (mean survival: high: 113 versus medium and low: 121 months, P<0.05); and (C) for intratumoral CD8+ T-cell number: high versus medium and low (mean survival: high: 170 vs. medium and low: 104 months, P=0.05). Disease-specific survival based on peritumoral CD4+ cells and TAM number is shown. Prognosis is depicted with Kaplan–Meier curves: (D) for peritumoral CD4+ cell number: high versus medium and low (mean survival: high: 150 vs. medium and low: 173 months, P=0.04), and (E) for TAM number: high versus medium and low (mean survival: high: 149 vs. medium and low: 175 months, P =0.04). P values derived from log-rank tests.
Table 2.
Univariate and multivariate analyses of DFS or DSS with immune parameters and clinicopathologic characteristics in patients with PanNET
| Months | HR (95%CI) | P-value | |
|---|---|---|---|
| Univariate analysis | |||
| DFS | |||
| WHO (Grade 1 vs Grade 2) | 163 vs 98 0.04 | ||
| T stage (1–2 vs 3–4) | 159 vs 44 <0.001 | ||
| Lymph node positivity
(Negative vs Positive) |
156 vs 47 <0.001 | ||
| Mitotic rate (<2 vs ≥2 mitoses/10HPF) |
159 vs 73 0.01 | ||
| Ki67LI (<3% vs ≥3%) | 149 vs 103 0.01 | ||
| Number of peritumoral CD4+
cells
(Median & low vs High) |
123 vs 113 0.02 | ||
| Number of total TAMs
(Median & low vs High) |
121 vs 113 <0.05 | ||
| Number of intratumoral CD8+ T cells (Median & low vs High) | 104 vs 170 0.05 | ||
| DSS | |||
| T stage (1–2 vs 3–4) | 194 vs 145 0.02 | ||
| Lymph node positivity (Negative vs Positive) |
191 vs 121 0.05 | ||
| Number of peritumoral CD4+
cells (Median & low vs High) |
173 vs 150 0.04 | ||
| Number of total TAM (Median & low vs High) |
175 vs 149 0.04 | ||
| Multivariate analysis | |||
| DFS | |||
| Number of total TAMs (Median & low vs High) |
1.1 (1.04–1.28) | 0.02 | |
| WHO (Grade 1 vs Grade 2) | 2.3 (1.06–5.44) | 0.04 | |
| T stage (1–2 vs 3–4) | 2.3 (1.29–4.13) | 0.01 | |
| Lymph node positivity (Negative vs Positive) |
2.8 (1.05–7.45) | 0.04 | |
| DSS | |||
| Number of total TAMs (Median & low vs High) | 1.1 (1.03–1.29) | 0.02 | |
DFS: disease-free survival; DSS:disease-specific survival; HR: hazard ratio.
The mean DSS was 190 months (95%CI: 181–198). Patients with T3 and T4 stages (145 vs 194 months, P=0.02), lymph node positivity (112 vs 191 months, P=0.05) and mitotic rates >2 mitoses/10HPF (HR=1.4, 95%CI: 1.1–1.8, P=0.02) had a shorter DSS. Additionally, high numbers of peritumoral CD4+ cells (≥45/HPF) (high: 150 vs. medium and low: 173 months, P=0.04, Fig. 3D) and TAMs (≥6/HPF) (high: 149 vs medium and low: 175 months, P=0.04, Fig. 3E) were associated with a shorter DSS (Table 2). Of note, no disease-specific deaths were observed during the follow-up period among patients with positive HLA-A expression (P=0.18), positive β2m expression (P=0.14), lack of B7-H3 expression by PanNET cells (P=0.28), and a high number of intratumoral infiltrating CD8+ T cells (≥5/HPF, P=0.16). However, none of these associations reached statistical significance. On the other hand, negative HLA-A (P=0.02) and β2m (P=0.03) expression, as well as a high number of TAMs (≥6/HPF, P=0.02) were significantly associated with poor survival in patients with nondetectable PD-L1 expression in their tumors (Fig. 4). None of the clusters was associated with DSS.
Figure 4.
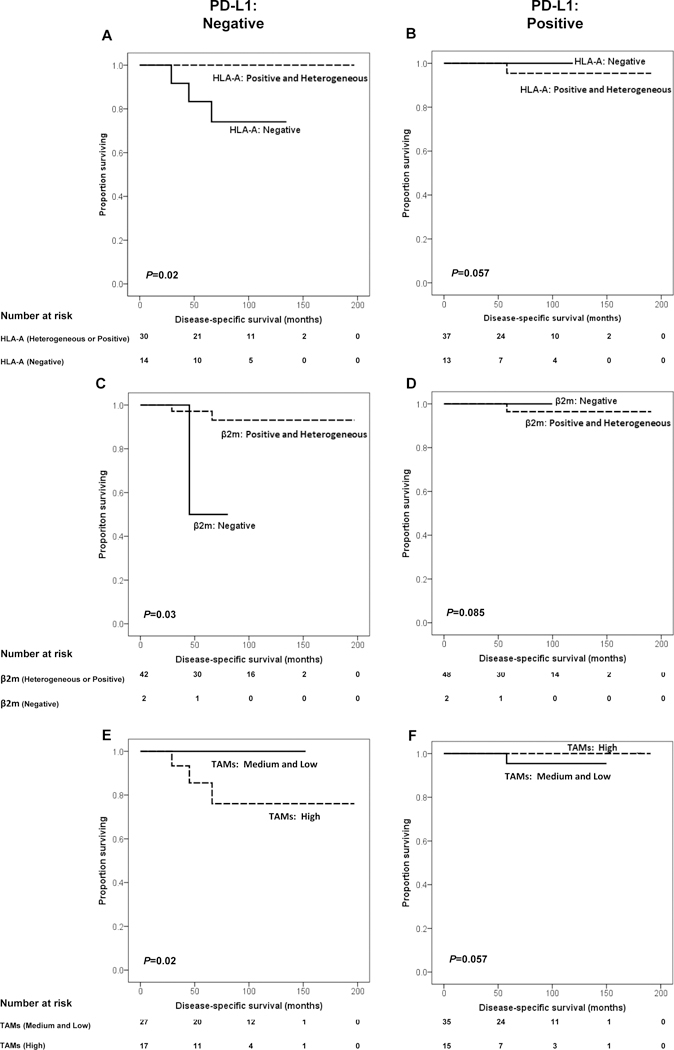
Impact of PD-L1 expression on the association of HLA-A and β2m expression by PanNET cells and of TAM number with DSS in patients with PanNET. A and B, Prognosis is depicted with Kaplan-Meier curves for HLA-A expression (positive and heterogeneous vs. negative) in PD-L1-negative (P=0.02) and positive (P=0.057) group. C and D, Prognosis is depicted with Kaplan-Meier curves for β2m expression (positive and heterogeneous vs. negative) in PD-L1–negative (P=0.03) and positive (P=0.085) group. E and F, Prognosis is depicted with Kaplan-Meier curves for TAM number (high vs. medium and low) in PD-L1-negative (P=0.02) and positive (P=0.057) group. P values derived from logrank tests.
On multivariate survival analyses, number of TAMs (HR=1.1, 95%CI: 1.04–1.28, P=0.02), WHO grade 2 (HR=2.3, 95%CI: 1.06–5.44, P=0.04), T stage>2 (HR=2.3, 95%CI: 1.29–4.13, P=0.01), and lymph node positivity (HR=2.8, 95%CI: 1.05–7.45, P=0.04) were all independent predictors of DFS (Table 2). Number of TAMs (HR=1.06, 95%CI: 1.01–1.12, P=0.026) and T stage>2 (HR=2.6, 95%CI: 1.2–5.4, P=0.012) continued to be independent predictors of DFS when only WHO grade 1 PanNETs were included in the multivariate analysis. Furthermore, the number of TAMs (HR=1.1, 95%CI: 1.03–1.29, P=0.02) was the sole independent predictor of DSS (Table 2).
Discussion
To improve our understanding of the role of the immune microenvironment in the clinical course of PanNET, we performed a comprehensive analysis of more than 100 well-annotated PanNETs. Tumor-infiltrating immune cells were identified in all the PanNETs analyzed. CD8+ T cells, CD4+ cells and macrophages were evaluated since they appear to influence DFS and DSS in other cancer types (36–38). PanNET cells appear to avail themselves of several multiple escape mechanisms to avoid destruction by the host’s immune system (Supplementary Table. S6). These include: i) abnormalities in HLA class I expression by PanNET cells, which may result in their defective recognition by cognate T cells, and ii) a defective effector phase of the immune response because of inhibitory signals released by suppressor cells and/or checkpoint molecules in the tumor microenvironment.
The number of TAMs was the sole predictor of DSS. A higher number of TAMs, along with WHO grade, T stage, and lymph node positivity were all independent predictors of DFS. When we analyzed only WHO grade I PanNETs, the number of TAMs and T stage continued to be independent predictors of DFS. The above data suggests that the extent of TAM infiltration may allow us to better stratify patients and identify those who have a poor prognosis despite a low WHO grade. In this regard, TAMs have been shown to dampen TA-specific immune responses in PDAC both in mice and in humans (39). This defect may reflect a reduction in the number of tumor-infiltrating CD8+ T cells associated with TAMs and/or the release of inhibitory signals by molecules such as members of the B7 family (40). An additional mechanism underlying the association of TAM and T cell infiltration with the clinical course of the disease is suggested by the results recently described in a mouse PDAC model (41). T cell infiltration was reactivated both at the epigenetic and at the functional level after TAM elimination, with a switch from IL-10-secreting T cells towards an effector/memory phenotype, as demonstrated by the increased percentage of IFNγ+ T cells in the tumor microenvironment. This switch could be at least in part mediated by inflammatory cytokines and chemokines secreted in response to trabectedin treatment (42). These results altogether imply that eliminating TAMs may have a beneficial effect on the clinical course of the disease. This possibility is being tested in a number of clinical trials which utilize chemotherapeutic agents as individual reagents or in combination with checkpoint inhibitors, as recently reviewed by Mantovani et al (43).
HLA class I expression, which is required to present TA-derived peptides to cognate T cells, was defective in about 70% of the PanNETs analyzed. This frequency, which is similar to that found by Sato et al (13) in 16 PanNETs, is at the upper limit of the frequency of HLA class I defects present in many other cancer types (30,44,45). As observed in other cancer types (46,47), HLA class I defects in PanNETs are clinically relevant, since they are associated with a poor prognosis. This association is likely to reflect the escape of PanNET cells from immune surveillance because of defective presentation by cancer cells of TA-derived peptides to cognate T cells. HLA class II expression was detected neither in our cohort of patients, nor in that of Ryschich et al (12) who analyzed tumors removed from 12 PanNET patients.
The significant correlation of HLA class I expression with STAT1 activation (Fig. 2D) and with RIG-I expression (Fig. 2F) suggests that these two pathways are involved in the regulation of their expression in PanNETs. This is not unique to PanNET. The STAT1 pathway (48) is involved in head and neck cancer and RIG-I in PDAC (49). Our results suggest that HLA class I downregulation in PanNET may be caused by epigenetic mechanisms. Therefore HLA class I expression may be restored by strategies which activate the STAT1 pathway (50) and/or induce RIG-I expression (51), an additional pathway which may be involved in PanNET. HLA class I may be also downregulated by the MAPKinase pathway. This pathway is activated in PanNETs (52) and its activation is associated with HLA class I downregulation in other cancer types (53–55). However, we were unable to identify a close association between ERK activation and HLA class I downregulation in the 15 PanNETs we analyzed (data not shown).
The last immune escape mechanism we identified in the present study is represented by the expression of inhibitory molecules, such as PD-L1 and B7-H3 on malignant and immune cells. PD-L1 was expressed in about 50% of the PanNETs analyzed; this frequency is similar to that described by Kim et al. in 14 PanNETs (16). In our PanNET cohort, PD-L1 expression by malignant cells was positively associated with high CD8+ T cell infiltration. Whether the latter plays a major role in PD-L1 expression by PanNET cells through the release of IFNγ in the tumor microenvironment is not known at present. However, PD-L1 expression by PanNET cells was not detected in a significant percentage of the tumors with lymphocyte infiltration analyzed. The lack of absolute concordance between PD-L1 expression by PanNET cells and presence of tumor lymphocyte infiltration may be due to the activity of cytokines, chemokines and oncogenic signaling pathways (NF-κB, AKT-mTOR and EGFR) in the tumor microenvironment (56,57). Of note, in the present study, PD-L1 was expressed on TAMs only in 3 cases, while in glioma, it was expressed on most tumor-infiltrating macrophages in the majority of the tumors analyzed (58).
B7-H3 was expressed in about 50% of the PanNETs analyzed. This frequency is similar to that described in breast (59), esophageal (60), lung (61), gallbladder (62), hepatic (63), colorectal (64), prostate (21), cervical (65), ovarian (18), endometrial cancers (66), osteosarcoma (67) and melanoma (68), but higher than that described in gastric cancer (22) and PDAC (23). B7-H3 expression was mainly identified on the surface and in the cytoplasm of PanNET cells. On the other hand, it was not detected in the nucleus of cancer cells, as it has been described for colorectal cancer cells (64). Furthermore, as described in other types of cancer (18,41,64,65,69), B7-H3 was found to be expressed on tumoral stromal cells and in tumor vasculature in more than 70% of the PanNETs analyzed.
B7-H3 expression is associated with a poor prognosis in many types of cancers (18–21). However, as observed in cervical cancer (65) and in melanoma (68), such an association was not found in PanNET, in spite of the association of B7-H3 expression with a higher mitotic count, a poor prognostic marker for survival. This outcome could potentially represent a type II error caused by the limited number of cases analyzed. It is unclear how B7-H3 mediates T cell infiltration. In PanNETs, like in lung cancer (61) and in melanoma (68), B7-H3 expression was not associated with CD8+ T cell infiltration. However, in evaluating the potential clinical relevance of B7-H3, one should consider not only its expression, but also its cellular distribution. The latter variable may affect its function and as a result its role in the clinical course of the disease, as well as its use as a target for immunotherapeutic strategies developed for the treatment of malignant diseases. In the present study, B7-H3 was localized to the PanNET cell cytoplasm in the majority of the cases analyzed. Nuclear staining of B7-H3 was not detected. Additionally, in the present study, B7-H3 expression was significantly associated with β2m expression. As the genes encoding these two markers are both located on chromosome 15 long arm in humans, the association we have found is compatible with the possibility that B7-H3 and β2m may play a costimulating role in the tumoral immune response.
An intriguing finding of our study is the association of positive HLA-A and β2m expression with favourable clinical course of the disease, only when PD-L1 expression was not detectable on PanNET cells. One might interpret these results by arguing that HLA class I expression may not play a major role in the interactions of PanNET cells with cognate T cells when their anti-tumor activity is inhibited by the binding of PD-L1 on PanNET cells to PD-1 expressed on cognate T cells. In contrast, HLA class I expression is necessary for cognate T cells to recognize and kill PanNET cells, when PD-L1 is not expressed. The association we have described and the mechanism we have proposed are not unique of PanNET, since similar observations have been made in intrahepatic cholangiocarcinoma (30) and in esophageal cancer (70). Furthermore the scenario we have described is likely to mimic what happens in patients with malignant disease when the PD-1/PD-L1 axis is disrupted with inhibitors. If our hypothesis is correct, our results imply that implementation of checkpoint inhibitor-based therapy should take into account HLA class I expression by cancer cells, since these antigens mediate the interaction between cancer cells and cognate T cells unleashed by this type of therapy. Expression of a functional HLA class I antigen processing machinery (APM) by cancer cells is a requirement for the successful application of checkpoint inhibitor-based immunotherapy, since this machinery plays a crucial role in the generation and presentation of tumor antigen-derived peptides to cognate T cells. This possibility is supported by the recently described association between HLA class I expression on targeted tumor cells and response to therapy with anti-CTLA4 therapy in patients with melanoma (71). On the other hand, HLA class I independent immunotherapeutic strategies such as antibody-based immunotherapies should be considered, when HLA class I APM is not fully functional or its function can not be restored in cancer cells.
It is noteworthy that the results we have presented have to be interpreted with caution, since our study has some limitations. They include its retrospective nature, its performance at one single academic medical center and the lack of validation of the described results in an independent patient cohort. However, the low frequency of PanNET in the population is an obstacle to the collection of a large number of tumors from an independent patient cohort. Therefore we hope that the results we have described will represent a useful reference for future studies.
In conclusion, the immune system appears to play a role in the clinical course of the disease in PanNET, since – among other findings – the high infiltration of TAMs is an independent predictor of poor prognosis. This study provides a strong rationale to design TAM-targeting therapies, as well as to use HLA class I expression status as a criterion to select PanNET patients to be treated with checkpoint inhibitors. Additionally, an immune profile with intact HLA class I expression and low TAM expression is likely to improve DSS in PD-L1 negative PanNET patients. A better understanding of the PanNet immune microenvironment may sharpen our ability to stratify patients in a more effective way and potentially develop novel treatment strategies.
Supplementary Material
Translational Relevance.
PanNETs have a wide range of clinical presentations and recurrence-free survivals after surgical resection. The available prognostic biomarkers have significant limitations. This study evaluated the role of immune cells and molecules in the clinical course of PanNET. Low tumor associated macrophage (TAM), low peritumoral CD4+ cell and high intratumoral CD8+ T cell infiltration was associated with prolonged disease-free and/or disease-specific survival. The association of HLA-A expression by PanNET cells with the clinical course of the disease, only in patients with no detectable PD-L1 expression on their tumors, is compatible with the role of the susceptibility of PanNET cells to recognition by host immune system in the clinical course of the disease. Therefore, testing of PanNET patients for HLA-A and PD-L1 expression may identify those who may benefit from T-cell based therapy. Furthermore, the association of TAM infiltration with poor prognosis suggests that TAM-targeting strategies may be beneficial in PanNET patients.
Acknowledgments
Funding:This study was supported by the Martin Loeffler Family, NCI R21 CA164756 (PI: SF and CRF), NCI RO3 CA231766 (PI: SF, CRF and DV) and National Natural Science Foundation of China 81201948 (PI: LC).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008;15(2):409–27 doi 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135(5):1469–92 doi 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol 2015;21(32):9512–25 doi 10.3748/wjg.v21.i32.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dromain C, Deandreis D, Scoazec JY, Goere D, Ducreux M, Baudin E, et al. Imaging of neuroendocrine tumors of the pancreas. Diagn Interv Imaging 2016;97(12):1241–57 doi 10.1016/j.diii.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39(6):707–12 doi 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd RV Osamura R, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2017. [Google Scholar]
- 7.Liu JB, Baker MS. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg Clin North Am 2016;96(6):1447–68 doi 10.1016/j.suc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375(18):1767–78 doi 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161(2):205–14 doi 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei IH, Harmon CM, Arcerito M, Cheng DF, Minter RM, Simeone DM. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann Surg 2014;260(6):1088–94 doi 10.1097/SLA.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, et al. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene 2012;31(11):1459–67 doi 10.1038/onc.2011.337. [DOI] [PubMed] [Google Scholar]
- 12.Ryschich E, Autschbach F, Eisold S, Klar E, Buchler MW, Schmidt J. Expression of HLA class I/II antigens and T cell immune response in human neuroendocrine tumors of the pancreas. Tissue Antigens 2003;62(1):48–54. [DOI] [PubMed] [Google Scholar]
- 13.Sato S, Tsuchikawa T, Nakamura T, Sato N, Tamoto E, Okamura K, et al. Impact of the tumor microenvironment in predicting postoperative hepatic recurrence of pancreatic neuroendocrine tumors. Oncol Rep 2014;32(6):2753–9 doi 10.3892/or.2014.3530. [DOI] [PubMed] [Google Scholar]
- 14.Katz SC, Donkor C, Glasgow K, Pillarisetty VG, Gonen M, Espat NJ, et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB (Oxford) 2010;12(10):674–83 doi 10.1111/j.1477-2574.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis 2017;8(8):e3004 doi 10.1038/cddis.2017.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim ST, Ha SY, Lee S, Ahn S, Lee J, Park SH, et al. The Impact of PD-L1 Expression in Patients with Metastatic GEP-NETs. J Cancer 2016;7(5):484–9 doi 10.7150/jca.13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27(1):111–22 doi 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol 2010;23(8):1104–12 doi 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inamura K, Yokouchi Y, Kobayashi M, Sakakibara R, Ninomiya H, Subat S, et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer 2017;103:44–51 doi 10.1016/j.lungcan.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer immunology, immunotherapy : CII 2010;59(8):1163–71 doi 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer research 2007;67(16):7893–900 doi 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 22.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 2006;12(3):457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009;9:463 doi 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias-Santos D, Ferrone CR, Zheng H, Lillemoe KD, Fernandez-Del Castillo C. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery 2015;157(5):881–7 doi 10.1016/j.surg.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Villani V, Mahadevan KK, Ligorio M, Fernandez-Del Castillo C, Ting DT, Sabbatino F, et al. Phosphorylated Histone H3 (PHH3) Is a Superior Proliferation Marker for Prognosis of Pancreatic Neuroendocrine Tumors. Ann Surg Oncol 2016;23(Suppl 5):609–17 doi 10.1245/s10434-016-5171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol 1998;35(3):177–88. [DOI] [PubMed] [Google Scholar]
- 27.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol 2003;171(4):1918–26. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino MA, Ng AK, Russo C, Ferrone S. Heterogeneous distribution of the determinants defined by monoclonal antibodies on HLA-A and B antigens bearing molecules. Transplantation 1982;34(1):18–23. [DOI] [PubMed] [Google Scholar]
- 29.Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S. Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods 1993;161(2):239–56. [DOI] [PubMed] [Google Scholar]
- 30.Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, et al. PD-L1 and HLA Class I Antigen Expression and Clinical Course of the Disease in Intrahepatic Cholangiocarcinoma. Clin Cancer Res 2016;22(2):470–8 doi 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrido F CT, Accolla RS, Bensa JC, Bodmer W, Dohr G, Drouet M, Fauchet R, Ferrara GB, Ferrone S, Giacomini P, Kageshita T, Koopman L, Maio M, Marincola M, Mazzilli C, Morel PA, Murray A, Papasteriades CRH, Salvaneschi L, Stern PL, Ziegler A. HLA and cancer: 12th International Histocompatibility Workshop study. Genetic Diversity of HLA Functional and Medical Implication Proc Twelfth International Histocompatibility Workshop and Conference Volume II. Sevres, France: EDK; 1977. p 445–52. [Google Scholar]
- 32.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389(10064):67–76 doi 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph RW, Millis SZ, Carballido EM, Bryant D, Gatalica Z, Reddy S, et al. PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation. Cancer Immunol Res 2015;3(12):1303–7 doi 10.1158/2326-6066.CIR-15-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev 2017;276(1):26–39 doi 10.1111/imr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, et al. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res 2013;19(7):1816–26 doi 10.1158/1078-0432.CCR-12-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremble LF, Forde PF, Soden DM. Clinical evaluation of macrophages in cancer: role in treatment, modulation and challenges. Cancer immunology, immunotherapy : CII 2017;66(12):1509–27 doi 10.1007/s00262-017-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol 2016;2(10):1354–60 doi 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 38.Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014;110(6):1595–605 doi 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331(6024):1612–6 doi 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009;206(6):1327–37 doi 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, et al. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017;31(4):501–15 e8 doi 10.1016/j.ccell.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borgoni S, Iannello A, Cutrupi S, Allavena P, D’Incalci M, Novelli F, et al. Depletion of tumor-associated macrophages switches the epigenetic profile of pancreatic cancer infiltrating T cells and restores their anti-tumor phenotype. Oncoimmunology 2018;7(2):e1393596 doi 10.1080/2162402X.2017.1393596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14(7):399–416 doi 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speetjens FM, de Bruin EC, Morreau H, Zeestraten EC, Putter H, van Krieken JH, et al. Clinical impact of HLA class I expression in rectal cancer. Cancer immunology, immunotherapy : CII 2008;57(5):601–9 doi 10.1007/s00262-007-0396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol 1999;154(3):745–54 doi 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol 2007;601:123–31. [DOI] [PubMed] [Google Scholar]
- 47.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer immunology, immunotherapy : CII 2007;56(2):227–36 doi 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR, et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res 2013;19(4):798–808 doi 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duewell P, Steger A, Lohr H, Bourhis H, Hoelz H, Kirchleitner SV, et al. RIG-I-like helicases induce immunogenic cell death of pancreatic cancer cells and sensitize tumors toward killing by CD8(+) T cells. Cell Death Differ 2014;21(12):1825–37 doi 10.1038/cdd.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. STAT1-Induced HLA Class I Upregulation Enhances Immunogenicity and Clinical Response to Anti-EGFR mAb Cetuximab Therapy in HNC Patients. Cancer Immunol Res 2015;3(8):936–45 doi 10.1158/2326-6066.CIR-15-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo X, Liu T, Shi H, Wang J, Ji P, Wang H, et al. Respiratory Syncytial Virus Infection Upregulates NLRC5 and Major Histocompatibility Complex Class I Expression through RIG-I Induction in Airway Epithelial Cells. J Virol 2015;89(15):7636–45 doi 10.1128/JVI.00349-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schimmack S, Lawrence B, Svejda B, Alaimo D, Schmitz-Winnenthal H, Fischer L, et al. The clinical implications and biologic relevance of neurofilament expression in gastroenteropancreatic neuroendocrine neoplasms. Cancer 2012;118(10):2763–75 doi 10.1002/cncr.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, et al. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol 2013;191(12):6261–72 doi 10.4049/jimmunol.1301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabbatino F, Wang Y, Scognamiglio G, Favoino E, Feldman SA, Villani V, et al. Antitumor Activity of BRAF Inhibitor and IFNalpha Combination in BRAF-Mutant Melanoma. J Natl Cancer Inst 2016;108(7) doi 10.1093/jnci/djv435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, et al. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol Res 2016;4(11):936–47 doi 10.1158/2326-6066.CIR-16-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer research 2015;75(11):2139–45 doi 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer research 2016;76(2):227–38 doi 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 58.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res 2013;19(12):3165–75 doi 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachawal SV, Jensen KC, Wilson KE, Tian L, Lutz AM, Willmann JK. Breast Cancer Detection by B7-H3-Targeted Ultrasound Molecular Imaging. Cancer research 2015;75(12):2501–9 doi 10.1158/0008-5472.CAN-14-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song J, Shi W, Zhang Y, Sun M, Liang X, Zheng S. Epidermal growth factor receptor and B7-H3 expression in esophageal squamous tissues correlate to patient prognosis. Onco Targets Ther 2016;9:6257–63 doi 10.2147/OTT.S111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boland JM, Kwon ED, Harrington SM, Wampfler JA, Tang H, Yang P, et al. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin Lung Cancer 2013;14(2):157–63 doi 10.1016/j.cllc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Liu CL, Zang XX, Huang H, Zhang H, Wang C, Kong YL, et al. The expression of B7-H3 and B7-H4 in human gallbladder carcinoma and their clinical implications. Eur Rev Med Pharmacol Sci 2016;20(21):4466–73. [PubMed] [Google Scholar]
- 63.Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer immunology, immunotherapy : CII 2012;61(11):2171–82 doi 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ingebrigtsen VA, Boye K, Nesland JM, Nesbakken A, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer 2014;14:602 doi 10.1186/1471-2407-14-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brustmann H, Igaz M, Eder C, Brunner A. Epithelial and tumor-associated endothelial expression of B7-H3 in cervical carcinoma: relation with CD8+ intraepithelial lymphocytes, FIGO stage, and phosphohistone H3 (PHH3) reactivity. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 2015;34(2):187–95 doi 10.1097/PGP.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 66.Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol 2012;124(1):105–11 doi 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, et al. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One 2013;8(8):e70689 doi 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res 2011;17(10):3100–11 doi 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 69.Mesri M, Birse C, Heidbrink J, McKinnon K, Brand E, Bermingham CL, et al. Identification and characterization of angiogenesis targets through proteomic profiling of endothelial cells in human cancer tissues. PLoS One 2013;8(11):e78885 doi 10.1371/journal.pone.0078885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito S, Okano S, Morita M, Saeki H, Tsutsumi S, Tsukihara H, et al. Expression of PD-L1 and HLA Class I in Esophageal Squamous Cell Carcinoma: Prognostic Factors for Patient Outcome. Ann Surg Oncol 2016;23(Suppl 4):508–15 doi 10.1245/s10434-016-5376-z. [DOI] [PubMed] [Google Scholar]
- 71.Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med 2018;10(450) doi 10.1126/scitranslmed.aar3342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


