Abstract
Inflammatory bowel diseases, primarily Crohn’s disease and ulcerative colitis, are chronic inflammatory disorders of the gastrointestinal tract with unknown etiology. The majority of current therapeutic agents focus on controlling proinflammatory molecules. The neuropeptide nociceptin/orphanin FQ (N/OFQ) has been described as a potential immunomodulator for inflammatory bowel diseases. In this study, we asked whether the small molecule N/OFQ antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB612111) would inhibit the development of dextran sodium sulfate-induced colitis in C57BL/6 mice. Inhibition of the N/OFQ receptor (NOP) by SB612111 significantly ameliorated the clinical disease course in these animals, as indicated by reduced fecal bleeding, improved recovery from diarrhea and weight loss, and a reduction in histopathological alterations. In addition, the inflammatory response in the colon was diminished, as demonstrated by reduced cytokine protein and messenger RNA expression for CXCL1/keratinocyte-derived chemokine, interferon-γ, interleukin-1β, interleukin-6, and tumor necrosis factor-α, some of which are known targets for the treatment of this devastating disease. Our results strongly support a role for the receptor–ligand pair NOP-N/OFQ in the pathogenesis of colitis. We conclude that inhibition of NOP receptors with small molecule inhibitors may constitute a novel, urgently needed approach for the treatment of inflammatory bowel diseases.
Keywords: Nociceptin/orphanin FQ, N/OFQ receptor, Inflammatory bowel disease, Dextran sodium sulfate-induced colitis
1. Introduction
Inflammatory bowel diseases affect about 1 million people and account for an estimated $6.3 billion in annual medical costs in the United States (Kappelman et al., 2008). Annual incidence for inflammatory bowel diseases has been rising worldwide, with highest incidences occurring in Europe and North America (Molodecky et al., 2012). While Crohn’s disease and ulcerative colitis account for the majority of inflammatory diseases of the digestive system, inflammatory bowel diseases also include additional noninfectious inflammations of the bowel (Fiocchi, 1998; Strober et al., 2007; Xavier and Podolsky, 2007). Although the etiology is not clear, inflammatory bowel diseases are commonly thought to be mediated by uncontrolled inflammatory responses in the bowel.
Currently approved medications, which have been used for decades, include small molecule salicylates, corticosteroids, and general immunosuppressants (Benchimol et al., 2008; Feagan, 2003; Sandborn, 2006; Siegel and Sands, 2005). More recent approaches have focused on the use of biologics targeting proinflammatory cytokines (Rutgeerts et al., 2004; Yun and Hanauer, 2009).
Nociceptin/orphanin FQ (N/OFQ) is a 17 amino acid neuropeptide that binds to NOP, the fourth member of the opioid receptor family (Meunier et al., 1995; Reinscheid et al., 1995). NOP receptors do not bind to opioids with high affinity, and N/OFQ binds with only low affinity to the classical μ-, δ-, and κ-opioid (MOP, DOP, KOP) receptors (Gintzler et al., 1997). More recent studies suggest that N/OFQ may be a target for treating inflammatory bowel diseases (Kato et al., 2005).
The first indication that NOP receptors play a role in immune cells was reported by Peluso et al., who demonstrated that NOP receptor messenger RNA was expressed in monocytes and T and B lymphocytes (Peluso et al., 1998). These findings were supported by subsequent studies that demonstrated that the NOP receptor ligand N/OFQ binds to peripheral blood mononuclear cells, monocytes, T lymphocytes, and B lymphocytes (Arjomand et al., 2002; Hom et al., 1999; Peluso et al., 2001; Williams et al., 2007).
Like the NOP receptor, its N/OFQ ligand is expressed by neutrophils, and N/OFQ messenger RNA has been detected in human B lymphocytes (Arjomand et al., 2002; Fiset et al., 2003). Kato et al. provided further evidence of a potential immunomodulatory role for NOP receptors in demonstrating that NOP receptor knockout mice developed less severe dextran sodium sulfate-induced colitis than wild type mice (Kato et al., 2005). These results suggest that treatment of inflammatory bowel diseases with small molecule inhibitors targeting the NOP receptor-N/OFQ interaction could be effective in ameliorating the disease.
In this study, we investigated whether NOP receptor inhibition with a small molecule drug would diminish the inflammatory response in inflammatory bowel diseases. Our data suggest that inhibition of the NOP receptor decreases the severity of symptoms in inflammatory bowel diseases and could represent a novel therapeutic approach for treating these devastating diseases.
2. Materials and methods
2.1. Reagents
The high-affinity, high-selectivity NOP receptor antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB612111) (Zaratin et al., 2004) was synthesized in-house. Cytokines were measured using the mouse proinflammatory cytokine kit (K15012, Meso Scale Discovery, Gaithersburg, MD). Custom oligonucleotides were obtained from the Protein and Nucleic Acid Facility (Stanford University, Stanford, CA).
2.2. Colitis model
All animal work was approved by SRI’s Institutional Animal Care and Use Committee in full compliance with all regulations of the National Institutes of Health Office of Laboratory Animal Welfare. To induce colitis, 6–8 week old female C57BL/6 mice were given 3.5% dextran sodium sulfate (36–50 kDa; MPBio, Solon, OH) solution in their drinking water for 5 consecutive days ad libitum. Body weights were recorded daily. Test articles were administered once daily intraperitoneally or via oral gavage. Clinical disease pathogenesis was monitored once daily after disease onset on day 3 until day 12. Diarrhea and fecal bleeding were monitored and scored daily as follows: weight loss (0=no weight loss; 1=0–5% weight loss; 2=5–10% weight loss; 3=10–15% weight loss; 4>15% weight loss), diarrhea (0=normal stool; 2=loose stool; 4=diarrhea), fecal bleeding (0=no blood; 1=occult blood/green guaiac test result [Hemoccult Sensa; Beckman, Brea, CA]; 2=occult blood/blue guaiac test result; 3=bloody stool; 4=bloody anus). At the end of the study, colons were collected and processed for histopathology, protein, and RNA analysis.
2.3. Histopathology
Freshly prepared colon segments were embedded in O.C.T. (Tissue-Tek, Sakura, Torrance, CA), and snap frozen as described (Alt et al., 2002). Cross-sections 6 μm thick were cut, dried and stained with hematoxylin and eosin (American Mastertech, Lodi, CA). Histopathological alterations were scored by a board-certified pathologist in a blinded fashion. Microscopic changes were coded by the most specific topographic and morphologic diagnosis; Systematized Nomenclature of Medicine (SNOMED) and National Toxicology Program terminology manuals were used as guidelines. In brief, gradable observations of decreased mucous cells, increased lymphocyte or mixed leukocyte infiltration, edema, and ulceration/erosion were scored as follows: 0=not observed; 1=minimal; 2=mild; 3=moderate; and 4=marked. The scores for all observations for each individual animal were added together to produce a cumulative histopathology score.
2.4. Immunoassay
Colon tissue was minced using a two scalpel technique, snap frozen, and stored at ≤−60 °C until processed for cytokine analysis. The samples were thawed in 2 ml microtubes and mixed by vortexing with approximately 0.2 ml glass beads (Sigma-Aldrich, St. Louis, MO) and 0.8 ml lysis buffer (50 mM Tris-Cl− pH 7.3, 150 mM sodium chloride, 50 μM ethylenediamintetraacetate, and 1X protease inhibitor [Thermo Fisher Scientific, Wilmington, DE]). Samples were subsequently homogenized three times at 4 °C for 5 min using a Bullet Blender (Next Advance, Averill Park, NY). Debris was removed by two sequential centrifugations at 4 °C, and the supernatants were stored at ≤−60 °C. Protein content of the samples was measured using a bicinchoninic acid kit (Thermo Scientific Pierce, Rockford, IL) according to the manufacturer’s instructions.
A multiplex immunoassay (Meso Scale Discovery, Gaithersburg, MD) was used to measure cytokine levels in the tissue lysate samples in duplicate. In brief, pre-coated assay plates were treated with blocking solution. Samples or protein standards were added, and the plates were incubated for 2 h at room temperature. Plates were washed with PBS/0.05% Tween-20 using an automated plate washer (ELx405; BioTek, Winooski, VT), and incubated for 2 h at room temperature with a detector antibody mix. Plates were washed again, and read with a SECTOR Imager 2400 (Meso Scale Discovery). Cytokine concentrations were calculated using MSD Workbench 3 software (Meso Scale Discovery).
2.5. Quantitative polymerase chain reaction (PCR) analysis
Colon tissue samples were minced, snap frozen and stored at ≤−60 °C until processed for quantitative PCR analysis using an RNeasy Mini Kit (Qiagen, Valencia, CA) modified from the manufacturer’s instructions. In brief, colon samples were resuspended in 0.6 ml RLT lysis buffer (Qiagen), 0.2 ml glass beads were added, and the tissue samples were homogenized three times at 4 °C for 5 min using a Bullet Blender. The homogenized samples were centrifuged, one volume of 70% ethanol was added to the supernatant, and the mixture was applied to RNeasy Mini spin columns. Columns were washed according to the manufacturer’s instructions, and RNA was eluted twice using 0.030 ml nuclease-free water. RNA content was measured using a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Wilmington, DE). RNA templates (~100 ng) were amplified with a reverse transcription kit (A3500 kit; Promega, Madison, WI) using anchored oligo-dT primers (IDT, Coralville, IA) according to the manufacturer’s instructions. Complementary DNA samples were stored at ≤−10 °C until further analysis.
Intron-spanning primers were designed for the quantitative PCR reactions (Table 1); quantitative PCR amplification reactions (initial denaturing at 95 °C for 15 min; 55 cycles denaturing at 95 °C for 15 s, 60 °C for 30 s, elongation at 72 °C for 10 s; and final melting point analysis) were performed using the QuantiTect SYBR Green PCR Kit (Qiagen) in a LightCycler 480 (Roche Applied Science; Indianapolis, IN). Results were verified by melting point analysis, and data was normalized to housekeeping gene expression (hypoxanthine–guanine phosphoribosyltransferase[HPRT]).
Table 1.
Primers used for quantitative PCR analysis.
| Forward primer | Reverse primer | |
|---|---|---|
| CXCL1 | 5′-CCCTCAGGGCCCCACT-3′ | 5′-TCCGTTACTTGGGGACACCTT-3′ |
| HPRT | 5′-GTATACCTAATCATTATGCCGAGGAT-3′ | 5′-TTATAGCCCCCCTTGAGCACA-3′ |
| Interferon-γ | 5′-CCATCAGCAACAACATAAGCGTC-3′ | 5′-GCTTGGCGCTGGACCTG-3′ |
| IL-1β | 5′-GACGGACCCCAAAAGATGAAG-3′ | 5′-GCTTCTCCACAGCCACAATGA-3′ |
| IL-6 | 5′-TACCACTTCACAAGTCGGAGG-3′ | 5′-TGTTTTCTGCAAGTGCATCATCGTT-3′ |
| IL-10 | 5′-GACCCTCAGGATGCGGC-3′ | 5′-CTTGTAGACACCTTGGTCTTGG-3′ |
| IL-17 | 5′-GCTCCAGAAGGCCCTCAG-3′ | 5′-GCGGCACTGAGCTTCCC-3′ |
| IL-23-p19 | 5′-CCAGCGGGACATATGAATCTAC-3′ | 5′-GCCAGACCTTGGCGGATC-3′ |
| ICAM | 5′-CGGGGAGGACAGCAGTC-3′ | 5′-TCCTTGCCTACTTGCTGCCA-3′ |
| MAdCAM | 5′-GGTCCCATGGTAGAGGGC-3′ | 5′-CCACAGGCGGTAGGCAAG-3′ |
| N/OFQ | 5′-GCCTGCTCTCCAGCGTG-3′ | 5′-CATGACTTTGGTGCATACAGTCC-3′ |
| NOP | 5′-GGCCCAGCTTCTGAAGAGG-3′ | 5′-ACAGGTTCCCTTGAAAGTGGC-3′ |
| VCAM | 5′-GTGGGTTTTGAGGATGAACACTC-3′ | 5′-ATGCACAAGTGGCCCACTCAT-3′ |
| TNF-α | 5′-GGGGTGATCGGTCCCCA-3′ | 5′-TGCTCCTCCACTTGGTGGTTT-3′ |
2.6. Statistical analysis
Statistical calculations were performed using GraphPad Prism 4.0 (San Diego, CA).
3. Results
3.1. Inhibition of NOP receptor-N/OFQ interaction ameliorates colitis in dextran sodium sulfate-treated mice
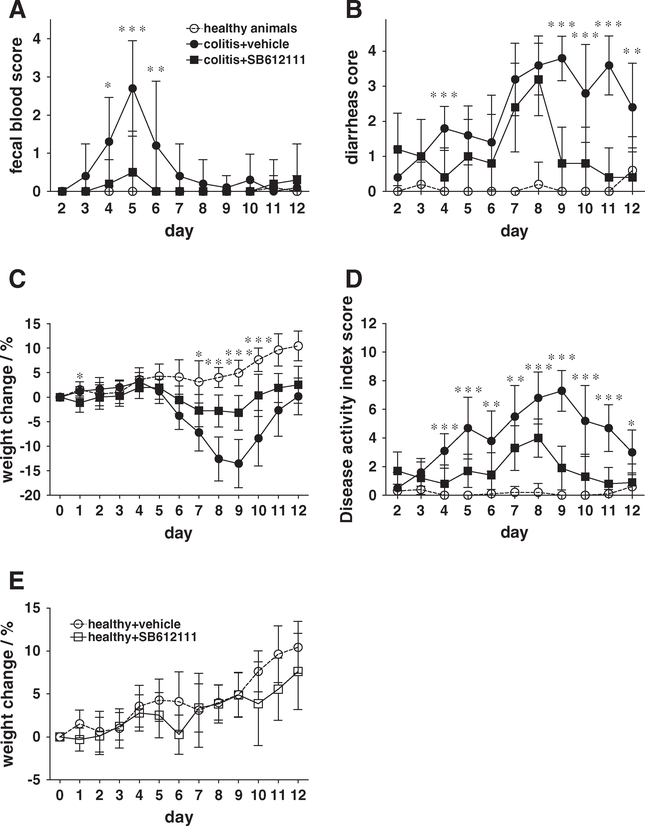
Previous data indicated that N/OFQ affects primarily neutrophils and macrophages (Fiset et al., 2003; Kaminsky and Rogers, 2008; Serhan et al., 2001; Trombella et al., 2005). Therefore, we used a dextran sodium sulfate-induced inflammatory bowel disease model in which colitis is mediated by innate immune responses. In this model, we examined whether pharmacological inhibition of the NOP receptor-N/OFQ interaction with small molecule inhibitors would result in reduced inflammatory bowel disease pathogenesis after feeding C57BL/6 mice with dextran sodium sulfate in their drinking water for 5 days. Clinical disease pathogenesis was monitored during the treatment and subsequent recovery period for a total of 12 days. Dextran sodium sulfate treatment resulted in fecal bleeding, diarrhea, and weight loss (Fig. 1). When animals were treated intraperitoneally once daily with the high-affinity and high-selectivity NOP receptor antagonist SB612111 (Zaratin et al., 2004) at 30 mg/kg starting at day 0, fecal bleeding was significantly reduced by days 4–6 (Fig. 1A). SB612111 treatment at 30 mg/kg was the most effective dose in these studies (data for 10 mg/kg and 60 mg/kg groups not shown). Treated animals also recovered earlier from diarrhea (starting at day 9) and weight loss (starting at day 7) compared to vehicle-treated animals (Fig. 1B and C, respectively). This earlier recovery resulted in a significant reduction in overall disease activity starting at day 8 (Fig. 1D). To confirm that SB612111 did not affect food or water uptake, which could have affected the outcome of these experiments, we treated naïve, healthy C57BL/6 mice once daily for 12 days with 30 mg/kg SB612111. No statistical differences in body weight changes were observed when comparing SB612111-treated and vehicle-treated control animals (Fig. 1E). We observed similar amelioration of fecal bleeding, diarrhea, and body weight loss when administering SB612111 intraperitoneally or via oral gavage, resulting in an overall improved disease activity index (Fig. 2).
Fig. 1.
Inhibition of N/OFQ significantly improved disease activity in dextran sodium sulfate-induced colitis in C57BL/6 mice. C57BL/6 mice received dextran sodium sulfate for 5 days, and disease activity (fecal bleeding, diarrhea, and weight loss) was monitored for an additional 7 days (12 days total). Starting on day 0, one group of mice received daily intraperitoneal injections of the specific NOP receptor antagonist SB612111 at 30 mg/kg. Treated animals (solid squares) showed less severe fecal bleeding (A), faster recovery from diarrhea (B), and a reduction in weight loss (C) compared with vehicle-treated control animals (solid circles), resulting in an improved disease activity index score (D). Body weight changes were comparable in SB612111-treated and vehicle-treated healthy animals (E). Healthy age-matched controls injected daily with vehicle are shown for comparison (open circles). Mean and standard deviation are shown (n=10). Statistical analysis was performed by one-way analysis of variance (ANOVA) and Dunnett’s test (*P<0.05, **P<0.01, ***P<0.001). One out of two representative experiments is shown.
Fig. 2.
Inhibition of N/OFQ by oral application of SB612111 significantly improved disease activity in dextran sodium sulfate-induced colitis in C57BL/6 mice. C57BL/6 mice received dextran sodium sulfate for 5 days, and disease activity (fecal bleeding, diarrhea, and weight loss) was monitored for an additional 7 days (12 days total). One group was dosed daily with SB612111 at 30 mg/kg starting on Day 0. Treated animals (solid squares) showed less severe fecal bleeding (A), faster recovery from diarrhea (B), and a reduction in weight loss (C) compared with vehicle-treated control animals (solid circles), resulting in an improved disease activity index score (D). Healthy age-matched controls that received vehicle daily via oral gavage are shown as a comparison (open circles). Mean and standard deviation are shown (n=10). Statistical analysis was performed by one-way analysis of variance (ANOVA) and Dunnett’s test (*P<0.05, **P<0.01, ***P<0.001).
3.2. Effect of treatment with SB61211 on histopathology in dextran sodium sulfate-treated mice
We then explored whether pharmacological inhibition of the NOP receptor-N/OFQ interaction with SB612111 would improve the histopathological alterations observed during colitis. At the end of a 12 day dextran sodium sulfate-induced colitis study, colon samples from dextran sodium sulfate-treated animals showed increased histopathological signs of colitis: increased inflammation, increased edema, and ulceration/erosion, compared to colons from control animals (Fig. 3). Treatment with SB612111 did not result in a significant reduction in the cumulative histopathology score nor in the individual parameters such as the numbers of lymphocytes in the lamina propria, edema, and ulceration/erosion of the epithelium (Fig. 3).
Fig. 3.
Histopathology observations after SB612111 treatment. Colitis was induced with dextran sodium sulfate in animals that were treated with SB612111 (30 mg/kg) or vehicle once daily. Colon tissues were prepared 12 days after study initiation and stained with hematoxylin and eosin. Histopathological alterations in decreased mucous cells, increased lymphocyte or mixed leukocyte infiltration, edema, and erosion/ulceration were scored in a blinded fashion using the following scale: 0=normal, 1=minimal, 2=mild, 3=moderate, 4=marked. Dextran sodium sulfate-treated animals that received either vehicle (B) or SB612111 (C), and healthy animals are shown (A). This figure shows images from animals with representative histopathology scores (A–C) and cumulative histopathology scores summarizing the observed histopathological alterations, as well as individual histopathological alterations (D). Bar=100 μm.
3.3. Effect of SB61211-treatment on N/OFQ, NOP, and cell adhesion molecule expression
When measuring N/OFQ and NOP receptor expression by immunofluorescent staining, neither was detectable in the colon sections, although they were detectable in spinal cord sections. Since this could be due to limited sensitivity of the commercially available antibodies, we tested the expression of N/OFQ and NOP messenger RNA in colon extracts by quantitative PCR. Neither N/OFQ nor NOP were detectable in significant amounts in colon extracts 2, 5, 8, or 12 days after colitis induction, although they were detectable in brain extracts: The crossing point for N/OFQ was 28.8, for NOP was 29.1, and for HPRT was 21.3.
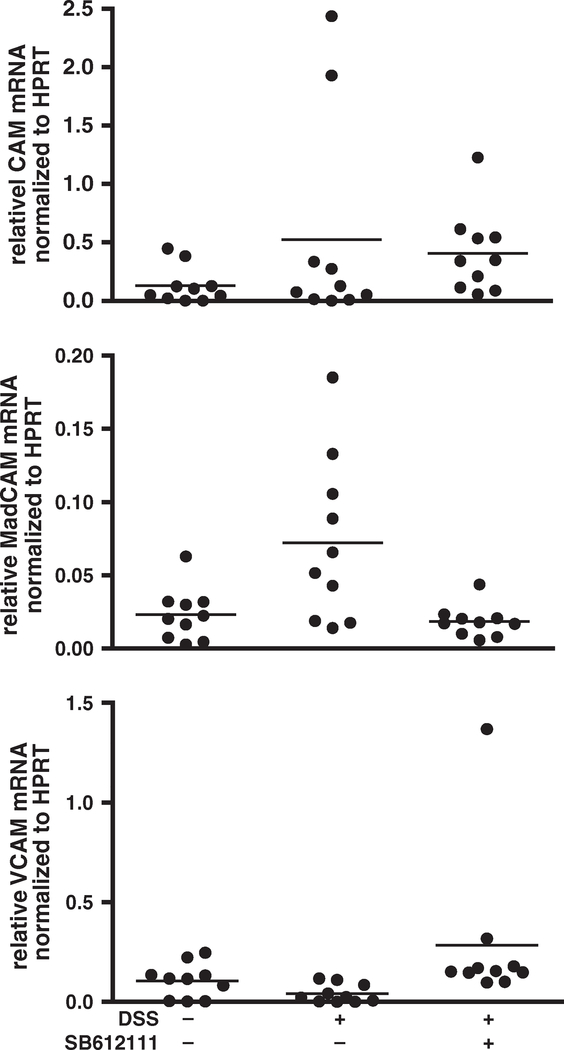
A role for N/OFQ–NOP in endothelial cells has been described before (Carvalho et al., 2008; Williams et al., 2008). Since expression of cell adhesion molecules by endothelial cells is important for recruitment of lymphocytes into inflamed tissues, we examined whether there was a difference in expression of intercellular cell adhesion molecule (ICAM)-1, mucosal addressin CAM (MAdCAM)-1, or vascular CAM (VCAM)-1 after treatment with SB61211. We did not observe any statistically significant expression changes of ICAM-1, MAdCAM-1, or VCAM-1 in colon lysates from dextran sodium sulfate-treated animals 12 days after colitis induction (Fig. 4).
Fig. 4.
No statistically significant differences were observed between ICAM-1, MAdCAM-1, and VCAM-1 messenger RNA expression in colon extracts in dextran sodium sulfate-induced colitis. Inflammatory bowel disease was induced by treating C57BL/6 mice with dextran sodium sulfate for 5 days. Animals were treated daily with SB612111 at 30 mg/kg. Colons were excised after 12 days, and the amounts of ICAM-1, MAdCAM-1, and VCAM-1 messenger RNA were measured. No changes were observed during dextran sodium sulfated-induced colitis. Observations per individual animal and group means are shown (n=10). Statistical analysis was performed by one-way ANOVA and Dunnett’s test (**P<0.01). One out of two representative experiments is shown.
3.4. Inhibition of NOP receptor and N/OFQ reduces cytokine production in dextran sodium sulfate-induced colitis
To determine whether the improved clinical disease and histopathology following treatment with SB612111 resulted from reduced inflammation in the colon, we measured inflammatory cytokines by immunoassay from colon samples of dextran sodium sulfate-treated animals 12 days after commencement of the study and normalized the results to total protein amounts. We found that CXCL1/keratinocytederived chemokine (KC), interferon-γ, interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α protein amounts significantly increased after dextran sodium sulfate-treatment, whereas daily injections with SB612111 reduced the cytokine concentrations in the colon 3–16-fold (Fig. 5A). The reduction in protein correlated with local cytokine messenger RNA production in the colon as determined by quantitative PCR from complementary DNA preparations isolated from colonic tissue. Colonic messenger RNA encoding for interferon-γ, IL-1β, IL-6, and TNF-α increased significantly by more than 7-fold 12 days after colitis induction. Daily treatment with SB612111 reduced cytokine messenger RNA expression (Fig. 5B), suggesting that NOP receptor inhibition diminishes the local cytokine expression during colitis.
Fig. 5.
Inhibition of N/OFQ resulted in significantly reduced cytokine amounts in colon extracts in dextran sodium sulfate-induced colitis. Inflammatory bowel disease was induced in C57BL/6 mice by treating them with dextran sodium sulfate for 5 days. Animals were treated daily with the specific N/OFQ antagonist SB612111 at 30 mg/kg. Colons were excised after 12 days, and cytokine amounts were quantified by immunoassay or PCR. (A) The amount of CXCL1, IFN-γ, IL-1β, IL-6, IL-10, and TNF-α protein was significantly increased in colon tissue from animals treated with dextran sodium sulfate; this effect was diminished in animals that had been treated with SB612111. (B) The amount of IFN-γ, IL-1β, IL-6, and TNF-α messenger RNA was significantly increased in colon tissue from animals treated with dextran sodium sulfate; this effect was diminished in animals that had been treated with SB612111. Observations per individual animal and group means are shown (n=10). Statistical analysis was performed by one-way ANOVA and Dunnett’s test (**P<0.01). One out of two representative experiments is shown.
We also asked whether proinflammatory TH17 cytokines were affected by SB612111 treatment. No significant increase of IL-17 or IL-23-p19 was observed at day 12 after colitis induction (Fig. 6A); consequently we did not see any ameliorative effect from SB612111 (Fig. 6A). To determine whether IL-17 and IL-23 increased at all during the induction of dextran sodium sulfate-induced colitis, we measured these two cytokines 2, 5, 8, and 12 days after colitis induction. No significant changes in IL-17 or IL-23 messenger RNA expression were observed (Fig. 6B).
Fig. 6.
IL-17 and IL-23-p19 messenger RNA expression in the inflamed colon. Inflammatory bowel disease was induced in C57BL/6 mice by treating them with dextran sodium sulfate for 5 days. Animals were treated daily with the specific N/OFQ antagonist SB612111 at 30 mg/kg. Colons were excised after 12 days, and cytokine amounts were quantified by PCR. (A) When normalized to HPRT, IL-17 and IL-23(p19) messenger RNA were either detectable in only minimal amounts or undetectable. No increase was observed during the induced colitis. Observations per individual animal and group means are shown. (B). IL-17 and IL-23-p19 messenger RNA levels in colon extracts were detected on days 0, 2, 5, 8, and 12. No significant increase in the amount of IL-17 or IL-23-p19 messenger RNA was observed. Statistical analysis was performed by one-way ANOVA and Dunnett’s test.
4. Discussion
In our study, treatment with SB612111 ameliorated the disease course in dextran sodium sulfate-induced colitis. Our findings concur with the observations reported by Kato et al. that NOP receptor-deficient mice exhibited reduced body weight loss, diarrhea and fecal bleeding when compared to wild type animals subjected to dextran sodium sulfate-induced colitis (Kato et al., 2005). Kato et al. also reported increased expression of N/OFQ by immunohistochemistry in the colon during colitis (Kato et al., 2005), which we did not observe in our studies using several commercial antibodies for N/OFQ and NOP. Since these antibodies detected N/OFQ and NOP in the central nervous system, we considered that expression levels in the colon were too low to be detected by immunohistochemistry. We then used quantitative PCR to measure the expression of N/OFQ and NOP in colonic extracts; only minimal amounts of N/OFQ were detected in the colonic extracts compared to the expression levels in the brain. A possible explanation may be that N/OFQ is expressed in the central nervous system and then axonally transported into the intestine, a mechanism that has been previously reported for other neurotransmitters (Dockray et al., 1981).
In dextran sodium sulfate-induced colitis, initial epithelial damage triggers an exaggerated innate immune response that causes an inflammatory response in the colon (Egger et al., 2000; Strober et al., 2002; Wirtz et al., 2007; Yan et al., 2009). Although not required for the disease induction in this model, the adaptive response is triggered during the disease course (Dieleman et al., 1994; Strober et al., 2002). While all current in vivo models of inflammatory bowel disease fail to mimic every aspect of the disease, currently used therapies that modulate the immune response have been found to be efficacious in the dextran sodium sulfate-induced colitis model (Melgar et al., 2008).
Many in vitro observations suggest that N/OFQ and its receptor NOP play a functional role in the innate immune response; for example, Fiset observed neutrophils secreting N/OFQ upon degranulation (Fiset et al., 2003). Moreover, neutrophils treated with N/OFQ showed changes in tyrosine-phosphorylation and cAMP-production as well as increased cell migration (Fiset et al., 2003). Monocytes treated with N/OFQ not only showed increased migration (Trombella et al., 2005), but also altered chemokine expression that may be involved in recruiting additional leukocytes into inflamed tissues (Kaminsky and Rogers, 2008). CXCL1 is a chemokine critical for the recruitment of neutrophils into inflamed sites (Kobayashi, 2008). We observed that increased CXCL1 chemokine expression in the inflamed colon was reduced after treatment with SB612111. These in vitro observations suggest that N/OFQ may alter the recruitment of neutrophils and monocytes into inflamed tissues and that inhibition of N/OFQ may reduce inflammation and therefore ameliorate inflammatory bowel diseases. To test whether NOP inhibition also affects the adaptive immune response, we measured the expression of IL-17, a TH17-produced cytokine, and IL-23, a cytokine that promotes TH17 survival and proliferation (Bettelli et al., 2007). We did not observe significant increases in colonic IL-17, or IL-23 mRNA expression during DSS-induced colitis in vivo. This observation is not surprising since DSS-induced colitis is commonly considered an innate immunity-mediated colitis model (Egger et al., 2000; Strober et al., 2002; Wirtz et al., 2007; Yan et al., 2009). This observation suggests that the ameliorative effect of NOP receptor inhibition in colitis results from modulation of innate immune responses, however this does not exclude a potential role of N/OFQ–NOP in adaptive immune responses which remains to be elucidated.
The remarkable increase in fecal bleeding and edema observed in our present study may be due to increased vascular permeability caused directly or indirectly by N/OFQ (Brookes et al., 2007). In animal models of sepsis and in septic patients, increased N/OFQ is correlated with increased disease severity, possibly through a similar mechanism involving the blood vessels (Carvalho et al., 2008; Williams et al., 2008). Kato reported reduced expression of MAdCAM-1 in intestinal blood vessels and reduced numbers of cells expressing MAdCAM-1 ligand in NOP receptor-deficient mice after dextran sodium sulfate-induced colitis (Kato et al., 2005) which we did not observe after SB612111-treatment, possibly because the reduction of MAdCAM-1 is less pronounced after pharmacological inhibition when compared to a genetic deletion of NOP.
The beneficial effect of NOP receptor inhibition on weight change is unlikely to be a result of neuromodulatory feeding effects, since we did not see any weight gain in naïve animals treated with SB612111. Moreover, others have reported that NOP receptor activation, but not NOP receptor inhibition, resulted in increased food intake (Economidou et al., 2006).
As described above, we used SB612111 to inhibit the NOP receptor-N/OFQ interaction. SB61211 is a highly specific, highaffinity (Ki=0.33 nM) NOP receptor antagonist, with more than 100-fold lower binding affinity to opioid receptors and only low affinity binding to α1A−, α2A−, α2C−, α3-adrenergic, and H2-histimine receptors when tested against a large panel of receptors (Zaratin et al., 2004). These binding properties indicate that the beneficial effects of SB612111 treatment for colitis predominantly resulted from NOP receptor inhibition. The reduced disease pathogenesis correlated with decreased cytokine production in the colon. CXCL1, IL-1β, IL-6, and TNF-α are produced by a large variety of cell types, including mononuclear phagocytic cells and neutrophils as well as tissue parenchymal cells such as epithelial cells, endothelial cells, and fibroblasts (Commins et al., 2010; Iida and Grotendorst, 1990). In contrast, interferon-γ is predominantly produced by macrophages, T and NK cells (Becker et al., 1994; Commins et al., 2010). The fact that anti-TNF-α therapy has shown encouraging results for the treatment of human inflammatory bowel diseases points out the importance of these cytokines in inflammatory bowel diseases (Rutgeerts et al., 2004; Yun and Hanauer, 2009) whereas medications targeting interferon-γ or IL-6 receptor are currently undergoing clinical trials (Bosani et al., 2009). However, as with most biological therapeutics, the risk of developing immunogenic side effects, such as infusion reactions and serum sickness-like reactions, is of major concern, particularly for the treatment of chronic diseases. Small molecule therapeutics are devoid of such side effects, and could allow oral application, and therefore their development would be advantageous (Baert et al., 2003; Farrell et al., 2003).
5. Conclusions
Our results strongly indicate a role for NOP receptor-N/OFQ in the disease pathogenesis of inflammatory bowel diseases. NOP receptor inhibition with SB612111 significantly ameliorated the disease course in our colitis model, and decreased the production of several inflammatory cytokines that are known targets for the treatment of these devastating diseases. Although additional studies are necessary to explore these effects, our results suggest that inhibition of NOP receptors with small molecule inhibitors may be a novel, safe, and urgently needed approach for the treatment of inflammatory bowel disease.
Acknowledgments
We would like to thank SRI’s Immunology and Inflammation group, including Linda Dousman, Nahoko Dunlap, Ken Shew, ThanhThuy Tran, and Maja Zukic, for their help. We greatly appreciate the expert technical assistance of Dominic Dinh, Carol Hou, Eunice Kwon, and Willma Polgar. Additionally, we would like to thank Dr. Pankaj Jay Pasricha for the insightful discussions of the project, Dr. David Fairchild for his assistance with the histopathology data and Dr. Paul Stein for his helpful discussion of the manuscript.
Role of the funding source
This work was funded internally by SRI International.
References
- Alt C, Laschinger M, Engelhardt B, 2002. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood–brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol 32, 2133–2144. [DOI] [PubMed] [Google Scholar]
- Arjomand J, Cole S, Evans CJ, 2002. Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. J. Neuroimmunol 130, 100–108. [DOI] [PubMed] [Google Scholar]
- Baert F, Noman M, Vermeire S, Van Assche G, D’Haens G, Carbonez A, Rutgeerts P, 2003. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med 348, 601–608. [DOI] [PubMed] [Google Scholar]
- Becker S, Quay J, Koren HS, Haskill JS, 1994. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am. J. Physiol 266, L278–L286. [DOI] [PubMed] [Google Scholar]
- Benchimol EI, Seow CH, Steinhart AH, Griffiths AM, 2008. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev CD006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK, 2007. Th17: the third member of the effector T cell trilogy. Curr. Opin. Immunol 19, 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosani M, Ardizzone S, Porro GB, 2009. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics 3, 77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brookes ZL, Stedman EN, Guerrini R, Lawton BK, Calo G, Lambert DG, 2007. Proinflammatory and vasodilator effects of nociceptin/orphanin FQ in the rat mesenteric microcirculation are mediated by histamine. Am. J. Physiol. Heart Circ. Physiol 293, H2977–H2985. [DOI] [PubMed] [Google Scholar]
- Carvalho D, Petronilho F, Vuolo F, Machado RA, Constantino L, Guerrini R, Calo G, Gavioli EC, Streck EL, Dal-Pizzol F, 2008. The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med. 34, 2284–2290. [DOI] [PubMed] [Google Scholar]
- Commins SP, Borish L, Steinke JW, 2010. Immunologic messenger molecules: cytokines, interferons, and chemokines. J. Allergy Clin. Immunol 125, S53–S72. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO, 1994. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107, 1643–1652. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Gregory RA, Tracy HJ, Zhu WY, 1981. Transport of cholecystokininoctapeptide-like immunoreactivity toward the gut in afferent vagal fibres in cat and dog. J. Physiol 314, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Policani F, Angellotti T, Massi M, Terada T, Ciccocioppo R, 2006. Effect of novel NOP receptor ligands on food intake in rats. Peptides 27, 775–783. [DOI] [PubMed] [Google Scholar]
- Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW, 2000. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62, 240–248. [DOI] [PubMed] [Google Scholar]
- Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P, 2003. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 124, 917–924. [DOI] [PubMed] [Google Scholar]
- Feagan BG, 2003. Maintenance therapy for inflammatory bowel disease. Am. J. Gastroenterol 98, S6–S17. [DOI] [PubMed] [Google Scholar]
- Fiocchi C, 1998. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115, 182–205. [DOI] [PubMed] [Google Scholar]
- Fiset ME, Gilbert C, Poubelle PE, Pouliot M, 2003. Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochemistry 42, 10498–10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintzler AR, Adapa ID, Toll L, Medina VM, Wang L, 1997. Modulation of enkephalin release by nociceptin (orphanin FQ). Eur. J. Pharmacol 325, 29–34. [DOI] [PubMed] [Google Scholar]
- Hom JS, Goldberg I, Mathis J, Pan YX, Brooks AI, Ryan-Moro J, Scheinberg DA, Pasternak GW, 1999. [(125)I]orphanin FQ/nociceptin binding in Raji cells. Synapse 34, 187–191. [DOI] [PubMed] [Google Scholar]
- Iida N, Grotendorst GR, 1990. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol. Cell. Biol 10, 5596–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky DE, Rogers TJ, 2008. Suppression of CCL2/MCP-1 and CCL5/RANTES expression by nociceptin in human monocytes. J. Neuroimmune Pharmacol 3, 75–82. [DOI] [PubMed] [Google Scholar]
- Kappelman MD, Rifas-Shiman SL, Porter CQ, Ollendorf DA, Sandler RS, Galanko JA, Finkelstein JA, 2008. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 135, 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Tsuzuki Y, Hokari R, Okada Y, Miyazaki J, Matsuzaki K, Iwai A, Kawaguchi A, Nagao S, Itoh K, Suzuki H, Nabeshima T, Miura S, 2005. Role of nociceptin/orphanin FQ (Noc/oFQ) in murine experimental colitis. J. Neuroimmunol 161, 21–28. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, 2008. The role of chemokines in neutrophil biology. Front. Biosci 13, 2400–2407. [DOI] [PubMed] [Google Scholar]
- Melgar S, Karlsson L, Rehnstrom E, Karlsson A, Utkovic H, Jansson L, Michaelsson E, 2008. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol 8, 836–844. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. , 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535. [DOI] [PubMed] [Google Scholar]
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG, 2012. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54 (e42; quiz e30). [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C, 1998. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J. Neuroimmunol 81, 184–192. [DOI] [PubMed] [Google Scholar]
- Peluso J, Gaveriaux-Ruff C, Matthes HW, Filliol D, Kieffer BL, 2001. Orphanin FQ/nociceptin binds to functionally coupled ORL1 receptors on human immune cell lines and alters peripheral blood mononuclear cell proliferation. Brain Res. Bull 54, 655–660. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr., Civelli O, 1995. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Van Assche G, Vermeire S, 2004. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology 126, 1593–1610. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, 2006. Treatment of ulcerative colitis with oral mesalamine: advances in drug formulation, efficacy expectations and dose response, compliance, and chemoprevention. Rev. Gastroenterol. Disord 6, 97–105. [PubMed] [Google Scholar]
- Serhan CN, Fierro IM, Chiang N, Pouliot M, 2001. Cutting edge: nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epilipoxin A4. J. Immunol 166, 3650–3654. [DOI] [PubMed] [Google Scholar]
- Siegel CA, Sands BE, 2005. Review article: practical management of inflammatory bowel disease patients taking immunomodulators. Aliment. Pharmacol. Ther 22, 1–16. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS, 2002. The immunology of mucosal models of inflammation. Annu. Rev. Immunol 20, 495–549. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P, 2007. The fundamental basis of inflammatory bowel disease. J. Clin. Invest 117, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombella S, Vergura R, Falzarano S, Guerrini R, Calo G, Spisani S, 2005. Nociceptin/ orphanin FQ stimulates human monocyte chemotaxis via NOP receptor activation. Peptides 26, 1497–1502. [DOI] [PubMed] [Google Scholar]
- Williams JP, Thompson JP, McDonald J, Barnes TA, Cote T, Rowbotham DJ, Lambert DG, 2007. Human peripheral blood mononuclear cells express nociceptin/ orphanin FQ, but not mu, delta, or kappa opioid receptors. Anesth. Analg 105, 998–1005 (table of contents). [DOI] [PubMed] [Google Scholar]
- Williams JP, Thompson JP, Young SP, Gold SJ, McDonald J, Rowbotham DJ, Lambert DG, 2008. Nociceptin and urotensin-II concentrations in critically ill patients with sepsis. Br. J. Anaesth 100, 810–814. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF, 2007. Chemically induced mouse models of intestinal inflammation. Nat. Protoc 2, 541–546. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK, 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434. [DOI] [PubMed] [Google Scholar]
- Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, Merlin D, 2009. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4, e6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun L, Hanauer S, 2009. Selecting appropriate anti-TNF agents in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol 3, 235–248. [DOI] [PubMed] [Google Scholar]
- Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA, 2004. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). J. Pharmacol. Exp. Ther 308, 454–461. [DOI] [PubMed] [Google Scholar]