Abstract
Background
Ischemia-reperfusion (I/R) leads to kidney injury. Renal I/R frequently occurs in kidney transplantations and acute kidney injuries. Recent studies reported that miR-30 stimulated immune responses and reductions in renal I/R related to anti-inflammation. Our study investigated the effects of miR-30c-5p on renal I/R and the relationship among miR-30c-5p, renal I/R, and macrophages.
Material/Methods
Sprague Dawley rats received intravenous tail injections of miR-30c-5p agomir. Then a renal I/R model were established by removing the left kidney and clamping the right renal artery. Serum creatinine (Cr) was analyzed using a serum Cr assay kit, and serum neutrophil gelatinase associated lipocalin (NGAL) was measured using a NGAL ELISA (enzyme-linked immunosorbent assay) kit. Rat kidney tissues were analyzed using hematoxylin and eosin staining. THP-1 cells treated with miR-30c-5p agomir and miR-30c-5p antagomir were measured with quantitative reverse transcription-polymerase chain reaction. Protein levels were analyzed by western blot.
Results
MiR-30c-5p agomir reduced serum Cr, serum NGAL, and renal I/R injury. MiR-30c-5p agomir inhibited the expression of CD86 (M1 macrophage marker), inducible nitric oxide synthase (iNOS), and tumor necrosis factor-alpha (TNF-α) and promoted the expression of CD206 (M2 macrophage marker), interleukin (IL)-4, and IL-10 in rat kidneys. MiR-30c-5p agomir reduced the expression of CD86 and iNOS, and increased the expression of CD206 and IL-10 in THP-1 cells.
Conclusions
We preliminarily demonstrated that miR-30c-5p agomir might decrease renal I/R through transformation of M1 macrophages to M2 macrophages and resulted in changes in inflammatory cytokines.
MeSH Keywords: Inflammation, miR-30c-5p, M1 Macrophage, M2 Macrophage, Renal Ischemia-Reperfusion
Background
Ischemia-reperfusion (I/R) leads to multiple organs injury, including lung injury [1], myocardial injury [2] and kidney injury [3]. Acute kidney injury frequently occurs in aortic surgery with suprarenal clamping [4]. Renal I/R is one expression of kidney injury due to blood supply variation and is one cause of acute tubular necrosis (ATN). The loss of vascular fluid and interstitial fluid in the kidney is responsible for poor perfusion and damaged kidney function after ischemia [5]. Endothelial cell dysfunction accompanying kidney ischemia and renal cell swelling is one major cause of the no-reflow phenomenon in the kidney, and when relieved, can prevent kidney injury [5,6]. In addition, renal I/R takes place during kidney transplantation, which delays kidney graft function in clinical practice [7]. Therefore, many studies investigated method to relieve renal I/R damage [8,9].
MiR-30 belongs to the microRNA (miRNA) family and includes 5 members (miR-30a, miR-30b, miR-30c, miR-30d, and miR-30e) and 6 mature miRNAs; it is encoded by 6 genes located on chromosomes 1, 6, and 8 [10]. Most studies have shown that miR-30 acts as a suppressor in tumors and can inhibit multiple tumors, such as hepatocellular carcinoma [11], lung cancer [12], and colorectal cancer [13]. A recent study demonstrated that non-infective systemic inflammatory response syndrome (SIRS) reflected changes in miR-30a-5p and miR-30d-5p, and that these circulating inflammatory-relevant miRNAs were not from red blood cell damage caused hemolysis or coagulopathy, but were activated immune cells produced by SIRS-related miRNAs [14]. Chen et al. reported high-mobility group box 1 (HMG1) aggravated renal I/R by stimulating inflammation and immunologic response [15]. In addition, Yarijani et al. pointed out that anti-inflammation protected the kidney against injury resulting from I/R [16]. Our study hypothesis was based on the aforementioned studies that suggested that there was one relationship among miR-30c-5p, renal I/R, and immune responses. In our study, we first investigated the relationship between overexpression of miR-30c-5p and renal I/R, then explored the corresponding immune reactions.
Material and Methods
I/R model of kidney in rat
Sprague Dawley rats were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, Guangdong, China). The left kidney was removed, and the right renal artery was clamped through right flank incisions by atraumatic vascular clamp after the rats were anesthetized with pentobarbital (3%, 50 mg/kg; Beizhuo, Shanghai, China) by intraperitoneal injection. Subsequently, the clamp was removed after an ischemic time of 40 minutes. Wounds were closed and sterilized after the end of the operation. In addition, the rats were supplemented with physiological saline. The rats were normally fed for 96 hours after the operation. The Sham group (n=10) had an incision but did not have the left kidney removed or the right renal artery clamped. Rats (n=10) in the normal control (NC) group and rats in the miR-30c-5p agomir (5′-UGUAAACAUCCUACACUCUCAGC-3′) group were administered miR-30c-5p agomir (10 nmol/20 g weight) by intravenous tail injection before the I/R operation. Our animal study was approved by the Institutional Animal Care and Use Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University.
Colorimetry
The blood samples were drawn at 24 hours, 48 hours, and 96 hours after surgery. Creatinine (Cr) assay kit (sarcosine oxidase method) (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) determined the concentration of Cr in blood samples with automatic chemical analysis (Mindray, Shenzhen, Guangdong, China) at the wavelength of 570 nm. The manipulation was performed following the product’s specifications.
Enzyme-linked immunosorbent assay (ELISA)
The blood samples were drawn at 24 hours, 48 hours, and 96 hours after surgery. Rat neutrophil gelatinase associated lipocalin (NGAL) ELISA kit (Elabscience, Wuhan, Hubei, China) measured the concentration of NGAL in blood samples with Multiskan™ FC (Thermo Scientific, Waltham, MA, USA) at the wavelength of 450 nm, following the product’s specifications.
Hematoxylin and eosin (H&E) stain
Rats from each group were sacrificed 96-hours post operation. Kidney tissues from rats were collected for histopathology [17]. Kidney tissues were fixed with 10% buffered formalin (Solarbio, Beijing, China). Then the tissues were dehydrated, cleared, embedded in paraffin and sectioned (thickness of 3–4 μm). Kidney tissue sections were stained with hematoxylin and eosin (H&E, Solarbio, Beijing, China).
Cell culture and cell transfection
The THP-1 cell line, which originated from a 1-year old infant with acute monocytic leukemia, was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). THP-1 cells were cultured with RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% 10 000 units/mL penicillin and 10 000 μg/mL streptomycin (Gibco, Carlsbad, CA, USA). Phorbol myristate acetate (PMA, 100 ng/mL) (Sigma-Aldrich, St. Louis, MO, USA) was used to induce THP-1 cells for 48 hours. MiR-30c-5p agomir (5′-UGUAAACAUCCUACACUCUCAGC-3′), miR-30c-5p antagomir, NC agomir, and NC antagomir mixed with lipofectamine solution (Invitrogen, Carlsbad, CA, USA) was dissolved in free FBS RPMI-1640 medium. The mixed solutions were used to treat the PMA-induced cells for 3 hours, and then the cells were cultured with normal medium for 48 hours.
Western blot
Proteins from the cells or kidney tissues were harvested using lysis buffer (Thermo Scientific, Waltham, MA, USA), and the lysates were centrifuged at 4°C, 12 000 rad/min for 15 minutes. To measure the concentration of protein, a BCA kit (Thermo Scientific, Waltham, MA, USA) was used, and the quantified proteins were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred on PVDF membranes (Sigma-Aldrich, St. Louis, MO, USA). Afterward, the protein membranes were stained with 1x Ponceau (Solarbio, Beijing, China), then blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA) dissolved in TBST. The protein membranes were then incubated with primary antibodies, including CD86 antibody (ab239075, ab112490, Abcam, Cambridge, MA, USA), CD206 (#91992, Cell Signaling Technology, Danvers, MA, USA) (P22897, Elabscience, Wuhan, Hubei, China), inducible nitric oxide synthase (iNOS) (ab136918, Abcam, Cambridge, MA, USA), interleukin (IL)-10 (#12163, Cell Signaling Technology, Danvers, MA, USA) and β-actin (ab8227, Abcam, Cambridge, MA, USA), overnight at 4°C in a box containing antibody solution. The protein membranes were washed with TBST solution 3 times after primary antibody incubation and were then incubated with anti-rabbit antibody tagged with horse radish peroxidase (HRP) (ab7090, Abcam, Cambridge, MA, USA) for 2 hour at the room temperature. The protein membranes were detected with enhanced chemiluminescence (ECL) kit (Sigma-Aldrich, St. Louis, MO, USA) after the membranes were washed, as described in a previous study [18].
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNAs from kidney tissue or THP-1 cells were homogenized with TRIzol reagent (Sigma-Aldrich, St. Louis, MO, USA) using a homogenizer (IKA-WERK, Staufen, Germany). Total RNA was separated and extracted according to the manufacturer’s instructions. RNAs were synthesized by cDNA synthesis kit (Sigma-Aldrich, St. Louis, MO, USA). The operation followed the product’s specifications.
The sequences of PCR primers used are shown in Table 1 and were synthesized by Sangon Biotech (Shanghai, China). The conditions of PCR were: initial incubation at 95°C for 2 minutes, 40 cycles of 95°C for 30 seconds, 55°C for 60 seconds, and 72°C for 30 seconds. We used the 2(−ΔΔCt) method for analyzing the relative level of mRNA.
Table 1.
Oligonucleotide primer sequences for PCR.
| Primer target | Sequence |
|---|---|
| miR-30c-5p* | F: 5′-ACACTCCAGCTGGGTGTAAACATCCTACACTC-3′ |
| R: 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTT GAGGCTCAGAG-3′ | |
| U6* | F: 5′-GCTTCGGCAGCACATCTCATAAAAT-3′ |
| R: 5′-CGCTTCACGATTTGCGTGTCAT-3′ | |
| CD86* | F: 5′-TAGGGATAACCAGGCTCTAC-3′ |
| R: 5′-CGTGGGTGTCTTTTGCTGTA -3′ | |
| iNOS* | F: 5′-GGAGAGATTTTTCACGACACCC-3′ |
| R: 5′-CCATGCATAATTTGGACTTGCA-3′ | |
| CD206* | F: 5′-GGGACTCTGGATTGGACTCA-3′ |
| R: 5′-CCAGGCTCTGATGATGGACT-3′ | |
| IL-4* | F: 5′-TCGGCATTTTGAACGAGGTC-3′ |
| R: 5′-GAAAAGCCCGAAAGAGTCTC-3′ | |
| IL-10* | F: 5′-CGGGAAGACAATAACTG-3′ |
| R: 5′-CACGCTGGCTCAGCCACTC-3′ | |
| β-actin* | F: 5′-ATGGCAACGTCAAGGCTGAGA-3 |
| R: 5′CGCTCCTGGAAGATGGTGAT-3′ | |
| miR-30c-5p** | F: 5′-TGTAAACATCCTCGAC-3′ |
| R: 5′-ACATCCAGTGTAGCATA-3′ | |
| U6** | F: 5′-CTTCGGCAGCACATATACTAAAAT-3′ |
| R: 5′-CAGGGGCCATGCTAAATCTTC-3′ | |
| CD86** | F: 5′-TTGCAGAGGCAGCAAGATGG-3′ |
| R: 5′-ATGATGAGTGGCAGCAAGATGG-3′ | |
| iNOS** | F: 5′-GGAGCCAGCTCTGCATTATC-3′ |
| R: 5′-TTTTGTCTCCAAGGGACCAG-3′ | |
| CD206** | F: 5′-TTCGGACACCCATCGGAATTT-3′ |
| R: 5′-CACAAGCGCTGCGTGGAT-3′ | |
| IL-10** | F: 5′-CTCGGATCCAAGGCATGCACAGCTCAGC-3′ |
| R: 5′-CTCCTCGAGCCTGATGTCTCAGTTTCGTA-3′ | |
| β-actin** | F: 5′-CTGCAGGTCGACGATTGGACTCCGGTGACGGGGTCA-3′ |
| R: 5′-GGATCCTCTAGAGATTATGACCTGGCCGTCAGGCAG-3′ |
Primer target in rat;
Primer target in THP-1 cell line.
Statistical analysis
The values were presented as mean ± standard deviation (SD) using SPSS 22 (IBM, Armonk, NY, USA). THP-1 cell experiments were independently performed at least 3 times. Statistical analyses were performed by one-way analysis of variance (ANOVA) with Turkey’s multiple test for comparison by Graph Prism 6 (Graph Pad, La Jolla, CA, USA). P values <0.05 were considered to be statistically significant.
Results
MiR-30c-5p agomir decreased the contents of serum Cr and NGAL in serum of rats and attenuated renal I/R injury of rats
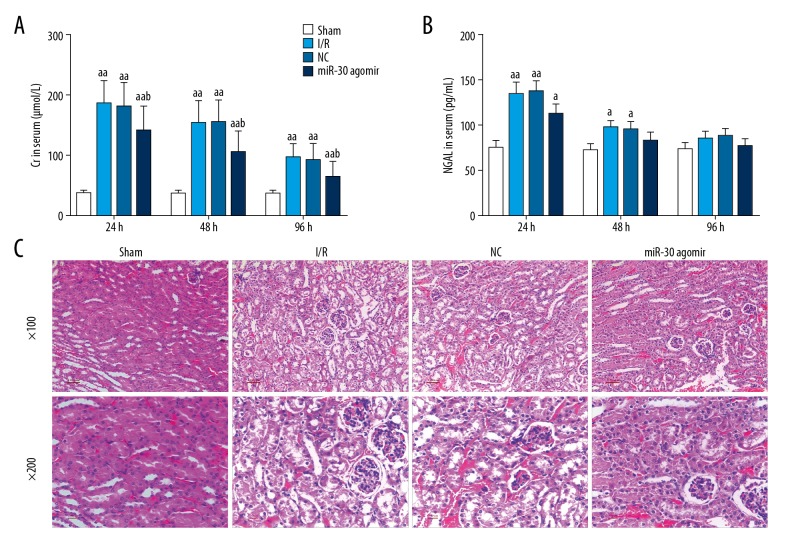
The contents of serum Cr and NGAL were decreased following the feeding time of rats after surgery (Figure 1A, 1B). Serum Cr and serum NGAL were reduced by miR-30c-5p agomir (Figure 1A, 1B). There was a statistical significance difference between the miR-30c-5p agomir group and the NC group in determination of serum Cr. Kidney tissue of the I/R group showed changes compared to the Sham group, including nuclear fragmentation and swelling (Figure 1C). H&E staining of the I/R group revealed that I/R led to injury of kidney tissue. MiR-30c-5p reduced the damage from I/R in kidney tissue (Figure 1C).
Figure 1.
MiR-30c-5p attenuated rat renal I/R and inhibited serum Cr and serum NGAL. After the rats had surgery to create a rate renal I/R model, the rats were fed normally. Serum samples were taken at 24 hours, 48 hours, and 96 hours after normal feeding. Serum Cr was measured by serum Cr assay kit using automatic chemical analysis at the wavelength of 570 nm (A). Serum NGAL was evaluated by ELISA kit (B). After the rats were sacrificed, the kidney tissues were removed, and the kidney injury was measured by H&E staining (C). MiR-30=miR-30c-5p. The values were presented as mean ±SD, and the data were analyzed by ANOVA with Turkey’s multiple test (a versus Sham group, b versus I/R group; a=b P<0.05, aa=bb P<0.01). I/R – ischemia-reperfusion; Cr – creatinine; NGAL – neutrophil gelatinase associated lipocalin; ELISA – enzyme-linked immunosorbent assay; H&E – hematoxylin and eosin; SD – standard deviation; ANOVA – analysis of variance.
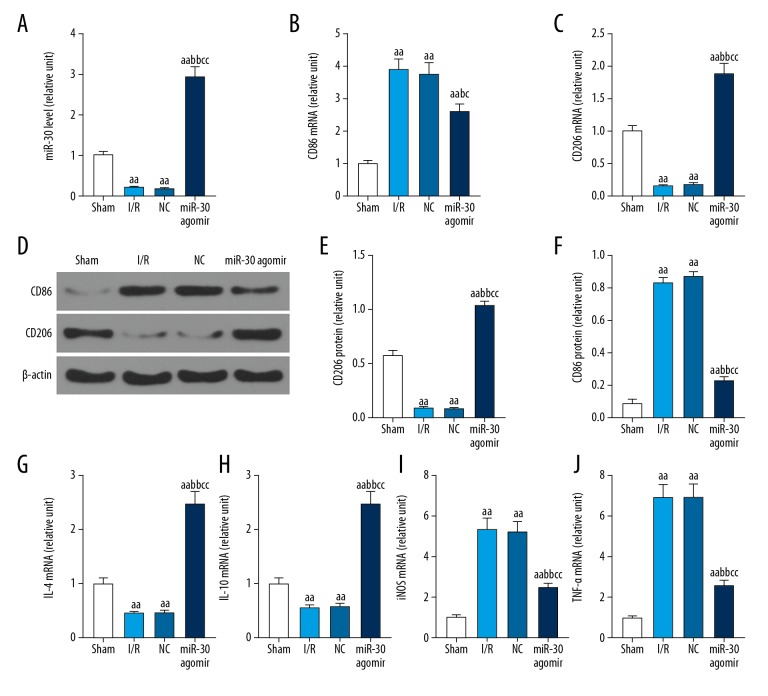
Effects of miR-30c-5p agomir on the expressions of CD86, CD206, IL-4, IL-10, iNOS, and tumor necrosis factor-alpha (TNF-α) in kidney tissue of rats treated with I/R model
I/R of the kidney caused lower expression of miR-30c-5p, CD206, IL-4, and IL-10, however, it caused increased expression of CD86, iNOS, and tumor necrosis factor-alpha (TNF-α) (Figure 2). MiR-30c-5p agomir promoted miR-30c-5p expression, inhibited expression of CD86, iNOS, and TNF-α, and increased expression of CD206, IL-4, and IL-10 in rats with renal I/R (Figure 3). Next, we investigated the effects of miR-30c-5p agomir on monocytes because miR-30c-5p agomir might vary inflammatory factors and activated monocytes.
Figure 2.
Effects of miR-30c-5p on the expression of CD86, CD206, IL-4, IL-10, iNOS, and TNF-α in kidney tissue of rats. After the rats were sacrificed, kidney tissues were removed, and the mRNA of miR-30c-5p, CD86, CD206, IL-4, IL-10, iNOS, and TNF-α were measured by qRT-PCR (A–C, G–J), and the protein levels of CD86 and CD206 were analyzed by western blot (D–F). miR-30=miR-30c-5p. The values were presented as mean ±SD, and the data were analyzed by ANOVA with Turkey’s multiple test (a versus Sham group, b versus I/R group, c versus NC group; a=b=c P<0.05, aa=bb=cc P<0.01). IL – interleukin; iNOS – inducible nitric oxide synthase; TNF-α – tumor necrosis factor-alpha; qRT-PCR – quantitative reverse transcription-polymerase chain reaction; ANOVA – analysis of variance.
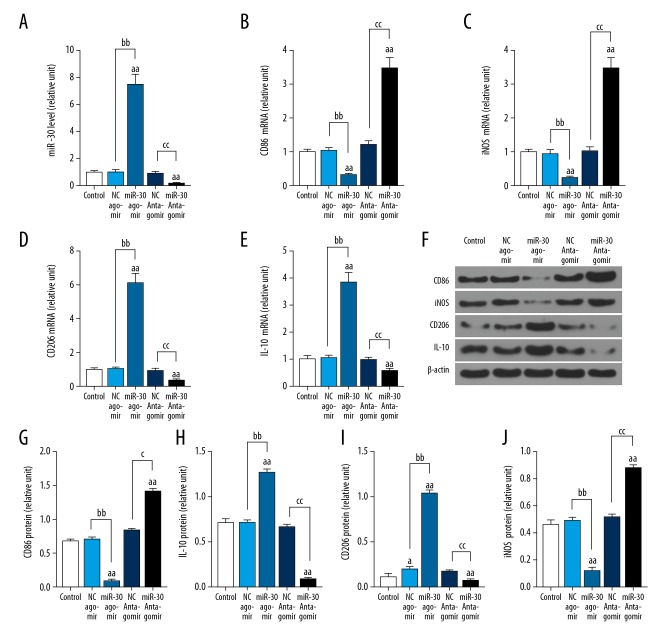
Figure 3.
Effects of miR-30c-5p on the expression of CD86, CD206, iNOS, and IL-10 in THP-1 cells. HTP-1 cells were induced with PMA. Cells were treated with miR-30c-5p agomir and miR-30c-5p antagomir for 3 hours, then the cells were cultured normally for 48 hours. The mRNA levels of miR-30c-5p, CD86, CD206, iNOS, and IL-10 were determined with qRT-PCR (A–E). The protein levels of CD86, CD206, iNOS, and IL-10 were detected with western blot (F–J). miR-30=miR-30c-5p. n=3. The values were presented as mean ±SD, and the data were analyzed by ANOVA with Turkey’s multiple test (a versus Control group; a=b=c P<0.05, aa=bb=cc P<0.01). IL – interleukin; iNOS – inducible nitric oxide synthase; PMA – phorbol myristate acetate; qRT-PCR – quantitative reverse transcription-polymerase chain reaction; SD – standard deviation; ANOVA – analysis of variance.
Effects of miR-30c-5p agomir and miR-30c-5p antagomir on the expression of CD86, CD206, IL-10, and iNOS in THP-1 cells
MiR-30c-5p agomir promoted miR-30c-5p expression, inhibited expression of CD86 and iNOS; however, it also enhanced levels of IL-10 and CD206 in THP-1 cells (Figure 3). MiR-30c-5p antagomir inhibited miR-30c-5p expression and promoted expression of CD86 and iNOS; however, it decreased the expression of IL-10 and CD206 in THP-1 cells (Figure 3).
Discussion
Serum Cr is a widely accepted biomarker of kidney injury or kidney toxicity and is used as one indicator to evaluate kidney function in clinical practice [19]. NGAL, a kidney tubular damage marker, is significantly high in type 2 diabetes patients with kidney injury and is a sensitive marker for kidney injury [20]. In our study, renal I/R decreased kidney function and damaged the kidney (Figure 1). MiR-30c-5p reduced kidney injury from I/R (Figure 1).
Kidney diseases are specifically classified as chronic and acute, and acute kidney injury is associated with bacterial infection, sepsis, and I/R injury. Acute kidney injury can develop into chronic kidney disease, and chronic kidney disease is typically associated with diabetic complications, obesity, and autoimmunity [21]. Inflammation and immune response are causal factors in development of acute and chronic kidney disease [21]. Studies have reported that IL-4 and IL-10, and T helper type 2 (Th2) cytokines in mice with crescentic glomerulonephritis inhibited the formation of glomerular crescent and protected kidney function [22,23]. In addition, the intake of IL-10 prolonged allograft rejection times and protected allografts [24]. A recent study reported that IL-4 polymorphisms were correlated with inflammatory bowel disease (IBD) [25], and patients with IBD were more likely to have kidney-related symptoms, including microscopic hematuria and proteinuria [26]. In our study, I/R caused rat kidney tissue to have lower expression of IL-4 and IL-10, and miR-30c-5p agomir reduced I/R injury in accordance with improvement of IL-4 and IL-10 (Figure 2).
Inducible nitric oxide synthase (iNOS) and tumor necrosis factor-alpha (TNF-α) are pro-inflammatory cytokines [27]. Chen et al. found that dexmedetomidine protected against hepatic I/R accompanying inhibition of pro-inflammatory mediators, including TNF-α, IL-6, and iNOS [28]. Renal I/R promoted the expression of iNOS and TNF-α, and miR-30c-5p agomir inhibited pro-inflammatory cytokines (Figures 2, 3). We thus, considered the questions: what kind of cells does miR-30c-5p agomir affect.
Macrophages are myeloid immune cells that are well known to be characterized by phagocytosis, and they are located throughout body tissues where they intake and degrade dead cells, foreign materials and fragments, and accelerate inflammatory processes [29]. Most studies have described 2 main polarized macrophages, T helper 1 (Th1) and Th2 immune response. One is a macrophage related to Th1 immune response that produces lots of inflammatory factors, including TNF-α, IL-12, and IL-6. The other one is a macrophage related to Th2 that repairs and remodels tissue by dissolving inflammation, and it can be induced by Th2 cytokines such as IL-10, IL-13, and IL-4 [30]. M2/M2-like macrophage function because surface molecules such as CD206 and CD163 are expressed in it [31]. M1 macrophages express CD86 and CD80 and has functional status [32,33]. A recent study reported that CD86 M1 macrophages had antitumor properties, and CD206 M2 macrophages had pro-tumoral behavior [34,35]. Our study preliminarily demonstrated that miR-30c-5p agomir promoted M1 macrophages transforming to M2 macrophages (Figure 2). In order to further investigate, we explored the effects of miR-30c-5p agomir on THP-1 cells, and showed that miR-30c-5p agomir promoted M1 THP-1 cells transformation to M2 THP-1 cells (Figure 3). However, we did not investigate the amount of M1 macrophages and M2 macrophages in the rat kidney tissue by immunohistochemistry or flow cytometry, which weakened the strength of our results.
Conclusions
In conclusion, our study findings preliminarily support that miR-30c-5p agomir decreased renal I/R by inflammatory response and reaction, including transformation of M1 macrophages to M2 macrophages and the accompanying changes of inflammatory factors. Thus, upregulation of miR-30c-5p exerted a reno-protective effect against renal I/R by reducing inflammation, which might contribute to identifying a promising molecular therapeutic target for treating renal I/R.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China [81700664]; the Shandong Provincial Natural Science Foundation of China [ZR2016HP38]; the Projects of Medical and Health Technology Development Program in Shandong Province [2016WS0714]; and the Science and Technology Project of Yantai [2016WS018]
Conflict of interest
None.
References
- 1.Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21(3):246–52. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skovsted GF, Kruse LS, Berchtold LA, et al. Myocardial ischemia-reperfusion enhances transcriptional expression of endothelin-1 and vasoconstrictor ETB receptors via the protein kinase MEK-ERK1/2 signaling pathway in rat. PLoS One. 2017;12(3):e0174119. doi: 10.1371/journal.pone.0174119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi DE, Jeong JY, Choi H, et al. ERK phosphorylation plays an important role in the protection afforded by hypothermia against renal ischemia-reperfusion injury. Surgery. 2017;161(2):444–52. doi: 10.1016/j.surg.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Sturm JT, Billiar TR, Luxenberg MG, Perry JF. Risk factors for the development of renal failure following the surgical treatment of traumatic aortic rupture. Ann Thorac Surg. 1987;43(4):425–27. doi: 10.1016/s0003-4975(10)62821-4. [DOI] [PubMed] [Google Scholar]
- 5.Mason J, Joeris B, Welsch J, Kriz W. Vascular congestion in ischemic renal failure: The role of cell swelling. Miner Electrolyte Metab. 1989;15(3):114–24. [PubMed] [Google Scholar]
- 6.Brodsky SV, Yamamoto T, Tada T, et al. Endothelial dysfunction in ischemic acute renal failure: Rescue by transplanted endothelial cells. Am J Physiol Renal Physiol. 2002;282(6):F1140–49. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli CE. The impact of cold ischemia time on renal transplant outcome. Kidney Int. 2015;87(2):272–75. doi: 10.1038/ki.2014.359. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Zang G, Jiang J, et al. Attenuating ischemia-reperfusion injury in kidney transplantation by perfusing donor organs with siRNA cocktail solution. Transplantation. 2016;100(4):743–52. doi: 10.1097/TP.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 9.Emal D, Rampanelli E, Stroo I, et al. Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2017;28(5):1450–61. doi: 10.1681/ASN.2016030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40(1):43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Tu K, Liu Q. Effects of microRNA-30a on migration, invasion and prognosis of hepatocellular carcinoma. FEBS Lett. 2014;588(17):3089–97. doi: 10.1016/j.febslet.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Kumarswamy R, Mudduluru G, Ceppi P, et al. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int J Cancer. 2012;130(9):2044–53. doi: 10.1002/ijc.26218. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S, Miao D, Wang M, et al. MiR-30-5p suppresses cell chemoresistance and stemness in colorectal cancer through USP22/Wnt/beta-catenin signaling axis. J Cell Mol Med. 2019;23(1):630–40. doi: 10.1111/jcmm.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caserta S, Mengozzi M, Kern F, et al. Severity of systemic inflammatory response syndrome affects the blood levels of circulating inflammatory-relevant micrornas. Front Immunol. 2017;8:1977. doi: 10.3389/fimmu.2017.01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CB, Liu LS, Zhou J, et al. Up-regulation of HMGB1 exacerbates renal ischemia-reperfusion injury by stimulating inflammatory and immune responses through the TLR4 signaling pathway in mice. Cell Physiol Biochem. 2017;41(6):2447–60. doi: 10.1159/000475914. [DOI] [PubMed] [Google Scholar]
- 16.Yarijani ZM, Pourmotabbed A, Pourmotabbed T, Najafi H. Crocin has anti-inflammatory and protective effects in ischemia-reperfusion induced renal injuries. Iran J Basic Med Sci. 2017;20(7):753–59. doi: 10.22038/IJBMS.2017.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tafuri WL, Melo MN, Paiva MC, et al. Kinetics of an experimental inflammatory reaction induced by Leishmania major during the implantation of paraffin tablets in mice. Virchows Archiv. 2000;437(4):429–35. doi: 10.1007/s004280000231. [DOI] [PubMed] [Google Scholar]
- 18.Dayem AA, Kim B, Gurunathan S, et al. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol J. 2014;9(7):934–43. doi: 10.1002/biot.201300555. [DOI] [PubMed] [Google Scholar]
- 19.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992;38(10):1933–53. [PubMed] [Google Scholar]
- 20.Siddiqi Z, Karoli R, Kaul A, et al. Evaluation of neutrophil gelatinase-associated lipocalin and cystatin C as early markers of diabetic nephropathy. Ann Afr Med. 2017;16(3):101–6. doi: 10.4103/aam.aam_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol. 2013;3(2):957–76. doi: 10.1002/cphy.c120028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitching AR, Tipping PG, Huang XR, et al. Interleukin-4 and interleukin-10 attenuate established crescentic glomerulonephritis in mice. Kidney Int. 1997;52(1):52–59. doi: 10.1038/ki.1997.303. [DOI] [PubMed] [Google Scholar]
- 23.Tipping PG, Kitching AR, Huang XR, et al. Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol. 1997;27(2):530–37. doi: 10.1002/eji.1830270226. [DOI] [PubMed] [Google Scholar]
- 24.Mulligan MS, Warner RL, McDuffie JE, et al. Regulatory role of Th-2 cytokines, IL-10 and IL-4, in cardiac allograft rejection. Exp Mol Pathol. 2000;69(1):1–9. doi: 10.1006/exmp.2000.2304. [DOI] [PubMed] [Google Scholar]
- 25.Ebrahimi Daryani N, Saghazadeh A, Moossavi S, et al. Interleukin-4 and interleukin-10 gene polymorphisms in patients with inflammatory bowel disease. Immunol Invest. 2017;46(7):714–29. doi: 10.1080/08820139.2017.1360343. [DOI] [PubMed] [Google Scholar]
- 26.Jang HM, Baek HS, Kim JE, et al. Renal involvement in children and adolescents with inflammatory bowel disease. Korean J Pediatr. 2018;61(10):327–31. doi: 10.3345/kjp.2018.06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169(2):203–14. doi: 10.1016/s0021-9150(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Ding T, Ma CG. Dexmedetomidine (DEX) protects against hepatic ischemia/reperfusion (I/R) injury by suppressing inflammation and oxidative stress in NLRC5 deficient mice. Biochem Biophys Res Commun. 2017;493(2):1143–50. doi: 10.1016/j.bbrc.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Varol C, Mildner A, Jung S. Macrophages: Development and tissue specialization. Ann Rev Immunol. 2015;33:643–75. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 30.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Boorsma CE, Draijer C, Melgert BN. Macrophage heterogeneity in respiratory diseases. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/769214. 769214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He S, Xie L, Lu J, Sun S. Characteristics and potential role of M2 macrophages in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3029–39. doi: 10.2147/COPD.S147144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskar A, Eilertsen J, Li W, Yuan XM. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem Biophys Res Commun. 2013;441(4):737–42. doi: 10.1016/j.bbrc.2013.10.115. [DOI] [PubMed] [Google Scholar]
- 34.Farooque A, Afrin F, Adhikari JS, Dwarakanath BS. Polarization of macrophages towards M1 phenotype by a combination of 2-deoxy-d-glucose and radiation: Implications for tumor therapy. Immunobiology. 2016;221(2):269–81. doi: 10.1016/j.imbio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Dong P, Ma L, Liu L, et al. CD86(+)/CD206(+), Diametrically polarized tumor-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int J Mol Sci. 2016;17(3):320. doi: 10.3390/ijms17030320. [DOI] [PMC free article] [PubMed] [Google Scholar]