Abstract
Background
Cataract is associated with increased apoptosis of the epithelial cells of the ocular lens. Previous studies have shown that microRNA-378a (miR-378a) has a role in the development of cataract, but the molecular mechanisms remain unclear. This study aimed to investigate the effects of miR-378a in human lens epithelial cells (HLECs) in vitro and normal lens tissues and cataract tissues.
Material/Methods
HLECs were grown in culture. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) and Western blot were used to examine gene expression levels. The MTT and TUNEL assay measured cell growth and apoptosis. Changes in the fluorescence ratio of ethidium to dihydroethidium (E: DHE) and in 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (C-H2DCFDA) were used to detect superoxide (O2−) and hydrogen peroxide (H2O2). The expression levels of miR-378a and the superoxide dismutase 1 gene (SOD1) were measured in normal human lens tissues and cataract tissues.
Results
Upregulation of miR-378a reduced the expression of SOD1. Levels of O2− were upregulated and H2O2 was slightly down-regulated by miR-378a. The use of a miR-378a mimic suppressed cell growth and enhanced apoptosis of HLECs, which were reversed by the use of a miR-378a inhibitor. SOD1 overexpression rescued the miR-378a-induced phenotypes of HLEC cells. Treatment with the PI3K inhibitor, LY294002, reversed miR-378a and ROS-regulated proliferation and apoptosis of HLEC cells. Also, miR-378a was upregulated, and SOD1 was down-regulated in human cataract tissues.
Conclusions
In HLECs, expression of miR-378a regulated ROS and PI3K/AKT signaling, and miR-378a was upregulated, and SOD1 was down-regulated in human cataract tissue.
MeSH Keywords: Apoptosis, Cataract, MicroRNAs, Phosphatidylinositol 3-Kinases
Background
Worldwide, cataract is one of the most common causes of loss of vision, with an estimated 16 million people being affected [1,2]. A cataract is characterized by opacity and loss of optical clarity of the lens [2]. Risk factors for cataract include age, exposure to ultraviolet light, and diabetes, but aging is the main cause [3]. The current treatment for cataract is surgery, with the replacement of the cataract with an intraocular lens [4]. However, there is a risk of postoperative blindness due to surgical complications [5]. Therefore, more effective treatments for cataract are still required.
The microRNAs are a form of endogenous RNA that are 20–24 nucleotides in length, which are involved in cell growth, apoptosis, cancer, and cataract [6–8]. Also, microRNA-378a (miR-378a) expression has been shown to have a tumor suppressive role in colorectal cancer [9]. A previous study showed that miR-378a inhibited the growth of tamoxifen-resistant breast cancer cells [10]. Furthermore, miR-378a has been shown to be upregulated in cataract [11]. However, the mechanism by which miR-378a modulates the development of cataract remains unclear.
Aerobic metabolism requires oxygen, which can be associated with the generation of free radicals and reactive oxygen species (ROS) [12]. Superoxide (O2−) and hydrogen peroxide (H2O2) are major ROS that can impair cellular components [13]. An increase in oxidative stress is believed to be the underlying cause for many human diseases, including cataract, and antioxidants may be potential therapeutic strategies for cataract [14]. ROS may also impact cell signaling proteins, including PI3K/AKT [15,16]. A previous study showed that blocking the increase in ROS inhibited the PI3K/Akt pathway and cell apoptosis in osteosarcoma cells [17]. Therefore, this study aimed to investigate the effects of miR-378a in human lens epithelial cells (HLECs) in vitro and normal lens tissues and cataract tissues.
Material and Methods
Ethical approval and patient consent
Human cataract lens tissue was obtained with written informed consent from patients. This study was approved by the Ethical Committee of the First Peoples’ Hospital of Changzhou Approval No: 20190130-006). This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Human tissue samples
Anterior lens capsule samples (n=25) were obtained from patients with cataract who had surgery at the first Peoples’ Hospital of Changzhou, China. Normal lens anterior capsular samples were collected from healthy postmortem eyes (n=25) donated to the Eye Bank of the First Peoples’ Hospital of Changzhou. Samples were immediately snap-frozen and stored at −80°C before RNA extraction. All samples were collected with consent from all patients.
Human lens epithelial cells (HLECs)
Human lens epithelial cells (HLECs) (SRA01/04) was obtained from the Tumor Center of the Chinese Academy of Medical Sciences, Beijing, China [18]. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. For PI3K inhibition, 50 μM of LY294002 was used to treat HLEC cells.
Generation of stable cell lines
Short-hairpin RNAs (shRNAs) were cloned into the pLKO.1 vector. The pLKO.1-shRNA, pPAX2, and pVSVG were co-transfected into 293T cells to generate lentiviruses. Then, we collected the supernatant with the virus at 24 h and 48 h after vector transfection. The virus was mixed with medium at a 1: 4 ratio and 2 μg/ml of puromycin was used to select the positive cells, and shNC was used as the scramble vector. The primer for shSOD1 was: GGGCAAAGGUGGAAAUGAA.
Cell transfection
The SOD1 cDNA was amplified and subcloned into the pcDNA4 vector. The resultant plasmid was transfected into cells using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA). For miR-378a mimic or inhibitor transfection, Lipofectamine RNAiMAX reagent was used (Life Technologies, Carlsbad, CA, USA). The miR-378a mimic or inhibitor was synthesized by GenePharma (Shanghai, China).
TUNEL assay
The TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche-11684795910) (Sigma-Aldrich, St. Louis MO, USA) to detect cell apoptosis [19], according to the manufacturer’s instructions.
Western blot
Total proteins were loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% dried skimmed milk powder and incubated with primary and secondary antibodies. The membranes were incubated with ECL solution. The following antibodies were used: anti-PI3K (Cell Signaling Technology, Danvers, MA, USA), anti-p-PI3K (Cell Signaling Technology, Danvers, MA, USA), anti-AKT (Cell Signaling Technology, Danvers, MA, USA), anti-p-AKT (Cell Signaling Technology, Danvers, MA, USA), anti-GAPDH (Proteintech, Manchester, UK).
Measurement of superoxide and hydrogen peroxide
Changes in the ethidium to dihydroethidium (E: DHE) fluorescence ratio and 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (C-H2DCFDA) (Sigma, St. Louis, MO, USA) were used for detection of superoxide (O2−) and hydrogen peroxide (H2O2), respectively, which were analyzed using standard assay kits, according to the manufacturer’s instructions [13,20]. The O2− and H2O2 levels were presented as units or μmol per milligram of protein.
RNA extraction and quantitative reverse transcription polymerase chain reaction (RT-qPCR) for microRNA-378a (miR-378a)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using reverse transcription reagent kit (Invitrogen, Carlsbad, CA, USA). RT-qPCR was performed using SYBR Green PCR kit (Takara, Minato-ku, Tokyo, Japan). GAPDH and U6 were used as internal controls. The relative expression levels of the genes were calculated by comparing them with the expression of GAPDH or U6 using the 2−ΔΔCT method. The primers used were as follows:
Forward, miR-378a: CTCCAGGTCCTGTGTGTTACC;
Reverse, miR-378a: GGCCTTCTGACTCCAAGT;
Forward, SOD1: GGTGTGGCCGATGTGTCTAT;
Reverse, SOD1: CAAGCCAAACGACTTCCAGC;
Forward, GAPDH: GCACCGTCAAGGCTGAGAAC;
Reverse, GAPDH-R: GCCTTCTCCATGGTGGTGAA;
Forward, U6: CTCGCTTCGGCAGCACATATACTA;
Reverse, U6: ACGAATTTGCGTGTCATCCTTGCG.
Statistical analysis
Each experiment was performed in triplicate. Data were presented as the mean ± standard deviation (SD). Comparison of the two groups was performed by the two-tailed unpaired Student’s t-test. Comparison between multiple groups was performed by one-way analysis of variance (ANOVA). A P-value <0.05 was considered to be statistically significant.
Results
microRNA-378a (miR-378a) regulated reactive oxygen species (ROS) in human lens epithelial cells (HLECs)
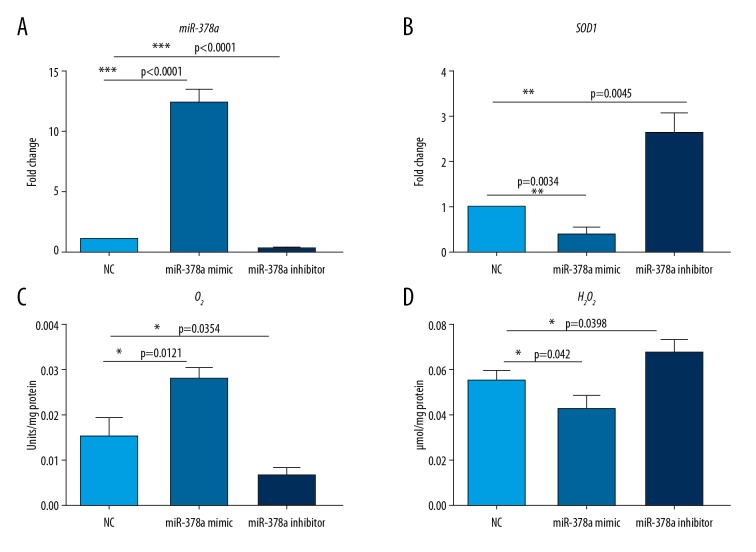
To determine the mechanism of microRNA-378a (miR-378a) in cataract, we transfected miR-378a mimic and inhibitor into HLECs. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) showed that the miR-378a mimic resulted in a reduced expression level of SOD1, while miR-378a inhibition promoted SOD1 gene expression (Figure 1A, 1B).
Figure 1.
microRNA-378a (miR-378a) regulates reactive oxygen species (ROS) in human lens epithelial cells (HLECs). (A) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis shows the level of microRNA-378a (miR-378a) in human lens epithelial cells (HLECs) transfected with NC, miR-378a mimic, and miR-378a inhibitor. All data are presented as the mean ±SD. The two-tailed unpaired Student’s t-test was performed for comparison of the two groups. (*** P<0.001). (B) RT-qPCR analysis shows the mRNA level of the SOD1 gene in HLEC cells transfected with NC, miR-378a mimic, and miR-378a inhibitor. All data are presented as the mean ±SD. The two-tailed unpaired Student’s t-test was performed for comparison of the two groups. (** P<0.01). (C, D) The levels of the ratio of ethidium to dihydroethidium (E: DHE) for superoxide (O2−), and 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (C-H2DCFDA) fluorescence for hydrogen peroxide (H2O2) in HLEC cells transfected with NC, miR-378a mimic, and miR-378a inhibitor. All data are presented as the mean ± SD. The two-tailed unpaired Student’s t-test was performed for comparison of the two groups. (* P<0.05).
Because SOD1 encodes the key enzyme involved in the regulation of levels of ROS, in this study, intracellular superoxide (O2−) and hydrogen peroxide (H2O2) levels were detected in HLEC cells that expressed the miR-378a mimic or inhibitor. The O2− levels were significantly increased, and H2O2 levels were slightly decreased in HLEC cells expressing the miR-378a mimic. However, O2− levels were significantly decreased, and H2O2 levels were slightly increased in miR-378a inhibited HLEC cells compared with NC cells (Figure 1C, 1D). These data indicate that miR-378a increased ROS levels (mainly O2− level) by suppressing SOD1 expression.
miR-378a inhibited cell proliferation and induced apoptosis of HLEC cells by modulating SOD1 expression
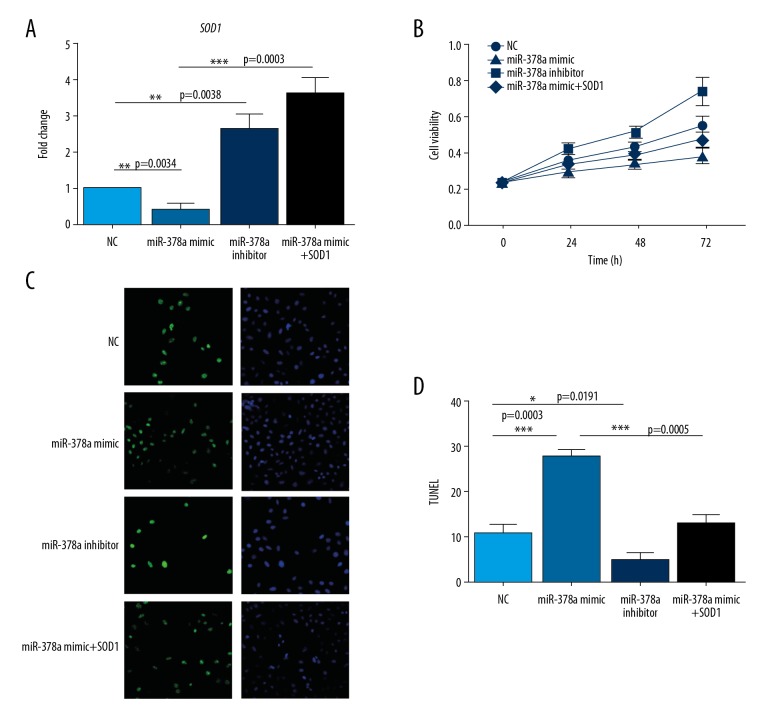
To investigate whether ROS was involved in miR-378a-mediated proliferation and apoptosis of HLEC cells, we first examined cell growth of HLEC cells expressing miR-378a mimic or inhibitor by the MTT method (Figure 2A, 2B). The miR-378a mimic significantly reduced the proliferation of HLEC cells relative to the negative control group. However, the miR-378a inhibitor promoted HLEC cells proliferation. Also, we measured the apoptosis rate of HLEC cells following the use of the miR-378a mimic or inhibition using the TUNEL assay (Figure 2C, 2D). The use of the miR-378a mimic increased the rate of cell apoptosis rate when compared with control cells, whereas the miR-378a inhibitor had the opposite effect.
Figure 2.
microRNA-378a (miR-378a) inhibits cell proliferation and induces apoptosis of human lens epithelial cells (HLECs) by modulating SOD1. (A) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis shows the mRNA level of SOD1 in human lens epithelial cells (HLECs) transfected with NC, miR-378a mimic, miR-378a inhibitor, miR-378a mimic plus SOD1. All data are presented as the mean ±SD. Comparison of the two groups was performed by one-way analysis of variance (ANOVA). (** P<0.01; *** P<0.001). (B) MTT assay shows cell viability of HLEC cells transfected with NC, miR-378a mimic, miR-378a inhibitor, miR-378a mimic, and SOD1 at different time points of 0, 24, 48, 72 h. (C) TUNEL assay shows cell apoptosis of HLEC cells transfected with NC, miR-378a mimic, miR-378a inhibitor, miR-378a mimic and SOD1. (D) Statistics of cell apoptosis rate of HLEC cells transfected with NC, miR-378a mimic, miR-378a inhibitor, miR-378a mimic plus SOD1. All data are presented as the mean ±SD. Comparison of the two groups were performed by ANOVA. (* P<0.05; *** P<0.001).
SOD1 was studied to determine its role in miR-378a-induced phenotypes of HLEC cells. Overexpression of SOD1 in HLEC cells reversed the inhibition of cell proliferation and enhanced apoptosis caused by the miR-378a mimic. These data suggest that miR-378a regulated proliferation and apoptosis of HLEC cells partially through ROS.
PI3K/AKT signaling was involved in miR-378a/ROS-regulated proliferation and apoptosis of HLEC cells
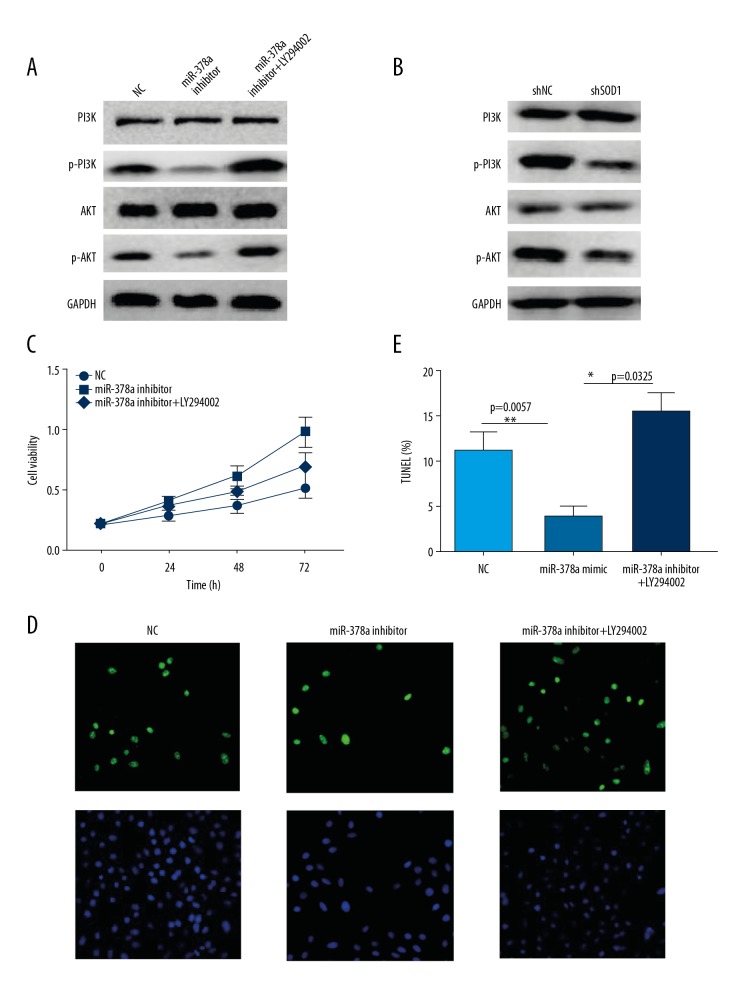
Because PI3K/AKT signaling was reported to be a crucial effector of ROS in some tumor cells, in this study, the role of PI3K/AKT was investigated in miR-378a/ROS-induced proliferation and apoptosis of HLEC cells. Western blot analysis showed that the phosphorylated forms of PI3K and AKT were reduced following miR-378a mimic or SOD1 knockdown in HLEC cells. However, p-PI3K and p-AKT levels were increased when the cells were transfected with the miR-378a inhibitor (Figure 3A, 3B).
Figure 3.
PI3K/AKT signaling is critical for microRNA-378a (miR-378a) and reactive oxygen species (ROS)-regulated proliferation and apoptosis of human lens epithelial cells (HLECs). (A) Western blot shows the levels of the indicated proteins in human lens epithelial cells (HLECs) transfected with NC, miR-378a mimic, miR-378a inhibitor. GAPDH acts as the loading control. (B) Western blot shows the levels of the indicated proteins in HLEC cells stably expressing shNC and shSOD1. GAPDH acts as the loading control. (C) MTT assay shows cell viability of HLEC cells transfected with NC, miR-378a inhibitor, miR-378a inhibitor plus LY294002 treatment at different time points of 0, 24, 48, 72 h. (D) TUNEL assay shows cell apoptosis of HLEC cells transfected with NC, miR-378a inhibitor, miR-378a inhibitor, and treatment with LY294002. (E) Statistical analysis of the cell apoptosis rate of HLEC cells transfected with NC, miR-378a inhibitor, miR-378a inhibitor and treatment with LY294002. All data are presented as the mean ± SD. Comparison of the groups was performed by one-way analysis of variance (ANOVA). (* P<0.05; ** P<0.01).
To determine the role of PI3K/AKT in miR-378a/ROS-regulated cell growth and apoptosis of HLEC cells, we used the PI3K inhibitor, LY294002, to treat the cells. The MTT assay showed that the PI3K inhibitor blocked the proliferation activity of miR-378a inhibited HLEC cells (Figure 3C). The TUNEL assay showed that the PI3K inhibitor, LY294002, promoted apoptosis of miR-378a inhibited HLEC cells (Figure 3D, 3E). These results indicated that PI3K/AKT signaling acted as a downstream effector of miR-378a/ROS-mediated proliferation and apoptosis.
miR-378a and SOD1 levels in human cataract lens samples
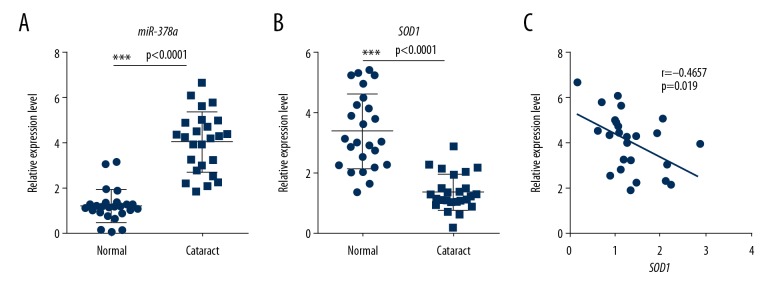
To determine whether the miR-378a/ROS pathway was involved in human lens tissue affected by cataract, we analyzed miR-378a and SOD1 expressions in human cataract lens and normal lens tissue. As shown in Figure 4A, 4B, the miR-378a level was significantly higher in cataract lens compared with normal lens. The SOD1 expression level was lower in cataract lens tissue compared with normal lens. Also, miR-378a expression was significantly negatively correlated with the levels of SOD1 (r=−0.4657; P=0.019) (Figure 4C). Therefore, in this study, miR-378a was regulated by ROS signaling.
Figure 4.
microRNA-378a (miR-378a) and SOD1 levels in human cataract lens samples. (A) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis shows the level of miR-378a in human normal lens and cataract lens samples (n=25). All data are presented as the mean ± SD. The two-tailed unpaired Student’s t-test was performed for comparison of the two groups. (*** P<0.001). (B) RT-qPCR analysis shows the mRNA level of SOD1 in the normal human lens and cataract lens samples (n=25). All data are presented as the mean ± SD. The two-tailed unpaired Student’s t-test was performed for comparison of the two groups. (*** P<0.001). (C) Spearman’s correlation analysis shows a negative correlation between miR-378a and SOD1 in human cataract lens samples (n=25). r=−0.4657, p=0.019.
Discussion
The findings from this study showed that in human lens epithelial cells (HLECs) in vitro and human cataract tissues, microRNA-378a (miR-378a) contributed to the generation of reactive oxygen species (ROS) by regulating the expression of the SOD1 gene. High ROS levels down-regulated PI3K/AKT signaling, which may have a role in cataract development. The findings from this study identified a new miR-34/ROS/PI3K/AKT signaling axis in cataract, but these preliminary findings require support from future studies.
Due to the global increase in the aging population, the prevalence of cataract has increased. Large-scale microarray analysis has identified several microRNAs that are deregulated in cataract [21]. From these studies, miR-378a, miR-15a, miR-16, and let-7 are upregulated, while miR-125b and miR-29a have been shown to be significantly down-regulated [22]. In the present study, several methods were used to identify the role of miR-378a in suppressing cell growth and inducing apoptosis in human lens epithelial cells (HLECs).
Oxidative stress represents a shift in the balance between oxidants and antioxidants, including superoxide dismutase and catalase, which results in increased reactive oxygen species (ROS) in the tissues [23]. The mechanisms involved in the induction of cataracts by free radicals or oxidants are still unclear, but a previous study showed that miR-21 modulated ROS levels by targeting SOD3 [24]. Currently, there has been little evidence to show that miR-378a regulates ROS levels. However, in this study, miR-378a was found to directly target the ROS-associated enzyme SOD1, which increased ROS levels.
An increase in ROS can influence cell signaling proteins and their functional consequences. In a previous study, signaling pathways were shown to be targeted by ROS, including NF-κB, MAPKs, PI3K/AKT, Wnt/β-catenin, and ion channel signaling [13,15,16]. To determine the role of PI3K/AKT signaling in cell proliferation and apoptosis of HLECs, we used the PI3K inhibitor, LY294002, to determine the effects on cell growth and apoptosis. The findings of the present study showed that the PI3K/AKT signaling pathway was involved with miR-378a and ROS in vitro in HLECs and in cataract tissue. Future studies should be undertaken to investigate downstream targets of miR-378a, and in vivo studies may confirm the role of miR-378a in the development of cataract.
Conclusions
This study aimed to investigate the effects of microRNA-378a (miR-378a) in human lens epithelial cells (HLECs) in vitro and normal lens tissues and cataract tissues. In HLECs, the expression of miR-378a regulated reactive oxygen species (ROS) and PI3K/AKT signaling, and miR-378a was upregulated, and SOD1 was down-regulated in human cataract tissue. The findings of this study require further investigation to determine the potential role of miR-378a in the diagnosis and treatment of cataract.
Acknowledgments
We thank Xujing Lv and Xun Pan for helpful discussions and critical reading of this manuscript.
Footnotes
Source of support: This study was supported by the Science and Technology Research Project of Changzhou (CJ20180067)
Conflict of interest
None.
References
- 1.Asbell PA, Dualan I, Mindel J, et al. Age-related cataract. Lancet. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 2.Thompson J, Lakhani N. Cataracts. Prim Care. 2015;42:409–23. doi: 10.1016/j.pop.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev. 1995;17:336–46. doi: 10.1093/oxfordjournals.epirev.a036197. [DOI] [PubMed] [Google Scholar]
- 4.Abdelkader H, Alany RG, Pierscionek B. Age-related cataract and drug therapy: Opportunities and challenges for topical antioxidant delivery to the lens. J Pharm Pharmacol. 2015;67:537–50. doi: 10.1111/jphp.12355. [DOI] [PubMed] [Google Scholar]
- 5.Apple DJ, Mamalis N, Loftfield K, et al. Complications of intraocular lenses. A historical and histopathological review. Surv Ophthalmol. 1984;29:1–54. doi: 10.1016/0039-6257(84)90113-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Wang Y, Li W, et al. MicroRNA-30a regulation of epithelial-mesenchymal transition in diabetic cataracts through targeting SNAI1. Sci Rep. 2017;7:1117. doi: 10.1038/s41598-017-01320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Jin H, Shi Y, Guo Y, Zhang H. Long non-coding RNA KCNQ1OT1 promotes cataractogenesis via miR-214 and activation of the caspase-1 pathway. Cell Physiol Biochem. 2017;42:295–305. doi: 10.1159/000477330. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Dai S, Zhen T, et al. Clinical and biological significance of miR-378a-3p and miR-378a-5p in colorectal cancer. Eur J Cancer. 2014;50:1207–21. doi: 10.1016/j.ejca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda K, Horie-Inoue K, Ueno T, et al. miR-378a-3p modulates tamoxifen sensitivity in breast cancer MCF-7 cells through targeting GOLT1A. Sci Rep. 2015;5:13170. doi: 10.1038/srep13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, Liu Z, Ma L, et al. MiRNAs regulate oxidative stress-related genes via binding to the 3′ UTR and TATA-box regions: A new hypothesis for cataract pathogenesis. BMC Ophthalmol. 2017;17:142. doi: 10.1186/s12886-017-0537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varma SD, Chand D, Sharma YR, et al. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res. 1984;3:35–57. doi: 10.3109/02713688408997186. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Huang Q, Wang Q, et al. Enhanced renal afferent arteriolar reactive oxygen species and contractility to Endothelin-1 are associated with canonical wnt signaling in diabetic mice. Kidney Blood Press Res. 2018;43:860–71. doi: 10.1159/000490334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiagarajan R, Manikandan R. Antioxidants and cataract. Free Radical Res. 2013;47:337–45. doi: 10.3109/10715762.2013.777155. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Wang X, Cheng S, et al. Reactive oxygen species mediate arsenic-induced cell transformation and tumorigenesis through Wnt/beta-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol Appl Pharmacol. 2011;256:114–21. doi: 10.1016/j.taap.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Wang X, Vikash V, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/4350965. 4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Sun N, Li X, et al. Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated down-regulation of the PI3K/Akt pathway. Oncol Rep. 2016;35:3648–58. doi: 10.3892/or.2016.4722. [DOI] [PubMed] [Google Scholar]
- 18.Fan F, Zhuang J, Zhou P, et al. MicroRNA-34a promotes mitochondrial dysfunction-induced apoptosis in human lens epithelial cells by targeting Notch2. Oncotarget. 2017;8:110209–20. doi: 10.18632/oncotarget.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun M, Geng D, Li S, et al. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol Chem. 2018;399:387–95. doi: 10.1515/hsz-2017-0255. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Zhao Z, Pang X, et al. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol Lett. 2017;277:115–22. doi: 10.1016/j.toxlet.2017.07.216. [DOI] [PubMed] [Google Scholar]
- 21.Kubo E, Hasanova N, Sasaki H, Singh DP. Dynamic and differential regulation in the microRNA expression in the developing and mature cataractous rat lens. J Cell Mol Med. 2013;17:1146–59. doi: 10.1111/jcmm.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Zheng H, Chan MT, Wu WKK. MicroRNAs: New players in cataract. Am J Transl Res. 2017;9:3896–903. [PMC free article] [PubMed] [Google Scholar]
- 23.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann NY Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Ng WL, Wang P, et al. MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNF alpha. Cancer Res. 2012;72:4707–13. doi: 10.1158/0008-5472.CAN-12-0639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]