Abstract
Background
Monocarboxylate transporter 4 (MCT4) is a critical element for glycolytic metabolism and malignant behaviors in many tumor cells. This study aimed to determine the expression level of MCT4 protein and its prognostic value in osteosarcoma.
Material/Methods
MCT4 expression was detected via immunohistochemical and Western blot analysis for 100 osteosarcoma patients. The correlation between MCT4 expression and clinical factors among the patients was analyzed using the chi-square test. Overall survival of osteosarcoma patients was estimated by Kaplan-Meier analysis. The prognostic value of MCT4 was evaluated using Cox regression analysis with adjustments for clinicopathological variables.
Results
MCT4 expression was significantly upregulated in osteosarcoma tissues compared with that in adjacent normal ones, detected via both immunohistochemical and Western blot analyses. High MCT4 expression showed a positive association with distant metastasis (P=0.000) and recurrence (P=0.000) of osteosarcoma. Kaplan-Meier analysis indicated that overall survival of osteosarcoma patients was significantly higher in the low MCT4 expression group than in the high expression group (log-rank test, P<0.001). Multivariate analysis indicated that MCT4 expression and clinical stage, which are tightly related to the prognosis of osteosarcoma, might be independent predictors of osteosarcoma prognosis.
Conclusions
High MCT4 expression appears to contribute to osteosarcoma progression and the upregulation of MCT4 may predict poor prognosis among osteosarcoma patients.
MeSH Keywords: Monocarboxylic Acid Transporters, Osteosarcoma, Prognosis
Background
As one of the most common primary bone tumors, osteosarcoma is usually diagnosed among children and adolescents, and accounts for about 20–35% of total malignant bone tumor cases [1–3]. This cancer is characterized by high malignancy and metastasis [4]. Most osteosarcomas metastasize to the lung and liver, leading to poor patient outcomes [5]. With the development of comprehensive therapy, the 5-year survival rate of osteosarcoma patients is significantly improved to as high as 60–70% [6,7]. However, once distant metastasis appears, the cure rate is very poor and 5-year survival rate is about 20% [8,9]. Moreover, fundamental molecular mechanisms of osteosarcoma development and progress are still obscure. Molecular bio-markers valuable in the prognosis of other diseases might be useful for the analysis of the mechanism of osteosarcoma. Therefore, novel and more reliable markers are needed to estimate the prognosis of osteosarcoma.
Monocarboxylate transporter 4 (MCT4), a member of the MCTs family, is an important factor for acid-resistant phenotype for several cancer cells and is a sensitive marker of aerobic glycolysis [10–13]. It sustains successive transition of pyruvate to lactate, after which glycolysis is maintained via exporting lactate [13]. In neuroendocrine prostate cancer, reduced MCT4 expression can suppress the secretion and glycolysis of lactic acid, thus inhibiting cell proliferation [14]. MCT4 inhibition can also boost glycolysis, and then lead to the death of cancer cells [15]. Upregulated MCT4 has been considered as an indicator for poor survival in many cancers, including lung cancer, breast cancer, head and neck cancer, colorectal cancer (CRC), and oral squamous cell carcinoma [16–20]. However, there have been few studies on the role of MCT4 in osteosarcoma.
In the present study, we detected MCT4 expression in osteosarcoma patients and analyzed its relationship with clinical factors of the cases, as well as assessing the prognostic value of MCT4 in osteosarcoma.
Material and Methods
Patients and tissue samples
We enrolled 100 patients with osteosarcoma at the First People’s Hospital of Zhangjiagang. These patients were diagnosed through histological examinations, and none of them had undergone any anti-cancer therapies before surgery resection. The hospital Ethics Committee approved this study. All patients signed written informed consents in advance.
Tumor tissues and adjacent normal tissues were obtained from each of the osteosarcoma patients and immediately frozen in liquid nitrogen. Then, the samples were stored at −80°C for subsequent experiments. Clinicopathological characteristics, including sex, age, tumor size, anatomical location, clinical stage, distant metastasis, recurrence, and histotypes, were collected from medical records. Follow-up lasted 5 years. Individuals were excluded from this study if their deaths were not related to osteosarcoma.
Immunohistochemistry
MCT4 protein expression was detected via immunohistochemistry for osteosarcoma tissues and adjacent normal tissues from 100 osteosarcoma patients whose conditions conformed to the descriptions in the literature [21]. Briefly, tumor tissues and adjacent tissues were fixed in 10% formaldehyde and embedded in paraffin. Then, the sample pieces (4 mm) were dewaxed with xylene and rehydrated using a graded alcohol series. Sodium citrate buffer (pH6.0) was used to retrieve antigen in a pressure cooker for 5 min, and then washed with 3% hydrogen peroxide. Afterwards, samples were cultivated in 10% goat serum at 25°C for 30 min. Later, the sections were incubated in MCT4 antibodies (Santa Cruz Biotech; rabbit monoclonal; dilution 1: 50) at 4°C overnight. After that, the sections were incubated using horseradish peroxidase (HRP)-conjugated sheep anti-rabbit or anti-mouse secondary antibodies (GTVision; Shanghai, China) at 25°C for 30 min. Samples were stained with 3, 3-diaminobenzidine (DAB) and Mayer’s hematoxylin. A previously assessed positive sample (osteosarcoma slide) was used as a positive control. Tissue sections were assessed under a 200× microscope. We randomly selected 10 fields to count tumor cells. The percentage of positive MCT4 cells was also calculated. MCT4 staining classifications were as followings: negative, 0; weak, 1; and strong, 2. A 5-point scale was used to classify the percentage of MCT4 positive cells (0=0%, 1=1–25%, 2=26–50%, 3=51–75%, and 4=76–100%). Grades 0–2 indicated low MCT4 gene expression and 3–4 indicated high MCT4 expression.
Western blot assay
MCT4 protein in osteosarcoma tissues and adjacent normal ones from 25 osteosarcoma patients were detected using Western blot following the descriptions in the literature [22]. Total proteins were separated with 10% SDS-PAGE gels. MCT4 and β-actin (reference) proteins were transferred onto PVDF membranes (Bio-Rad, CA, USA). Then, the membranes were cultured with rabbit polyclonal anti-MCT4 antibody (Abcam, Cambridge, MA, USA) and rabbit polyclonal anti-β-actin antibody (CWBiotech, Beijing, China) overnight at 4°C. Proteins were colored via chemiluminescence reagents (Hyperfilm ECL; Amersham Biosciences, Buckinghamshire, UK) and the signals were analyzed using an ImageQuant™ LAS 4000 mini biomolecular imager (Fujifilm, Tokyo, Japan). MCT4 expression levels were adjusted to β-actin.
Statistical analysis
Statistical analyses were carried out with SPSS (SPSS 18.0 version for Windows) statistical software. The levels of MCT4 are expressed as mean ± standard deviation (SD), and their differences between osteosarcoma tissues and adjacent normal ones were estimated by the t test. Functional influences of MCT4 expression on osteosarcoma clinical features were assessed through the χ2 test. Kaplan-Meier analysis with log-rank test were used to analyze the overall survival of the patients based on their MCT4 expression levels. Cox regression analysis was performed to assess the prognostic significance of clinical variables and MCT4 expression in osteosarcoma. All tests were two-tailed, and P values less than 0.05 indicated the statistical significance of results.
Results
MCT4 protein expression in tumor tissues and adjacent tissues
In immunohistochemistry, MCT4 protein was stained brown in the cytoplasm and a few nuclei of osteosarcoma tissues (Figure 1). Out of 100 patients, 65 osteosarcoma tissues (65.00%) showed positive MCT4 protein expression, while 27 (27.00%) adjacent normal ones were positive (Table 1). In comparison with adjacent normal tissues, MCT4 protein expression was distinctly increased in osteosarcoma tissues (P<0.05). These results suggest that MCT4 protein functions as an oncogene in osteosarcoma progression.
Figure 1.
Representative images for immunohistochemistry staining, specific to MCT4 expression in osteosarcoma tissues and adjacent normal tissues. From 100 patients, positive MCT4 protein expression was detected in 65 (65.00%) osteosarcoma tissues and in 27 (27.00%) adjacent normal tissues. The rate of positive MCT4 expression was significantly higher in osteosarcoma tissues than in adjacent normal ones. (A) OS tissue; (B) Adjacent normal tissue.
Table 1.
The positive expression of MCT4 in osteosarcoma tissues and normal bone tissues.
| Tissue | Cases (n) | MCT4 expression | χ2 | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Osteosarcoma | 100 | 35 | 65 | 29.066 | 0.000 |
| Normal | 100 | 73 | 27 | ||
Expression of MCT4 protein detected through Western blot
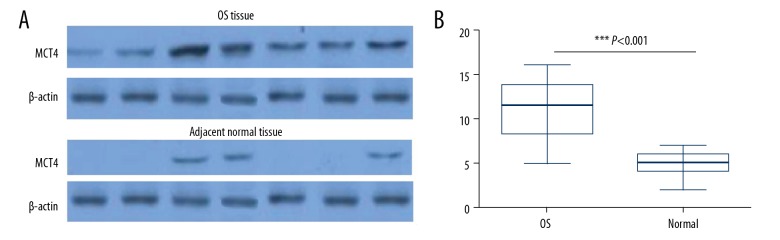
The other goal of the present study was to investigate whether MCT4 was detectable and to measure its differences between osteosarcoma tissue samples and adjacent normal tissues. Proteins were isolated from the frozen tissues, and Western blot analysis was used to detect the expression level of MCT4 protein. The results showed that relative MCT4 expression was significantly higher in tumor tissues (10.8500±0.75490) than in adjacent normal tissues (4.7500±0.31519) (P<0.05, Figure 2).
Figure 2.
The expression of MCT4 protein in osteosarcoma tissues and adjacent normal ones detected via Western blot. (A) The result of Western blot. (B) MCT4 protein was overexpressed in osteosarcoma tissues compared to adjacent normal ones (P<0.001).
Relationship between MCT4 expression and clinicopathologic factors of osteosarcoma patients
To assess the potential role of MCT4 in osteosarcoma development, we estimated the associations between MCT4 expression and clinicopathological features of osteosarcoma patients (Table 2). MCT4 expression was positively correlated with distant metastasis (P<0.001) and recurrence (P<0.001). The osteosarcoma patients with high MCT4 expression were more likely to undergo distant metastasis and recurrence. However, no obvious association was observed for MCT4 expression level with age, sex, tumor size, anatomical location, Enneking stage, or histotypes (P>0.05). Consequently, MCT4 appears to be closely correlated to osteosarcoma development.
Table 2.
Association between MCT4 expression and clinicopathological features of patients with osteosarcoma.
| Characteristics | Cases (n) | MCT4 expression | χ2 | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Sex | |||||
| Male | 54 | 20 | 34 | 0.214 | 0.644 |
| Female | 46 | 15 | 31 | ||
| Age | |||||
| ≤30 years | 60 | 20 | 40 | 0.183 | 0.669 |
| >30 years | 40 | 15 | 25 | ||
| Tumor size (cm) | |||||
| ≥5 cm | 57 | 23 | 34 | 1.668 | 0.196 |
| <5 cm | 43 | 12 | 31 | ||
| Anatomical location | |||||
| Tibia/femur | 55 | 20 | 35 | 0.100 | 0.752 |
| Elsewhere | 45 | 15 | 30 | ||
| Clinical stage (Enneking stage) | |||||
| I+II | 56 | 21 | 35 | 0.350 | 0.554 |
| III | 44 | 14 | 30 | ||
| Distant metastasis | |||||
| Yes | 52 | 2 | 50 | 46.217 | 0.000 |
| No | 48 | 33 | 15 | ||
| Recurrence | |||||
| Yes | 59 | 11 | 48 | 16.921 | 0.000 |
| No | 41 | 24 | 17 | ||
| Histotypes | |||||
| Osteogenic or chondrocytic | 62 | 18 | 44 | 2.554 | 0.110 |
| Fibrocytic or mixed | 38 | 17 | 21 | ||
Prognostic significance of MCT4 expression in osteosarcoma
Kaplan-Meier analysis and log-rank test were used to assess the influences of MCT4 expression on overall survival of patients with osteosarcoma (Figure 3). We found that the osteosarcoma patients with high expression of MCT4 had poorer survival than those with low expression (P<0.001), suggesting that MCT4 is associated with osteosarcoma prognosis. Subsequently, multivariate analysis results suggested that high MCT4 expression and advanced clinical stage (Enneking stage) can predict poor prognosis of osteosarcoma. MCT4 might be an independent prognostic indicator for osteosarcoma (HR=5.062, 95CI%=2.175–11.779, P=0.000), and a similar effect was also revealed for clinical stage (HR=2.395, 95CI%= 1.366–4.200, P=0.002, Table 3).
Figure 3.
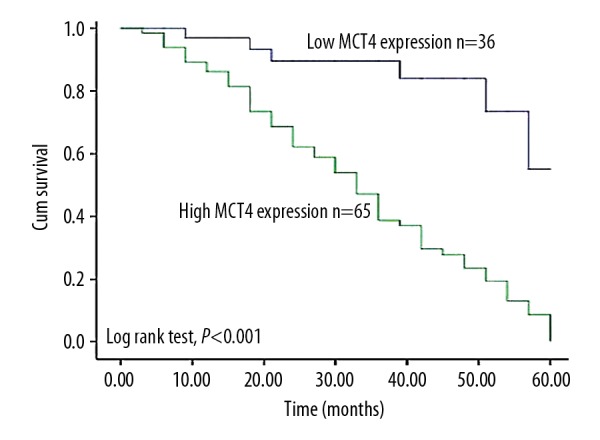
Kaplan-Meier analysis comparing the overall survival of the patients with different expressions of MCT4 in osteosarcoma tissues. Patients with high MCT4 expression had shorter overall survival than those with low expression (log-rank test, P<0.001).
Table 3.
Multivariate analysis adjusted for clinical factors for estimating the prognostic value of MCT4 in patients with osteosarcoma using Cox regression analysis.
| Variables | P Value | HR | 95%Cl |
|---|---|---|---|
| High MCT4 expression | 0.000 | 5.062 | 2.175–11.779 |
| Low MCT4 expression | – | – | – |
| Clinical stages (Enneking stage) | 0.002 | 2.395 | 1.366–4.200 |
Discussion
Although adjuvant chemotherapy has been dramatically improved, the prognosis of osteosarcoma patients is still not satisfactory, and over one-third of these patients eventually die of this disease [23]. Accumulating evidence demonstrates that osteosarcoma is a complex process regulated by the accumulating mutations in genes and signaling pathways. For instance, cryptochrome 2 (CRY2) can suppress the cell cycle, proliferation, and migration of osteosarcoma cells, which might be a potential target for cancer treatment [24]. Liu et al. reported that dysregulation of the ezrin/NF-κB signal pathway can activate epithelial-mesenchymal transition (EMT) in osteosarcoma, thus contributing to cancer progression and metastasis [25]. Designing the drugs or treatment methods specific to the genetic mutations in tumorigenesis may provide novel therapeutic methods for osteosarcoma [26]. Recently, a variety of molecular markers and therapeutic targets have been confirmed for osteosarcoma, including miRNAs, lncRNAs, and some specific genes. However, the molecular mechanisms of osteosarcoma remain unclear and there are no highly accurate bio-markers for the malignancy that are currently available.
MCT4 is a transmembrane protein involved in lactate metabolism. Growing evidence demonstrates its functional roles in tumorigenesis. For example, the MCT4 gene drives the proliferation ability of CRC cells [27]. Reduced MCT4 gene expression can decrease cell growth and increase apoptosis of urothelial carcinoma cells [28]. In esophageal squamous cell carcinoma, Cheng et al. found that the knockdown of MCT4 suppressed the proliferation of cancer cells and promoted apoptosis in vitro. The upregulation of MCT4 was reported to predict poor prognosis [29]. In the present study, we investigated the expression pattern of MCT4 protein in osteosarcoma, as well as its functional roles. Immunohistochemistry and Western blot analysis revealed significantly increased MCT4 expression in osteosarcoma tissue specimens. Next, to further analyze the possible function of MCT4 in the development of osteosarcoma, the relationship of MCT4 expression with clinical pathological variables was evaluated, showing that abnormal MCT4 expression was strongly associated with distant metastasis and recurrence, but not with sex, age, tumor size, or anatomical location. This suggests that MCT4 is involved in the onset and progression of osteosarcoma as an oncogene. It is generally accepted that MCT4 drives biological behaviors of cancer cells through glycolysis [30]. Whether MCT4 can control the fate of osteosarcoma cells through mediating glycolysis remains unclear and further studies are required to address this issue.
Given the functional roles of MCT4 in tumorigenesis, various studies have explored its prognostic significance in cancers. For example, Ohno et al. found that high MCT4 expression was an unfavorable prognostic biomarker for overall survival and disease-free survival in hepatocellular carcinoma [31]. MCT4 was found to be upregulated, and its positive expression was reported to be an adverse indicator for prognosis of colorectal cancer [32]. Doyen et al. found increased MCT4 expression predicts clinical outcome of triple-negative breast cancer [33], and its overexpression has also been detected in gastric cancer, acting as a prognostic marker [34]. In the present study, we investigated the prognostic value of MCT4 in osteosarcoma. Kaplan-Meier analysis confirmed that MCT4 level distinctly influenced the overall survival rate of osteosarcoma patients, and that patients with high MCT4 expression had shorter overall survival time than those with low expression. Finally, multivariate analysis with the adjustments for clinicopathological variables showed that MCT4 could be an independent prognostic factor for osteosarcoma.
Although we obtained these meaningful findings, several limitations in the present study should be noted. Firstly, the sample size was relatively small, which might have reduced the statistical power of our results. Secondly, the status of the MCT4 gene was not investigated, so the increase in its expression might be a result of many other factors, such as genetic or epigenetic factors. In addition, the molecular mechanisms of MCT4 in osteosarcoma etiology remains poorly defined. Therefore, our results should be verified and expanded by further research.
Conclusions
The expression of MCT4 is increased in osteosarcoma, and shows a close association with metastasis and recurrence. MCT4 may predict clinical outcomes of osteosarcoma, possibly being a prognostic biomarker for carcinoma.
Footnotes
Source of support: Departmental sources
References
- 1.Ham SJ, Schraffordt Koops H, van der Graaf WT, et al. Historical, current and future aspects of osteosarcoma treatment. Eur J Surg Oncol. 1998;24:584–600. doi: 10.1016/s0748-7983(98)93896-3. [DOI] [PubMed] [Google Scholar]
- 2.Papagelopoulos PJ, Galanis EC, Vlastou C, et al. Current concepts in the evaluation and treatment of osteosarcoma. Orthopedics. 2000;23:858–67. doi: 10.3928/0147-7447-20000801-11. ;quiz 568–69. [DOI] [PubMed] [Google Scholar]
- 3.Longhi A, Errani C, De Paolis M, et al. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat Rev. 2006;32:423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Yan S, Lu D, Dong F, Lian Y. Screening feature genes of osteosarcoma with DNA microarray: A bioinformatic analysis. Int J Clin Exp Med. 2015;8:7134–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchiya H, Tomita K, Mori Y, et al. Marginal excision for osteosarcoma with caffeine assisted chemotherapy. Clin Orthop Relat Res. 1999;358:27–35. [PubMed] [Google Scholar]
- 7.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamoureux F, Richard P, Wittrant Y, et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–18. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Wang Q, Wang GD, et al. miR-16 inhibits cell proliferation by targeting IGF1R and the Raf1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS Lett. 2013;587:1366–72. doi: 10.1016/j.febslet.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Dimmer KS, Friedrich B, Lang F, et al. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, et al. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle. 2012;11:1445–54. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinheiro C, Longatto-Filho A, Simoes K, et al. The prognostic value of CD147/EMMPRIN is associated with monocarboxylate transporter 1 co-expression in gastric cancer. Eur J Cancer. 2009;45:2418–24. doi: 10.1016/j.ejca.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, et al. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–39. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 14.Choi SYC, Ettinger SL, Lin D, et al. Targeting MCT4 to reduce lactic acid secretion and glycolysis for treatment of neuroendocrine prostate cancer. Cancer Med. 2018 doi: 10.1002/cam4.1587. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee ZW, Teo XY, Song ZJ, et al. Intracellular hyper-acidification potentiated by hydrogen sulfide mediates invasive and therapy resistant cancer cell death. Front Pharmacol. 2017;8:763. doi: 10.3389/fphar.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijer TW, Schuurbiers OC, Kaanders JH, et al. Differences in metabolism between adeno- and squamous cell non-small cell lung carcinomas: Spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer. 2012;76:316–23. doi: 10.1016/j.lungcan.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Lisanti MP, Sotgia F, Pestell RG, et al. Stromal glycolysis and MCT4 are hallmarks of DCIS progression to invasive breast cancer. Cell Cycle. 2013;12:2935–36. doi: 10.4161/cc.26280. [DOI] [PubMed] [Google Scholar]
- 18.Curry JM, Tuluc M, Whitaker-Menezes D, et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12:1371–84. doi: 10.4161/cc.24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotanda Y, Akagi Y, Kawahara A, et al. Expression of monocarboxylate transporter (MCT)-4 in colorectal cancer and its role: MCT4 contributes to the growth of colorectal cancer with vascular endothelial growth factor. Anticancer Res. 2013;33:2941–47. [PubMed] [Google Scholar]
- 20.Zhu J, Wu YN, Zhang W, et al. Monocarboxylate transporter 4 facilitates cell proliferation and migration and is associated with poor prognosis in oral squamous cell carcinoma patients. PLoS One. 2014;9:e87904. doi: 10.1371/journal.pone.0087904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Yang J, Fan T, et al. RhoE functions as a tumor suppressor in esophageal squamous cell carcinoma and modulates the PTEN/PI3K/Akt signaling pathway. Tumour Biol. 2012;33:1363–74. doi: 10.1007/s13277-012-0384-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Zhou W, Zhang F, et al. Knockdown of IRX2 inhibits osteosarcoma cell proliferation and invasion by the AKT/MMP9 signaling pathway. Mol Med Rep. 2014;10:169–74. doi: 10.3892/mmr.2014.2215. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013;25:398–406. doi: 10.1097/CCO.0b013e3283622c1b. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Li Y, Zhou L, et al. Cryptochrome 2 (CRY2) suppresses proliferation and migration and regulates clock gene network in osteosarcoma cells. Med Sci Monit. 2018;24:3856–62. doi: 10.12659/MSM.908596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Yang P, Zhang Z, et al. Ezrin/NF-kappaB pathway regulates EGF-induced epithelial-mesenchymal transition (EMT), metastasis, and progression of osteosarcoma. Med Sci Monit. 2018;24:2098–108. doi: 10.12659/MSM.906945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S, Zhu J, Xia K, et al. Cantharidin INHIBITS ANTI-APOPTOTIC Bcl-2 family proteins and induces apoptosis in human osteosarcoma cell lines MG-63 and MNNG/HOS via mitochondria-dependent pathway. Med Sci Monit. 2018;24:6742–49. doi: 10.12659/MSM.910294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HK, Lee I, Bang H, et al. MCT4 expression is a potential therapeutic target in colorectal cancer with peritoneal carcinomatosis. Mol Cancer Ther. 2018;17:838–48. doi: 10.1158/1535-7163.MCT-17-0535. [DOI] [PubMed] [Google Scholar]
- 28.Todenhofer T, Seiler R, Stewart C, et al. Selective inhibition of the lactate transporter MCT4 reduces growth of invasive bladder cancer. Mol Cancer Ther. 2018;17(12):2746–55. doi: 10.1158/1535-7163.MCT-18-0107. [DOI] [PubMed] [Google Scholar]
- 29.Cheng B, Chen X, Li Y, et al. Prognostic value of monocarboxylate transporter 4 in patients with esophageal squamous cell carcinoma. Oncol Rep. 2018;40:2906–15. doi: 10.3892/or.2018.6706. [DOI] [PubMed] [Google Scholar]
- 30.Bisetto S, Whitaker-Menezes D, Wilski NA, et al. Monocarboxylate transporter 4 (MCT4) knockout mice have attenuated 4NQO induced carcinogenesis; A role for MCT4 in driving oral squamous cell cancer. Front Oncol. 2018;8:324. doi: 10.3389/fonc.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohno A, Yorita K, Haruyama Y, et al. Aberrant expression of monocarboxylate transporter 4 in tumour cells predicts an unfavourable outcome in patients with hepatocellular carcinoma. Liver Int. 2014;34:942–52. doi: 10.1111/liv.12466. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama Y, Torigoe T, Inoue Y, et al. Prognostic significance of monocarboxylate transporter 4 expression in patients with colorectal cancer. Exp Ther Med. 2012;3:25–30. doi: 10.3892/etm.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyen J, Trastour C, Ettore F, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun. 2014;451:54–61. doi: 10.1016/j.bbrc.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Yan P, Li YH, Tang ZJ, et al. High monocarboxylate transporter 4 protein expression in stromal cells predicts adverse survival in gastric cancer. Asian Pac J Cancer Prev. 2014;15:8923–29. doi: 10.7314/apjcp.2014.15.20.8923. [DOI] [PubMed] [Google Scholar]