Abstract
Background
A retrospective study aimed to investigate the association between the CRP to albumin ratio and prognosis in patients with resectable non-metastatic breast cancer in terms of disease-free survival (DFS) and overall survival (OS) using propensity score matching.
Material/Methods
Patients with newly diagnosed resectable non-metastatic breast cancer (n=200) who underwent modified radical mastectomy between January 2008 to June 2013 included a group with an increased CRP to albumin ratio ≥0.029 (n=80) and a group with reduced CRP to albumin ratio <0.029 (n=120). Propensity score matching was used to estimate the prognostic role of the CRP to albumin ratio, and a 1: 1 matching using four covariates was performed to overcome selection bias. The prognostic significance of the CRP to albumin ratio was analyzed using receiver operating characteristic (ROC) curves. Kaplan-Meier survival analysis and a Cox proportional hazards model were conducted to identify the impact on DFS and OS.
Results
An increased CRP to albumin ratio was associated with increased age, post-menopausal status, and a high risk of recurrence or death in patients with breast cancer. An increased preoperative CRP to albumin ratio was significantly associated with reduced disease-free survival (DFS) and overall survival (OS) (all P<0.05). Multivariate analysis showed that an increased CRP to albumin ratio was an independent risk factor for long-term outcome and predicted reduced DFS (HR, 2.225; P=0.024) and OS (HR, 9.189; P=0.003).
Conclusions
Preoperative evaluation of the CRP to albumin ratio was an independent prognostic indicator in patients with resectable breast cancer.
MeSH Keywords: Breast Neoplasms, C-Reactive Protein, Disease-Free Survival, Propensity Score, Serum Albumin, Survival
Background
Worldwide, breast cancer remains the most primary malignancy in women [1]. Although surgery and adjuvant chemoradiotherapy can result in clinical benefit for patients, a proportion of patients develop relapse and metastases, resulting in the high rate of mortality from breast cancer [2]. Current methods used to evaluate patient prognosis in breast cancer include hormone receptor status, determination of the tumor node metastasis (TNM) stage, detection of circulating tumor cells, and gene profiling [3,4]. However, the identification of these prognostic factors can take time and can be costly. Therefore, the identification of reliable, economical, and practical prognostic markers remains a challenge for the prediction of individual patient survival that can improve patient management.
Recent studies have shown that inflammation and nutritional status are significantly associated with carcinogenesis and tumor progression [5]. Several inflammatory or nutritional indicators, including the neutrophil to lymphocyte ratio, the platelet to lymphocyte ratio, the prognostic nutritional index, and the Glasgow prognostic score, have been reported to be associated with poor prognosis in patients with several types of malignancy, including breast cancer [6,7]. Recently, the C-reactive protein (CRP) to albumin ratio, a novel inflammation and nutrition based index, has been reported to be an independent prognostic indicator in several cancers, including gastric cancer [8], ovarian cancer [9] and cervical cancer [10]. However, the prognostic role of the CRP to albumin ratio in patients with breast cancer remains unknown.
Propensity score matching is a statistical method that can be used to estimate the prognostic role of a diagnostic test by accounting for the covariates that predict patient prognosis. Therefore, this retrospective study aimed to investigate the association between the CRP to albumin ratio and prognosis in patients with breast cancer in terms of disease-free survival (DFS) and overall survival (OS) using propensity score matching.
Material and Methods
Study design and patients
This study was approved by the Ethics Committee of Zhongshan Hospital, Sun Yat-sen University. Signed informed consent was provided by each study participant. This retrospective study included 592 newly diagnosed female breast cancer patients without metastasis who underwent modified radical mastectomy between January 2008 and June 2013 at our institution in a maintained electronic database. A total of 200 patients, 80 with an increased CRP to albumin ratio, and 120 with reduced CRP to albumin ratio met the study criteria and were enrolled in the study.
Patients who were included in the study had a histopathologically confirmed diagnosis of primary breast cancer and underwent detailed preoperative evaluation, including physical examination, hematologic examination, color Doppler ultrasound, mammography, magnetic resonance imaging (MRI), and core needle breast biopsy. Exclusion criteria included patients with non-resectable or metastatic breast cancer, acute or chronic infection, immune diseases, a history of other primary malignancy, an initial diagnosis during pregnancy or lactation, patients who received neoadjuvant chemoradiotherapy, and patients with incomplete clinical records.
Clinical data
All blood specimens were collected and examined before patients received any treatment. Patient demographic and clinicopathological data collected included age, menstrual status, tumor volume, the presence of lymph node metastasis, histological grade (using the Nottingham criteria), the TNM stage, according to the criteria of the American Joint Committee on Cancer (AJCC) (7th edition), the estrogen receptor (ER), progesterone receptor (PR), and HER2 status, and the treatment protocol. Pretreatment serum CRP and albumin levels were analyzed. The CRP to albumin ratio was determined by dividing the CRP level by the albumin level. The neutrophil to lymphocyte ratio was obtained by dividing the neutrophil count by the lymphocyte count. The platelet to lymphocyte ratio was obtained by dividing the platelet count by the lymphocyte count. In the Glasgow prognostic score, patients with an increased CRP (>10 mg/L) and hypoalbuminemia (<35 g/L) were allocated a score of 2, and patients with one or neither of these abnormalities were allocated a score of 1 or 0 [6].
Treatment
All patients received standard treatment regimens with modified radical mastectomy, supplemented by radiotherapy, chemotherapy, endocrine therapy, and targeted therapy, if applicable, and with clinical follow-up according to the National Comprehensive Cancer Network (NCCN) clinical guidelines. Overall survival (OS) was defined as the period from initial pathological diagnosis to death (from any cause) or to the last clinical follow-up. Disease-free survival (DFS) was defined the period from initial diagnosis and relapse, death or new primary cancer. All study participants underwent three-monthly follow-up for the first three years, and then once a year until recurrence or death. The last patient follow-up date was December 2018. The survival status and clinicopathological parameters were accessed from the medical records and follow-up telephone interviews.
Propensity score matching analysis
Statistical analysis using propensity score matching was used to estimate the prognostic role of the CRP to albumin ratio by accounting for the covariates that predicted patient prognosis. To minimize selection bias of the retrospective study, a 1: 1 match was performed according to four related covariates, including age, menopausal status, tumor size, and tumor stage to generate propensity scores, and calculated via logistic regression model. Then nearest neighbor matching algorithm with a match tolerance of 0.2 was performed. Standardized differences between both groups no more than 20% were accepted. Finally, a total of 80 patients with a CRP to albumin ratio <0.029 and 120 patients with a CRP to albumin ratio ≥0.029 were included in the 80 matched pairs. The risk factors for patient prognosis were investigated between the two matched cohorts.
Statistical analysis
The categorical variables were presented as frequencies and percentages (%). The differences between the clinicopathological characteristics were analyzed using Pearson’s chi-squared (χ2) test or Fisher’s exact test. The optimal cut-off level was determined according to receiver operating characteristic (ROC) curve analysis based on Youden’s index. The survival status was calculated by Kaplan-Meier estimates and the log-rank test. Factors associated with survival outcome were evaluated using Cox univariate and multivariate proportional hazard regression analysis. P-values were two-tailed. Statistical significance was assumed to be P<0.05. All data management and statistical analysis were performed using SPSS version 23.0 software (SPSS, Chicago, IL, USA).
Results
Patient demographic and clinicopathological characteristics
As shown in Table 1, a total of 200 patients with non-metastatic breast cancer were included and evaluated. The median age was 51 years (range, 27–77 years), and 95 (47.5%) patients were postmenopausal. There were 58 (29.0%) patients with TNM stage I, 107 (53.5%) patients with TNM stage II, and 35 (17.5%) patients with TNM stage III breast cancer.
Table 1.
Clinicopathological characteristic of breast cancer patients associated with a low and high C-reactive protein (CRP) to albumin ratio (CAR).
| Characteristic | Before propensity score matching (n=200) | Before propensity score matching (n=160) | ||||
|---|---|---|---|---|---|---|
| CAR <0.029 (n=120) | CAR ≥0.029 (n=80) | P-value | CAR <0.029 | CAR ≥0.029 | P-value | |
| (n=80) | (n=80) | |||||
| Age group (yrs) | 0.033 | 0.154 | ||||
| ≤50 | 68 | 33 | 42 | 33 | ||
| >50 | 52 | 47 | 38 | 47 | ||
| Menopausal status | 0.009 | 0.113 | ||||
| Premenopausal | 72 | 33 | 43 | 33 | ||
| Postmenopausal | 48 | 47 | 37 | 47 | ||
| BMI (kg/m2)* | 20.84±2.61 | 20.42±2.42 | 0.261 | 21.29±2.07 | 20.41±2.42 | 0.016 |
| Tumor classification | 0.346 | 0.331 | ||||
| T1 | 51 | 26 | 35 | 26 | ||
| T2 | 66 | 51 | 43 | 51 | ||
| T3 | 3 | 3 | 2 | 3 | ||
| LN metastasis | 0.078 | 0.049 | ||||
| N0 | 80 | 39 | 54 | 39 | ||
| N1 | 20 | 19 | 12 | 19 | ||
| N2 | 16 | 16 | 13 | 16 | ||
| N3 | 4 | 6 | 1 | 6 | ||
| Vascular and neural invasion | 0.827 | 0.440 | ||||
| No | 96 | 65 | 61 | 65 | ||
| Yes | 24 | 15 | 19 | 15 | ||
| TNM staging | 0.142 | 0.342 | ||||
| I | 40 | 18 | 26 | 18 | ||
| II | 63 | 44 | 40 | 44 | ||
| III | 17 | 18 | 14 | 18 | ||
| Histological grade | 0.194 | 0.539 | ||||
| G1 | 26 | 12 | 14 | 12 | ||
| G2 | 81 | 53 | 56 | 53 | ||
| G3 | 13 | 15 | 10 | 15 | ||
| Histological type | 0.456 | 0.263 | ||||
| IDC | 109 | 75 | 71 | 75 | ||
| Others | 11 | 5 | 9 | 5 | ||
| ER status | 0.597 | 0.521 | ||||
| Positive | 69 | 49 | 45 | 49 | ||
| Negative | 51 | 31 | 35 | 31 | ||
| PR status | 0.817 | 0.635 | ||||
| Positive | 65 | 38 | 39 | 42 | ||
| Negative | 55 | 38 | 41 | 38 | ||
| HER2 status | 0.238 | 0.056 | ||||
| Positive | 34 | 29 | 18 | 29 | ||
| Negative | 86 | 51 | 62 | 51 | ||
| Ki67 | 0.096 | 0.440 | ||||
| Positive | 85 | 65 | 61 | 65 | ||
| Negative | 35 | 15 | 19 | 15 | ||
| Endocrinotherapy | 0.728 | 0.343 | ||||
| Yes | 63 | 44 | 38 | 44 | ||
| No | 57 | 36 | 42 | 36 | ||
| Targeted therapy | 0.431 | 0.292 | ||||
| Yes | 13 | 6 | 10 | 6 | ||
| No | 107 | 74 | 70 | 74 | ||
| Chemotherapy | 0.640 | 0.772 | ||||
| Yes | 113 | 74 | 73 | 74 | ||
| No | 7 | 6 | 7 | 6 | ||
| Radiotherapy | 0.105 | 0.063 | ||||
| Yes | 24 | 24 | 14 | 24 | ||
| No | 96 | 56 | 66 | 56 | ||
| Recurrence/metastasis | 0.002 | 0.007 | ||||
| Yes | 19 | 28 | 13 | 28 | ||
| No | 101 | 52 | 67 | 52 | ||
| Metastasis sites | <0.001 | 0.002 | ||||
| Liver | 0 | 7 | 0 | 7 | ||
| Brain | 1 | 0 | 1 | 0 | ||
| Lung | 3 | 6 | 2 | 6 | ||
| Bone | 8 | 1 | 5 | 1 | ||
| Others | 7 | 14 | 5 | 14 | ||
| Death | <0.001 | <0.001 | ||||
| Yes | 4 | 17 | 2 | 17 | ||
| No | 116 | 63 | 78 | 63 | ||
| NLR* | 2.48±1.14 | 2.47±1.36 | 0.983 | 2.53±1.17 | 2.47±1.36 | 0.782 |
| PLR* | 159.57±57.43 | 152.76+66.62 | 0.442 | 161.22±64.67 | 152.76±66.62 | 0.416 |
| GPS* | 0.013 | 0.085 | ||||
| 0 | 118 | 71 | 78 | 71 | ||
| 1 | 2 | 8 | 2 | 8 | ||
| 2 | 0 | 1 | 0 | 1 | ||
CAR – C-reactive protein to albumin ratio; LN – lymph node; TNM – tumor-node-metastasis; IDC – infiltrative ductal carcinoma; ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor 2.
Values are shown as the means ± standard deviations.
Bold values indicate statistical significance; GPS – Glasgow prognostic score; PLR – platelet to lymphocyte ratio; NLR – neutrophil to lymphocyte ratio.
The histopathological subtypes included 184 (92.0%) patients with invasive ductal carcinoma (IDC), and 16 patients with other subtypes. The histological grades included G1 in 38 cases (19.0%), G2 in 134 cases (67.0%), and G3 in 28 cases (14.0%). Breast cancer tumor hormonal status and molecular markers included 118 (59.0%) that were estrogen-receptor (ER)-positive, 103 (51.5%) that were progesterone receptor (PR)-positive, 63 (31.5%) that were HER-2 positive, and 150 (75.0%) that were Ki-67-positive. In 39 (19.5%) cases the tumors showed vascular and neural invasion.
Postoperatively, there were 107 (53.5%) patients who underwent endocrine therapy, 19 (9.5%) patients underwent targeted therapy, 187 (93.5%) patients received chemotherapy, and 48 (24.0%) patients received radiotherapy.
Cut-off values for the C-reactive protein (CRP) to albumin ratio
According to receiver operating characteristic (ROC) curve analysis, the optimal cut-off value for the C-reactive protein (CRP) to albumin ratio for overall survival (OS) was 0.029 with a sensitivity of 81.0% and a specificity of 65.9%. The area under the curve (AUC) was 0.790 (95% CI, 0.685–0.894) (P<0.001) (Figure 1). Also, the CRP to albumin ratio had significantly higher AUC values compared with the neutrophil to lymphocyte ratio of 0.545 (95% CI, 0.432–0.658) (P=0.502), the platelet to lymphocyte ratio of 0.463 (95% CI, 0.308–0.617) (P=0.576), and the Glasgow prognostic score of 0.577 (95% CI, 0.435–0.718) (P=0.251), respectively.
Figure 1.
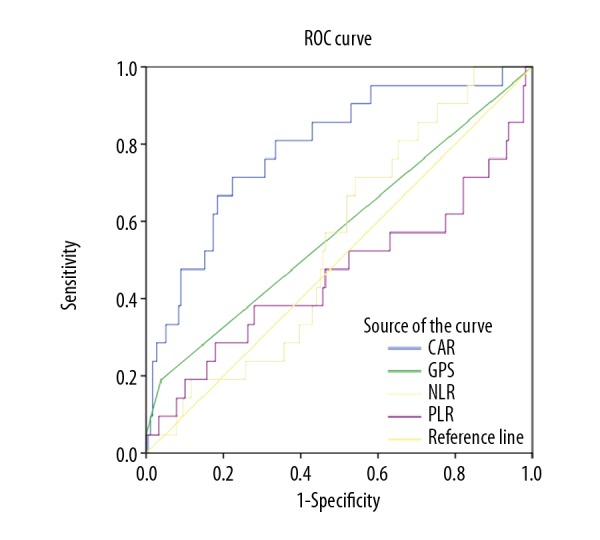
Receiver operating characteristic (ROC) curve analysis of the C-reactive protein (CRP) to albumin ratio for survival status of breast cancer patients. ROC – receiver operating characteristic; CI – confidence interval; CAR – C-reactive protein/albumin ratio; PLR – platelet to lymphocyte ratio; NLR – neutrophil to lymphocyte ratio; GPS – Glasgow prognostic ccore.
High and low CRP to albumin ratio and prognosis
The total of 200 patients with breast cancer were classified into the high CRP to albumin ratio subgroup ≥0.029 (n=80; 40.0%) and the low CRP to albumin ratio subgroup <0.029 (n=120; 60.0%). The associations between the CRP to albumin ratio and clinicopathological characteristics are shown in Table 1. Compared with the low CRP to albumin ratio group, patients were significantly older in high CRP to albumin ratio group, and there were more patients with postmenopausal status. There were no significant differences for tumor characteristics and method of treatment between the two groups. A 1: 1 propensity score matching analysis based on four related covariates was performed to balance the differences and reduce selection bias between two groups. In the propensity score matching model, the CRP to albumin ratio was found to be significantly correlated with body mass index (BMI) (P=0.0161) and the presence of lymph node metastases (P=0.049). There were no significant differences between the groups for age, menopausal status, therapy method, and other tumor characteristics. An increased CRP to albumin ratio level was significantly associated with cancer recurrence and mortality before and after propensity score matching.
Survival analysis
At a median postoperative follow-up of 60 months (range, 10–120 months) 47 (23.5%) patients experienced relapse, and 179 (89.5%) patients were alive until the last follow-up. Six patients were lost follow-up. In present breast cancer patient cohorts, the 3-year, 5-year and 10-year DFS and OS rates were 88.3%, 77.2%, 62.8% and 95.7%, 88.4%, 79.7%, respectively.
After propensity matching, patients with a higher CRP to albumin ratio had a 5-year OS rate of 73.4% (median OS, 96.0 months; range, 86.2–105.8 months) and a DFS rate of 59.8% (median DFS, 81.6 months; range, 70.8–92.4 months). For patients with a lower CRP to albumin ratio, the 5-year OS and DFS rate were 97.1% (median OS, 109.8 months; range, 106.7–112.8 months) and 79.2% (median DFS, 94.7 months; range, 86.6–103.0 months), respectively. Patients with a lower preoperative CRP to albumin ratio had a favorable prognosis for both OS and DFS (Figures 2, 3).
Figure 2.
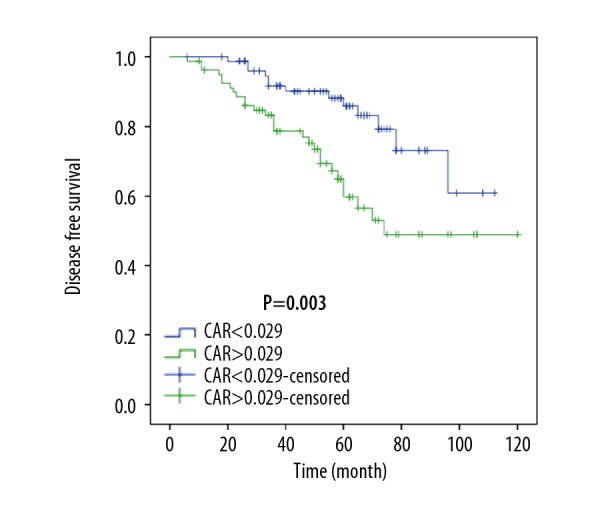
Disease-free survival (DFS) after propensity score matching. CAR – C-reactive protein/albumin ratio.
Figure 3.
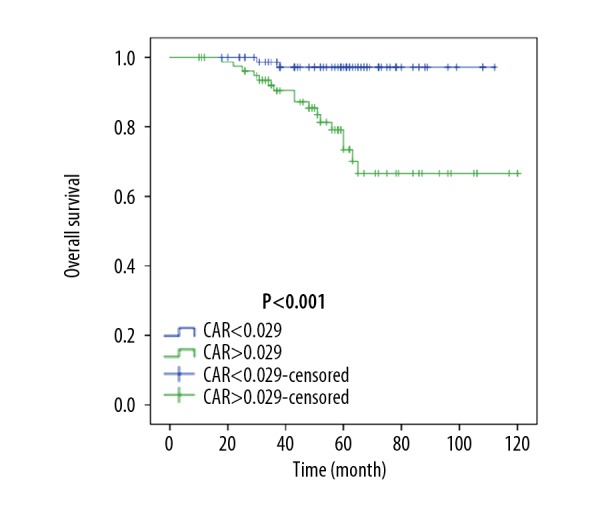
Overall survival (OS) after propensity score matching. CAR – C-reactive protein/albumin ratio.
Univariate and multivariate analysis for DFS and OS
Univariate analysis showed that tumor size, T3 (HR, 5.023; 95% CI, 1.113–22.673; P=0.036), the presence of lymph node metastasis, N1(HR, 2.329; 95% CI, 1.033–5.250; P=0.042), N2 (HR, 3.376; 95% CI, 1.568–7.189; P=0.002), N3 (HR, 10.258; 95% CI, 3.301–31.876; P<0.001), the requirement for postoperative adjuvant radiotherapy (HR, 3.738; 95% CI, 2.000–6.988; P<0.001), and higher CRP to albumin ratio (HR, 2.592; 95% CI, 1.340–5.013; P=0.005) were significantly associated with DFS (Table 2). The presence of lymph node metastases, N3 (HR, 14.275; 95% CI, 4.236–48.103; P<0.001), and a high CRP to albumin ratio (HR, 10.200; 95% CI, 2.353–44.229; P=0.002) were significantly associated with reduced OS (Table 2).
Table 2.
Univariate Cox regression analyses of risk factors associated with disease-free survival (DFS) and overall survival (OS) after propensity score matching.
| Characteristic | DFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (years) | ||||||
| ≤50 | Ref | Ref | ||||
| >50 | 0.728 | (0.394–1.345) | 0.311 | 0.958 | (0.389–2.358) | 0.925 |
| Menopausal status | ||||||
| Premenopausal | Ref | Ref | ||||
| Postmenopausal | 0.687 | (0.372–1.272) | 0.232 | 0.918 | (0.373–2.263) | 0.853 |
| Tumor classification | ||||||
| T1 | Ref | Ref | ||||
| T2 | 1.392 | (0.715–1.392) | 0.331 | 1.093 | (0.423–2.822)) | 0.854 |
| T3 | 5.023 | (1.113–22.673) | 0.036 | 3.632 | (0.438–30.115) | 0.232 |
| LN metastasis | ||||||
| N0 | Ref | Ref | ||||
| N1 | 2.329 | (1.033–5.250) | 0.042 | 0.724 | (0.156–3.358) | 0.680 |
| N2 | 3.376 | (1.586–7.189) | 0.002 | 1.547 | (0.476–5.026) | 0.468 |
| N3 | 10.258 | (3.301–31.876) | <0.001 | 14.275 | (4.236–48.103) | <0.001 |
| Vascular and neural invasion | ||||||
| Yes | 1.314 | (0.625–2.762) | 0.472 | 1.173 | (0.388–3.544) | 0.777 |
| No | Ref | Ref | ||||
| Histological grade | ||||||
| G1 | ref | Ref | ||||
| G2 | 1.010 | (0.442–2.309) | 0.980 | 0.795 | (0.259–2.444) | 0.689 |
| G3 | 0.871 | (0.275–2.758) | 0.814 | 0.596 | (0.109–3.264) | 0.551 |
| Histological type | ||||||
| IDC | 2.354 | (0.566–9.792) | 0.239 | 23.393 | (0.026–2.385) | 0.365 |
| Others | Ref | Ref | ||||
| ER status | ||||||
| Positive | 1.384 | (0.729–2.627) | 0.320 | 1.327 | (0.522–3.376) | 0.553 |
| Negative | Ref | Ref | ||||
| PR status | ||||||
| Positive | 1.276 | (0.685–2.376) | 0.443 | 1.326 | (0.533–3.297) | 0.544 |
| Negative | Ref | Ref | ||||
| HER2 status | ||||||
| Positive | 1.345 | (0.695–2.603) | 0.378 | 0.991 | (0.357–2.757) | 0.987 |
| Negative | Ref | Ref | ||||
| Ki67 | ||||||
| Positive | 1.258 | (0.580–2.726) | 0.561 | 1.511 | (0.440–5.188) | 0.512 |
| Negative | Ref | Ref | ||||
| Endocrinotherapy | ||||||
| Yes | 1.681 | (0.895–3.158) | 0.106 | 1.764 | (0.693–4.487) | 0.234 |
| No | Ref | Ref | ||||
| Targeted therapy | ||||||
| Yes | 0.660 | (0.204–2.140) | 0.489 | 0.429 | (0.057–3.214) | 0.410 |
| No | Ref | Ref | ||||
| Chemotherapy | ||||||
| Yes | 0.974 | (0.301–3.159) | 0.966 | 0.442 | (0.129–1.520) | 0.195 |
| No | Ref | Ref | ||||
| Radiotherapy | ||||||
| Yes | 3.738 | (2.000–6.988) | <0.001 | 1.096 | (0.362–3.316) | 0.871 |
| No | Ref | Ref | ||||
| CAR | ||||||
| <0.029 | Ref | Ref | ||||
| ≥0.029 | 2.592 | (1.340–5.013) | 0.005 | 10.200 | (2.353–44.229) | 0.002 |
CAR – C-reactive protein to albumin ratio; OS – overall survival; DFS – disease-free survival; HR – hazard ratio; CI – confidence interval; LN – lymph node; ER – estrogen receptor; PR – progesterone receptor; HER2 – human epidermal growth factor receptor 2; Ref – reference. Bold values indicate statistical significance.
As shown in Table 3, multivariate analysis included the variables that were found to be significant in univariate analysis. The results showed that a higher CRP to albumin ratio (HR, 2.225; 95% CI, 1.113–4.451; P=0.031) was an independent prognostic predictor of DFS. Lymph node metastases, N3 (HR, 7.974; 95% CI, 2.355–26.998; P=0.001), and a higher CRP to albumin ratio (HR, 9.189; 95% CI, 2.079–40.621; P=0.003) were independently associated with reduced OS.
Table 3.
Multivariate Cox regression analysis of risk factors related to disease-free survival (DFS) and overall survival (OS) after propensity score matching.
| Characteristic | DFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Tumor classification | ||||||
| T1 | Ref | |||||
| T2 | 1.016 | (0.506–2.042) | 0.964 | |||
| T3 | 2.789 | (0.508–15.311) | 0.238 | |||
| LN metastasis | ||||||
| N0 | Ref | Ref | ||||
| N1 | 1.514 | (0.614–3.730) | 0.368 | 0.493 | (0.106–2.296) | 0.367 |
| N2 | 2.038 | (0.78–5.317) | 0.145 | 1.254 | (0.385–4.086) | 0.707 |
| N3 | 3.678 | (0.894–15.127) | 0.071 | 7.974 | (2.355–26.998) | 0.001 |
| Radiotherapy | ||||||
| Yes | 1.958 | (0.870–4.409) | 0.105 | |||
| No | Ref | |||||
| CAR | ||||||
| <0.029 | Ref | Ref | ||||
| ≥0.029 | 2.225 | (1.113–4.451) | 0.024 | 9.189 | (2.079–40.621) | 0.003 |
CAR – C-reactive protein to albumin ratio; OS – overall survival; DFS – disease-free survival; HR – hazard ratio; CI – confidence interval; LN – lymph node; ref – reference. Bold values indicate statistical significance.
Discussion
This aim of this retrospective study was to investigate the association with the C-reactive protein (CRP) to albumin ratio and prognosis in patients with breast cancer in terms of disease-free survival (DFS) and overall survival (OS) using propensity score matching. Propensity score matching was used to estimate the prognostic role of the CRP to albumin ratio by accounting for the covariates that predict patient prognosis. The findings showed that an increased preoperative CRP to albumin ratio was an independent prognostic biomarker associated with reduced OS and DFS in patients with resectable non-metastatic breast cancer. To our knowledge, this study was the first to investigate the clinical value of the CRP to albumin ratio and patient prognosis in breast cancer.
Previously published studies have shown that systemic inflammation and nutritional status have a role in the prognosis of cancer patients. Among the tumor and host interactions, tumor growth and progression are associated with tissue damage and necrosis, activation of the immune response and the production of pro-inflammatory cytokines and chemokines including interleukin (IL)-1, IL-6, vascular endothelial growth factor (VEGF), and tumor necrosis factor (TNF) [11]. Tumor angiogenesis, cell proliferation, invasion, metastasis, and drug resistance are associated with increased circulating levels of inflammatory cytokines. Currently, some inflammation-associated parameters, including the neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and Glasgow prognostic score, which can be assessed by routine blood testing, have been reported to have prognostic significance in malignancy, including breast cancer [12–14].
CRP as an acute phase protein that is synthesized by hepatocytes and is regulated by inflammatory cytokines [9]. Increased serum levels of CRP concentrations have been reported to be associated with the activation of the inflammatory response in malignancy [15,16]. Several epidemiological studies have shown that increased CRP levels are associated with increased risk of breast cancer [17]. In breast cancer, circulating CRP can accelerate angiogenesis by increasing the levels of vascular growth factors and interleukins, promoting tumor cell invasion and metastasis by binding to integrins in the inflammatory microenvironment [18,19]. Increased serum levels of CRP detected before treatment may indicate the degree of tumor aggressiveness and may be associated with treatment resistance and adverse outcomes in patients with breast cancer [20–22].
The CRP to albumin ratio has previously been reported to be a prognostic biomarker for cardiotoxicity during anticancer therapy [23]. Increased CRP levels are usually associated with the reduction in levels of serum albumin due to the suppression of its rate of synthesis in the liver. Serum albumin levels are a commonly used indicator of nutritional status and are also associated with immune status. Malnutrition and hypoalbuminemia are commonly found in patients with cancer, including patients with breast cancer. The pathogenesis of hypoalbuminemia might be due to sustained cancer-associated systemic inflammation and the activation of inflammatory cytokines [24]. Nutritional deficiency suppresses immune defense mechanisms, compromise the response to treatment, and contribute to unfavorable outcomes in patients with cancer [24]. Also, several previously published studies have shown that low preoperative serum albumin concentrations are an independent indicator of poor prognosis in patients with malignancy, including breast cancer [9,25,26]. Improvement of nutritional status has a positive impact on quality of life, tolerance to antineoplastic treatment, and survival of patients with breast cancer [27].
The CRP to albumin ratio is a more comprehensive form of serum marker that reflects both the inflammatory and nutritional status of the patient and has previously been identified as a novel promising prognosis marker in patients with serious infectious disease and several types of cancer [7–10,28]. The findings from a recent meta-analysis assessed the relationship between the CRP to albumin ratio and prognosis in cancer and showed that a high CRP to albumin ratio before treatment was associated with an increased risk of relapse and mortality in patients with nasopharyngeal cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, lung cancer, oral cancer, and renal cancer [29]. Also, compared with other inflammation-based prognosis scoring systems, the CRP to albumin ratio showed more effective prognostic value and a more accurate discriminatory ability [30].
However, until the present study, there have been no previous studies on the prognostic value of measuring the CRP to albumin ratio in patients with breast cancer. In the patient cohort investigated in the present study, an increased CRP to albumin ratio was associated with increased patient age, post-menopausal status, and a high risk of cancer recurrence, or death. There was no significant difference between the CRP to albumin ratio and other tumor features, possibly due to the small study sample size. To minimize potential confounding bias, propensity score matching was used to create 61 matched patients with comparable baseline characteristics. The results confirmed that breast cancer patients with an increased CRP to albumin ratio had reduced DFS and OS before and after propensity score matching. Multivariate analysis showed that the preoperative serum CRP to albumin ratio was shown to be an independent predictive marker for the long-term patient outcome (p<0.001), which was in accordance with the results for other types of tumors [8–10]. Patients with breast cancer patients and a CRP to albumin ratio of ≥0.029 had a 2.6-fold increased risk of recurrence and 10.2-fold increased mortality risk compared with patients with a CRP to albumin ratio <0.029. Multivariate analysis also showed that lymph node metastasis status was an independent prognostic indicator of OS.
The results of the present study showed that patients with operable, non-metastatic breast cancer with an increased preoperative CRP to albumin ratio were more likely to have a reduced prognosis and should undergo more frequent follow-up, possibly with supportive anti-inflammatory and nutritional therapy. Compared with traditional prognostic indicators, the CRP to albumin ratio is a simple, economical, and objective biomarker that is routinely available from a routine laboratory blood test at the time before surgical treatment and throughout the treatment process.
This study had several limitations. This was a retrospective cohort study, and selection bias might exist even with the use of propensity score matching to minimize this. The study included a relatively small sample size and short duration of follow-up. Given that many factors may be involved in systemic inflammation and in nutritional status, further comparative studies are necessary to determine the best predictor of outcome among breast cancer patients. The cut-off values for the CRP to albumin ratio remain to be defined as they were relatively lower in this study compared with previous studies in patients with other types of malignancy. The possible reason for this finding might be that the majority of patients enrolled in the present study had early-stage breast cancer. Future prospective, large-scale, multicenter, controlled studies are required to further evaluate the prognostic role of the CRP to albumin ratio to identify high-risk groups in patients with breast cancer that may allow clinicians to choose the best possible adjuvant therapy strategy for individual patients with breast cancer.
Conclusions
The findings of this study showed that using propensity model analysis an increased preoperative serum C-reactive protein (CRP) to albumin ratio was significantly associated with increased recurrence and reduced long-term prognosis in patients with resectable non-metastatic breast cancer. The CRP to albumin ratio can be easily and rapidly performed at low cost, may be used as a prognostic indicator routinely in preoperative clinical assessment and has the potential to contribute to improved management of individual patients with resectable breast cancer.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.McDonald ES, Clark AS, Tchou J, et al. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57(Suppl 1):9S–16S. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 3.Leong SP, Tseng WW. Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: Clinical significance and biologic implications. Cancer J Clin. 2014;64(3):195–206. doi: 10.3322/caac.21217. [DOI] [PubMed] [Google Scholar]
- 4.Rakha EA, Ellis IO. Modern classification of breast cancer: Should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18(4):255–67. doi: 10.1097/PAP.0b013e318220f5d1. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. doi: 10.1186/s12885-015-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Sun X, Liu J, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4):339–45. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285. doi: 10.1186/s12885-017-3220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Liu K, Ye B, et al. Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with stage IB-IIA cervical cancer. Cancer Med. 2018;7(1):105–13. doi: 10.1002/cam4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YJ, Yao K, Lu MX, et al. Prognostic value of the C-reactive protein to albumin ratio: A novel inflammation-based prognostic indicator in osteosarcoma. Onco Targets Ther. 2017;10:5255–61. doi: 10.2147/OTT.S140560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–58. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang K, Lei J, Li C, et al. Comparison of the prognostic values of selected inflammation based scores in patients with medullary thyroid carcinoma: A pilot study. J Surg Oncol. 2017;116(3):281–87. doi: 10.1002/jso.24683. [DOI] [PubMed] [Google Scholar]
- 14.Kijima T, Arigami T, Uchikado Y, et al. Combined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinoma. Cancer Sci. 2017;108(2):193–99. doi: 10.1111/cas.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217–24. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SM, Lin J, Cook NR, et al. C-reactive protein and risk of breast cancer. J Natl Cancer Inst. 2007;99(11):890–94. doi: 10.1093/jnci/djk202. [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Liu S, Zhang S, et al. C-reactive protein and risk of breast cancer: A systematic review and meta-analysis. Sci Rep. 2015;5:10508. doi: 10.1038/srep10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim ES, Kim SY, Koh M, et al. C-reactive protein binds to integrin alpha2 and Fcgamma receptor I, leading to breast cell adhesion and breast cancer progression. Oncogene. 2018;37(1):28–38. doi: 10.1038/onc.2017.298. [DOI] [PubMed] [Google Scholar]
- 19.Kim ES, Cha Y, Ham M, et al. Inflammatory lipid sphingosine-1-phosphate upregulates C-reactive protein via C/EBPbeta and potentiates breast cancer progression. Oncogene. 2014;33(27):3583–93. doi: 10.1038/onc.2013.319. [DOI] [PubMed] [Google Scholar]
- 20.Allin KH, Nordestgaard BG, Flyger H, Bojesen SE. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: A cohort study. Breast Cancer Res. 2011;13(3):R55. doi: 10.1186/bcr2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asegaonkar SB, Asegaonkar BN, Takalkar UV, et al. C-reactive protein and breast cancer: New insights from old molecule. Int J Breast Cancer. 2015;2015 doi: 10.1155/2015/145647. 145647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frydenberg H, Thune I, Lofterod T, et al. Pre-diagnostic high-sensitive C-reactive protein and breast cancer risk, recurrence, and survival. Breast Cancer Res Treat. 2016;155(2):345–54. doi: 10.1007/s10549-015-3671-1. [DOI] [PubMed] [Google Scholar]
- 23.Onitilo AA, Engel JM, Stankowski RV, et al. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: A pilot study. Breast Cancer Res Treat. 2012;134(1):291–98. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 24.Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: What is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69(8):1151–76. doi: 10.1080/01635581.2017.1367947. [DOI] [PubMed] [Google Scholar]
- 25.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? J Parenter Enteral Nutr. 2003;27(1):10–15. doi: 10.1177/014860710302700110. [DOI] [PubMed] [Google Scholar]
- 27.Rahman MM, Ahsan MA, Monalisa NN, Rahman K. Influence of socioeconomic status and BMI on the quality of life after mastectomy in Bangladeshi breast cancer patients in a public hospital. Jpn J Clin Oncol. 2014;44(12):1150–57. doi: 10.1093/jjco/hyu144. [DOI] [PubMed] [Google Scholar]
- 28.Guo S, He X, Chen Q, et al. The C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patients. BMC Cancer. 2017;17(1):171. doi: 10.1186/s12885-017-3119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu HJ, Ma Y, Deng F, et al. The prognostic value of C-reactive protein/albumin ratio in human malignancies: An updated meta-analysis. Onco Targets Ther. 2017;10:3059–70. doi: 10.2147/OTT.S137002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamauchi Y, Safi S, Muley T, et al. C-reactive protein-albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA-N2 lung adenocarcinoma patients. Lung Cancer. 2017;114:62–67. doi: 10.1016/j.lungcan.2017.11.002. [DOI] [PubMed] [Google Scholar]


