Abstract
Multiple drugs of a new class of cancer treatments called immune checkpoint inhibitors, which work by enabling the immune system to attack tumour cells, have been approved for a variety of indications in recent years. Immune checkpoints, such as cytotoxic T-lymphocyte antigen-4 and programmed death-1, are part of the normal immune system and regulate immune activation. Treatment with inhibitors of these checkpoints can significantly improve response rates, progression-free survival and overall survival of patients with cancer; it can also result in adverse reactions that present similarly to other conditions. These immune-mediated adverse reactions (IMARs) are most commonly gastrointestinal, respiratory, endocrine or dermatologic. Although patients’ presentations may appear similar to other types of cancer therapy, the underlying causes, and consequently their management, may differ. Prompt recognition is critical because, with appropriate management, most IMARs resolve and patients can continue receiving immune checkpoint inhibitor treatment. Rarely, these IMARs may be life-threatening and escape detection from the usual evaluations in the emergency environment. Given the unusual spectrum and mechanism of IMARs arising from immune checkpoint inhibitors, emergency departmentED staff require a clear understanding of the evaluation of IMARs to enable them to appropriately assess and treat these patients. Treatment of IMARs, most often with high-dose steroids, differs from chemotherapy-related adverse events and when possible should be coordinated with the treating oncologist. This review summarises the ED presentation and management of IMARs arising from immune checkpoint inhibitors and includes recommendations for tools and resources for ED healthcare professionals.
Keywords: clinical management, emergency department, communications, clinical assessment
Introduction
The immune system balances complex interactions of danger-recognising defences against external threats, which include both infections and cancer, with tight regulation preventing immune-driven toxicity. This is achieved by distinguishing ‘self’ from ‘non-self’ and mounting an attack on invasive, infected or mutated cells, including tumour cells expressing ‘non-self’ antigens.1 Some tumours escape elimination by deploying natural immune regulatory molecules which, when released by non-cancerous cells, have a role in suppressing immune recognition and thereby protecting against autoimmunity. These molecules include immune checkpoints, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1), lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin-containing and mucin domain-containing molecule-3 (TIM-3).1–5
Drugs that inhibit immune checkpoints allow the immune system to detect and mount a defence against some cancers (table 1). Since the US Food and Drug Administration approval of ipilimumab to treat metastatic melanoma (in 2011), several intravenous immune checkpoint inhibitors (ICIs) have been approved to treat a range of cancers, including non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), melanoma, renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), microsatellite instability high (MSI-H)/mismatch repair deficient (dMMR) colorectal cancer (CRC), squamous cell carcinoma of the head and neck (SCCHN), urothelial carcinoma (UC), cervical cancer, Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma (table 1).6–11 Members of the ICI class can also be combined, including coadministration of nivolumab with ipilimumab for: (1) unresectable or metastatic melanoma; (2) intermediate- or poor-risk, previously untreated advanced RCC or (3) MSI-H/dMMR metastatic CRC that has progressed after chemotherapy (ie, fluoropyrimidine, oxaliplatin, irinotecan).
Table 1.
Target molecules/mechanism of action and current indications for ICIs6–11
| Target | Function of targeted checkpoint | Immune checkpoint inhibitor | FDA-approved tumour type* |
| CTLA-4 |
|
Ipilimumab (Yervoy) |
|
| PD-1 |
|
Nivolumab (Opdivo) Pembrolizumab (Keytruda) |
|
| PD-L1 |
|
Atezolizumab (Tecentriq) Durvalumab (Imfinzi) |
|
| Avelumab (Bavencio) |
|
*ICIs are FDA-approved for certain subtypes of patients with these tumour types.
CTLA-4, cytotoxic T-lymphocyte antigen-4; dMMR, mismatch repair deficient; FDA, US Food and Drug Administration; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; MSI-H, microsatellite instability-high; NSCLC, non-small cell lung cancer; PD-1, programmed death-1; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; SCLC, small cell lung cancer; UC, urothelial carcinoma.
ICIs can be administered for first-line therapy of unresectable or metastatic melanoma, NSCLC, and Merkel cell carcinoma, either as monotherapy or in combination with chemotherapy. Candidates for second-line ICI treatment include patients with metastatic, recurrent or locally advanced NSCLC, SCLC, SCCHN or UC who experience disease progression on or after platinum-based chemotherapy; RCC after antiangiogenic therapy; or progressive HCC after sorafenib. Patients with metastatic NSCLC and EGFR or ALK gene aberrations are eligible to receive medications targeting the PD-1/PD-1 ligand (PD-L1) axis after disease progression with a US-approved tyrosine kinase inhibitor for these abnormalities.
The ICIs are administered as intravenous infusions over 30–90 min, and dosages vary across tumour types. The infusion rate can be decreased (or the infusion interrupted) in the event of mild or moderate infusion reactions (or treatment discontinued in the event of severe or life-threatening reactions). When nivolumab and ipilimumab are combined, nivolumab is administered first (at a dose of 1 mg/kg for unresectable or metastatic melanoma or 3 mg/kg for advanced RCC), followed by ipilimumab (at a dose of 3 mg/kg for unresectable or metastatic melanoma or 1 mg/kg for RCC), for four doses (one every 3 weeks). After this weight-adjusted induction period, nivolumab is administered at 240 mg every 2 weeks (Q2W) or 480 mg Q4W until disease progression or unacceptable toxicity.6 7 Patients should be provided information on possible adverse events, including IMARs, with triggers for contacting the treating team and seeking emergency care. This would include symptoms of colitis (>3 stools per day), pneumonitis (worsening cough or shortness of breath) and other moderated changes in symptoms.
The efficacy and safety/tolerability of ICIs have been established in numerous pivotal clinical trials, where these agents have significantly improved various efficacy outcomes across multiple tumour types.6–11 However, the antitumour effects of ICIs may be accompanied by immune-mediated adverse reactions (IMARs) that resemble autoimmune diseases and can lead to organ dysfunction.2 These autoimmune conditions may not routinely present to the emergency physician under other circumstances. Early recognition with appropriate evaluation, therapy and triage for further inpatient versus outpatient management is vital to decreasing morbidity for this patient population.12 IMARs of ICIs may present similarly to adverse events associated with chemotherapy or targeted therapies, but they require different management.12 Although the oncology team is the first point of contact for patients experiencing IMAR symptoms, these may occur outside hours for the oncology team and many patients will seek help at their local emergency department ED.
Overview of presentation and management principles via case vignettes
Following are examples of cases that may be encountered in the ED, with consideration of consensus clinical practice guidelines to manage them.12–14
Vignette 1: ICI-associated colitis
A woman aged 48 years with metastatic melanoma who had been receiving an ICI (CTLA-4 inhibitor) for 4 months presented to the ED with worsening diarrhoea (four to six stools per day more than baseline), abdominal pain and blood in stool. She informed the ED that she was ‘on chemo’.
A complete blood count with differential and comprehensive metabolic panel were conducted, with testing for thyroid-stimulating hormone (TSH), erythrocyte sedimentation rate and C reactive protein. To rule out infectious causes, a stool culture was performed and assessed for the presence of Clostridium difficile; viruses, including cytomegalovirus; and ova and parasites, which were all negative. She received hydration and was discharged (with email communication to her oncologist) and referred for a gastroenterology consultation. The patient was subsequently prescribed antidiarrhoeal medications after the consult because of continued severity. When the diarrhoea did not abate, the patient underwent colonoscopy, which supported a diagnosis of immune-mediated colitis. Immunotherapy was immediately discontinued, and the patient treated with corticosteroids (prednisone at an initial daily dose of 1 mg/kg). She suffered from immune-mediated colitis for 8 months. Once symptoms returned to grade 1, corticosteroids were tapered over 4–6 weeks.
As illustrated in this vignette (consistent with grade 3 gastrointestinal [GI] toxicity), early recognition and prompt treatment of IMARs are essential to improve patient outcomes (table 2).15 16 Prompt administration of steroids to balance immune overactivation is also crucial and may prevent multiorgan failure or permanent treatment discontinuation.15 Systemic immune suppression should have been initiated sooner after ruling out infectious aetiologies. Intravenous steroids were indicated with the first ED evaluation and discharge on oral steroids with follow-up arranged within 1–2 days. Colonoscopy is considered but not always required unless needed for additional diagnostic information. CT of the abdomen and pelvis during workup (or after discharge or hospitalisation) may also be performed to identify immune-mediated colitis early.13 It is also important in this case to be mindful that GI metastases, which can develop secondary to melanoma, could also be responsible for the patient’s signs and symptoms.
Table 2.
Critical IMARs requiring intervention and management12–14 18
| IMAR* | Possible presenting signs/symptoms | Recommended workup | Grade† | Management |
| Always inform the patient’s oncology team so that the ICI can be withheld or discontinued. Inform the on-call oncology coverage for the patient if a decision is made to initiate systemic immunosuppression | ||||
|
Colitis
prevalence 6–9: Anti-PD-(L)1: 1.5% Anti-CTLA-4: 8% Anti-PD-1+anti-CTLA-4: 7%–10% Rare but serious IMAR to consider: enterocolitis |
|
|
Grade 4: life-threatening consequences; urgent intervention indicated |
|
| Grade 3: >6 liquid stools per day OR within 1 hour of eating; limiting self-care ADL |
|
|||
Grade 2: 4–6 liquid stools per day over baseline, or ≥1 of:
|
|
|||
|
Dermatologic (rash, Stevens-Johnson syndrome or toxic epidermal necrolysis)
prevalence (all dermatologic toxicities) 6 7 10 12 28 29: Anti-PD-(L)1: 9%–11% Anti-CTLA-4: 29%–50% Anti-PD-1+anti-CTLA-4: 23% Rare but serious IMAR to consider: Stevens-Johnson syndrome |
|
|
Grade 4: skin sloughing >30% BSA with associated symptoms (eg, erythema, purpura, epidermal detachment) |
|
| Grade 3: rash >30% BSA with moderate or severe symptoms |
|
|||
| Grade 2: rash covering 10%–30% BSA, potentially symptoms of pruritus or tenderness |
|
|||
|
Adrenal insufficiency
prevalence: see hypophysitis, which causes central/secondary adrenal insufficiency and other endocrinopathies Primary adrenal insufficiency is rare but can be serious |
|
|
Grade 3/4: severe symptoms:
|
|
Grade 2: moderate symptoms:
|
|
|||
|
Hypophysitis
prevalence 8 10–13 19 29–31: Anti-PD-(L)1: <1% Anti-CTLA-4: ≤10%–17% Anti-PD-1+anti-CTLA-4: 9% |
|
|
Grade 3/4: severe mass effect symptoms:
|
|
Grade 2: moderate symptoms:
|
|
|||
|
Hypothyroidism
prevalence 6–8 10 11 29 31: Anti-PD-(L)1: 5%–9% Anti-CTLA-4: <1% Anti-PD-1+anti-CTLA-4: 22% |
|
|
Grade 4: life-threatening consequences; urgent intervention required |
|
| Grade 3: severe symptoms; limiting self-care ADL; hospitalisation indicated | ||||
| Grade 2: symptomatic; thyroid replacement indicated; limiting instrumental ADL | ||||
|
Hyperthyroidism
prevalence 6–8 10 28 29 31: Anti-PD-(L)1: 1%–5% Anti-CTLA-4: <1% Anti-PD-1+anti-CTLA-4: 8% |
|
|
||
| Grade 4: life-threatening consequences; urgent intervention required |
|
|||
| Grade 3: severe symptoms; limiting self-care ADL; hospitalisation indicated | ||||
| Grade 2: symptomatic; thyroid suppression therapy indicated; limiting instrumental ADL | ||||
|
Pneumonitis
prevalence (most common lung toxicity) 6–8 10 17 28–31: Anti-PD-(L)1:<1%–4% Severe: 1%–2% Anti-CTLA-4: <1% Anti-PD-1+anti-CTLA-4: 6% |
|
|
Grade 3/4: severe new symptoms; limiting self-care ADL; new/worsening hypoxia; life-threatening respiratory compromise; urgent intervention indicated (eg, tracheotomy or intubation) |
|
| Grade 2: symptomatic mild/moderate (cough, dyspnoea, chest pain); no hypoxia; vitals normal |
|
|||
|
Hepatitis
prevalence 6–8 10 28 29 31: Anti-PD-(L)1: <1%–2% Anti-CTLA-4: 11% Anti-PD-1+anti-CTLA-4: 13% |
|
|
Grade 3/4: AST, ALT>5× ULN; total bilirubin>3× ULN |
|
| Grade 2: AST, ALT>3 to ≤5× ULN; total bilirubin>1.5 to ≤3× ULN |
|
|||
|
Encephalitis
prevalence 6–8 10 28 31: Anti-PD-(L)1: <1% Anti-CTLA-4: <1% Anti-PD-1+anti-CTLA-4: <1% |
|
|
Grade 3/4: severe, limiting self-care and aids warranted |
|
| Grade 2: moderate, some interference with ADL, symptoms concerning to patient (ie, pain but no weakness or gait limitation) | ||||
|
Nephritis
prevalence 6 7 10 28 29: Anti-PD-(L)1:<1%–1% Anti-CTLA-4: <1% Anti-PD-1+anti-CTLA-4: 2% |
|
|
Grade 4: creatinine>6× ULN |
|
| Grade 3: creatinine>3× baseline or >3–6× ULN | ||||
| Grade 2: creatinine>1.5–3× baseline or >1.5–3× ULN |
|
|||
|
Pancreatitis
prevalence 6–8 28 31: Anti-PD-(L)1: <1% Anti-CTLA-4: 1.3% Anti-PD-1+anti-CTLA-4: <1% |
|
|
Grade 4: life-threatening consequences; urgent intervention indicated |
|
| Grade 3: severe pain; vomiting; medical intervention indicated | ||||
| Grade 2: elevated enzymes or radiographic findings only | ||||
|
Peripheral motor and sensory neuropathy
prevalence 28: Anti-PD-(L)1:<1% Anti-PD-1+anti-CTLA-4: <1% |
|
|
Grade 4: life-threatening consequences; urgent intervention indicated |
|
| Grade 3: severe symptoms; limiting self-care ADL | ||||
| Grade 2: moderate symptoms; limiting instrumental ADL |
|
|||
|
Myocarditis, pericarditis, arrhythmias
prevalence (all cardiac toxicities) 10–12 14 29 30: Anti-PD-(L)1: <1%–5% Anti-PD-1+anti-CTLA-4: <1% |
|
|
Grade 4: moderate-to-severe decompensation, intravenous medication or intervention required, life-threatening conditions |
|
| Grade 3: moderately abnormal testing or symptoms with mild activity | ||||
| Grade 2: abnormal screening tests with mild symptoms | ||||
|
Uveitis
prevalence 12: Any ICI: <1% |
|
|
Grade 4: best-corrected visual acuity of 20/200 or worse in the affected eye |
|
| Grade 3: anterior uveitis with 3+ or greater cells; intermediate posterior or pan-uveitis | ||||
| Grade 2: anterior uveitis with 1+ or 2+ cells | ||||
G1: mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; no intervention indicated.
G2: moderate; minimal, local or non-invasive intervention indicated; limiting age-appropriate instrumental ADL.
G3: severe or medically significant but not immediately life-threatening; hospitalisation or prolongation of hospitalisation indicated; disabling; limiting self-care ADL.
G4: life-threatening consequences; urgent intervention indicated.
*Prevalence of IMARs is for any-grade IMARs.
CTCAE grade definitions18:
ACTH, adrenocorticotropic hormone; ADL, activities of daily living; ALT, alanine aminotransferase; ANCA, antineutrophil cytoplasmic antibodies; AST, aspartate aminotransferase; BSA, body surface area; CBC, complete blood count; CK, creatine kinase; CNS, central nervous system; CRP, C reactive protein; CTCAE, Common Terminology Criteria for Adverse Events; CTLA-4, cytotoxic T-lymphocyte antigen-4; CXR, chest X-ray (roentgenogram); ECG, electrocardiogram; EEG, electroencephalography; ESR, erythrocyte sedimentation rate; HSV, herpes simplex virus; ICI, immune checkpoint inhibitor; ICU, intensive care unit; IMAR, immune-mediated adverse reaction; IVIG, intravenous immunoglobulin; LFT, liver function test; NSAID, non-steroidal anti-inflammatory drug; PCR, polymerase chain reaction; PD-1, programmed death-1; PD-L1, programmed death ligand 1; T4, thyroxine; TB, tuberculosis; TFT, thyroid function test; TPO, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; UEC, urea electrolytes and creatinine; ULN, upper limit of normal; UPCR, urine protein/creatinine ratio.
Given that the patient misconstrued her therapy as ‘chemo’, she may not have been appropriately counselled about the nature of her treatment and how to recognise and report IMARs, potentially delaying effective intervention.
Vignette 2: ICI-associated pneumonitis
A man aged 35 years came into the ED with a dry cough, worsening dyspnoea and chest pain but without fever. Patient history indicated that he was diagnosed with melanoma and was on anti-PD-1 therapy. As part of the initial workup, nasal swabs were tested for viral pathogens and pan-culture and sensitivity performed on blood, urine and sputum, all of which were negative. Further diagnostic workup included pulse oximetry and cross-sectional CT.17
More than one lobe of the lung and 45% of lung parenchyma were involved, with findings including ground-glass opacities. Bronchoscopy with bronchoalveolar lavage (BAL) was performed, and the BAL fluid showed lymphocytosis (a sign consistent with ICI-induced pneumonitis). No lung biopsy was performed to confirm pathology.17 Empirical antibiotics were instituted before pan-culture and sensitivity results were available and discontinued when these tests returned normal.
Based on the lack of infectious disease, the CT and bronchoscopy results and a history of ICI treatment for melanoma, a diagnosis of ICI-induced pneumonitis was made. The patient was treated with prednisone 1 mg/kg/day and admitted to hospital, where he experienced rapid improvement in symptoms after 3 days and was later discharged. On improvement of the patient’s symptoms to grade 1, the corticosteroid therapy was tapered by 5–10 mg/week over 4–6 weeks and ICI therapy reinstated. This case, which is consistent with grade 2 ICI-induced pneumonitis, illustrates the importance of ruling out infectious causes and establishing a diagnosis in a timely manner to allow prompt institution of effective treatment.17 Mild symptoms may be managed as an outpatient and bronchoscopy may be delayed and used if patients fail to respond to initial management with new or worsening infiltrates. Cancer progression, alveolar haemorrhage, radiation pneumonitis and pneumonia may mimic immune-mediated pneumonitis.
Taken together, the case vignettes underscore the importance of early IMAR recognition with appropriate evaluation, therapy and triage for further inpatient versus outpatient management to decrease morbidity for this patient population.12 IMARs of ICIs may present similarly to adverse events associated with chemotherapy or targeted therapies, but they require different management.12 Management of IMARs is also dependent on the severity of symptoms, as shown for these two case vignettes reflecting contemporary clinical practice guidelines.12–14 Although the index of clinical suspicion for IMARs may be elevated in a patient receiving ICIs, it is important to underscore the fact that other, potentially more common, aetiologies often must be ruled out. These include infection, effects of other medications and malignant transformation or metastasis.
Objectives
The goals of this review are to provide ED physicians with (1) information concerning key IMARs of ICIs (including their differential diagnosis, frequency, presentation, time of onset), focusing on common IMARs as well as less common but potentially life-threatening IMARs and (2) consensus recommendations and tools to identify and manage these IMARs in an ED or urgent-care setting.
To address these goals, we conducted a 5-year English-language literature search of PubMed using title and abstract terms including ‘immunotherapy’, ‘checkpoint’, ‘CTLA-4’, ‘PD-1/PD-L1’ (with abbreviations also spelled out) and individual ICI names. Selected articles were manually searched to identify important, previously published reports. To enrich the output, we searched online medical-information portals, including educational videos, and identified recent consensus clinical practice guidelines issued by major cancer and other patient care professional societies. US full prescribing information and other educational materials from life sciences companies for selected ICIs were also reviewed. Finally, websites for the ICIs were searched for educational guidelines to manage IMARs.
Recognition of IMARs
Frequency and presentation of IMARs in the ED
The frequency and timing of IMARs differ between ICIs, dosing schedule and regimen and cancer type. Current grading and tracking of IMARs rely on the use of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE V.5.0), in which adverse events are rated from grade 1 to 5, corresponding to mild, moderate, severe, life-threatening and death, respectively.18 Grade 2 or higher toxicity is generally correlated with the need for medical intervention. 13
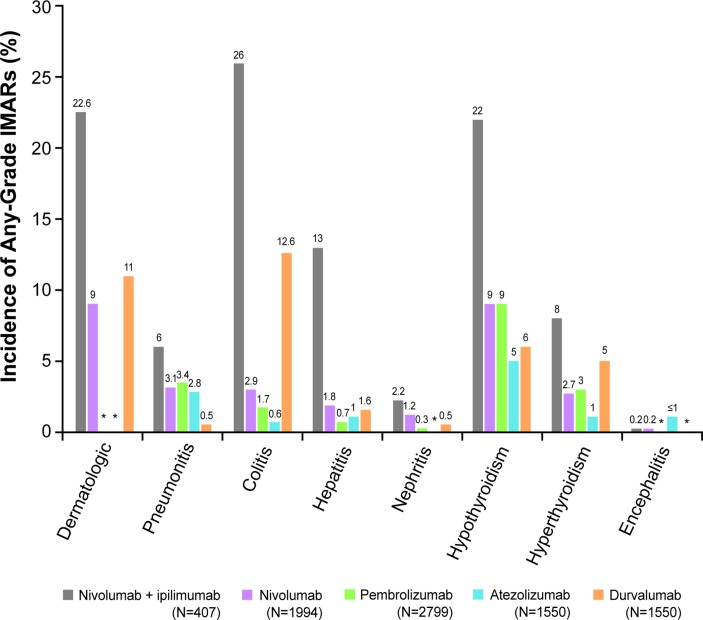
A higher-grade toxicity usually requires more urgent medical intervention.13 18 CTLA-4 inhibitors, such as ipilimumab, are associated with more frequent and severe (higher grade) IMARs than PD-1/PD-L1 inhibitor monotherapy.12 Combination therapy with two ICIs is associated with both earlier IMAR onset and noticeably higher levels of immune-related toxicity than either one alone (figure 1).2 14 19 In patients receiving nivolumab and ipilimumab in combination (for melanoma, RCC or MSI-H/dMMR CRC, respectively), 96%, 93% and 73% of patients had treatment-related adverse events, of which 55%, 46% and 32% (respectively) were grade 3 or higher.20–22 An increased frequency of adverse events may also be seen when ICIs are administered sequentially.14
Figure 1.
Frequency of IMARs following ICI treatment.2 7 8 10 11 14 19 28–31 *Data not available. ICI, immune checkpoint inhibitor; IMAR, immune-mediated adverse reaction.
Overall, the most frequent IMARs are those affecting the skin, endocrine system, GI tract and lungs.2 12 14 23 More rarely, neurologic, ocular, cardiovascular, haematologic and renal IMARs can occur.12 In some cases, these less common toxicities may be life-threatening and therefore require prompt diagnosis and treatment, particularly because initial presentation may be mild, with non-specific symptoms such as fatigue, headache and electrolyte disturbance.12 IMARs related to ICIs may present similarly to those related to chemotherapy (eg, diarrhoea and colitis), but may have different underlying causes and therefore require different diagnostic procedures, additional workup and different management.12 24
For instance, intestinal perforation may present as abdominal pain25; pneumonitis and myocarditis as complaints of shortness of breath14; myositis, Guillain-Barré syndrome, adrenal crisis, myocarditis, myasthenia gravis and diabetes as weakness/fatigue14; encephalitis and adrenal or thyroid crisis as confusion14 and uveitis and hypophysitis as vision changes.14
Time of onset
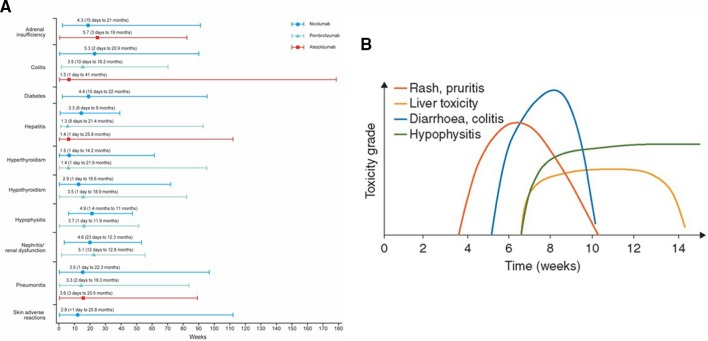
While they may occur with the first dose, IMARs related to ICIs most often occur after several infusions and may worsen later in therapy or after treatment discontinuation. IMARs have been documented as late as 1 year after treatment discontinuation.13 Immune-related toxicity risk generally progresses over time, as some IMARs appear to require the generation of an immune response to the ICI. This is in contrast to chemotherapy, where adverse events typically occur early and repetitively in the treatment course.12 Figure 2A shows the median (range) time of appearance of IMARs with anti-PD-1/PD-L1 treatment.7 8 11 Median time to onset of IMARs with anti-PD-1/PD-L1 antibodies is typically between 1 and 6 months; however, IMARs again may present as late as 41 months after treatment initiation.7 8 11 For ipilimumab (anti-CTLA-4), dermatologic IMARs typically present after 2–3 weeks of treatment, while GI and hepatic IMARs appear after 6–7 weeks and some endocrinopathies can appear 9 weeks or later after immunotherapy (figure 2B)26
Figure 2.
Kinetics of appearance of IMARs.2 7 8 11 14 19 26 45 (A) Median (range) IMAR symptom onset (months) following PD-1/PD-L1 inhibitor treatment across FDA-approved tumour types. (B) Timing of IMAR occurrence by toxicity grade following ipilimumab inhibitor treatment in melanoma. Figure 2B reprinted with permission from the Journal of Clinical Oncology. FDA, US Food and Drug Administration; IMAR, immune-mediated adverse reaction; PD-1, programmed death 1; PD-L1, programmed death ligand 1.
Management of IMARs
Because of the prolonged kinetics of ICIs, it is vital that patients presenting to the ED have oncology follow-up within 1–3 days after discharge. Toxicities may continue to evolve over days and weeks, from mild to severe, and may be associated with new organ dysfunction.13 Table 2 describes the potential signs and symptoms, evaluations and immediate intervention recommended for management of key IMARs, including the most common ICI-associated IMARs, along with less common but serious and potentially life-threatening toxicities. Table 2 includes additional testing to consider whenever the differential diagnosis remains unclear. High-dose steroids are often used as a first attempt at symptom control, with long tapers occurring over at least 4 weeks,13 14 in contrast with other immune-mediated events unrelated to immuno-oncology (I-O) therapy27; patients presenting to the ED may have already commenced steroidal therapy prescribed by their oncology team. Subsequent management strategies are therefore included in table 2 to provide guidance when treating these patients.
Identifying patients receiving ICI therapy
Because of the multitude of medications used in patients with cancer, it is important to inquire about the type of treatment patients are on (eg, immunotherapy, chemotherapy, targeted therapy), and determine which specific ICI(s) (eg, nivolumab, ipilimumab, pembrolizumab, atezolizumab, durvalumab, avelumab) patients are receiving. Patients will have received education on their treatment from their oncology team but may not distinguish immunotherapy from other types of anticancer therapy.13 14 Consider asking to see an alert card or other information the patient may have been provided. Alert cards contain information about symptoms of key IMARs and oncologist contact details (certain alert cards may contain QR codes allowing access to management guidelines).32
Furthermore, it is important to be aware of the potential delay in presentation of IMARs. Consider inquiring about the nature of ongoing and past treatment(s) in patients with a recent history of cancer.13 33 New agents are continuing to be developed that will add to both benefit and risk for oncology patients, including the possibility of new toxicities as these agents impact other immune regulatory receptors or molecules.
Reassure patients that IMARs are generally manageable, and that interrupted ICI treatment may be reinitiated at their oncologist’s discretion.12 13
Guidelines and other management tools
Various resources are available to ED clinicians. Guidelines and management tools are outlined below. In some cases, education may be provided by local oncologists and oncology nurses who are familiar with immunotherapy and related IMARs.33
Multidisciplinary guidance reflecting broad-based perspectives have been issued to guide clinicians on how to recognise, report and manage organ-specific toxicities related to immunotherapy. To date, guidelines have been published by the European Society of Medical Oncology,13 the Society for Immunotherapy of Cancer12 and other health authorities.24 These guidelines focus on the recognition and management of a wide array of IMARs, including asymptomatic or mild cases not discussed in this review. The guidelines also provide recommendations for additional evaluations, interrupting or permanently discontinuing ICI treatment and dosing of corticosteroid therapy.12–14
A variety of educational tools and resources to improve understanding of IMARs associated with I-O agents are available as well. Two continuing medical education videos by Healio34 and Medscape35 provide ED clinicians with valuable information on the identification and management of IMARs in patients with cancer. Furthermore, an education project review developed by the Association of Community Cancer Centers addresses real-world experiences and practical concerns with I-O delivery.33 Additional information on immunotherapy and IMARs in patients has also been developed by UpToDate,36 Access Medicine37 and the Oncology Nursing Society,38 including a speed talk video on ICIs.39 An interactive management tool is available from Clinical Care Options.40
Life science companies that manufacture and/or market ICIs have also developed educational tools to recognise and manage IMARs. These tools and management guides provide detailed information concerning the incidence of specific IMARs in the clinical trial setting. Moreover, these tools inform decisions about interrupting or permanently discontinuing I-O agents and instruct on the use of corticosteroids to mitigate each type of IMAR:
BAVENCIO (avelumab) safety information.41
IMFINZI (durvalumab) IMAR handbook.29
KEYTRUDA (pembrolizumab) management of IMARs.30
OPDIVO (nivolumab) digital safety tool and IMAR management guide.42
OPDIVO (nivolumab) IMAR management guide.28
TECENTRIQ (atezolizumab) management of IMARs.31
YERVOY (ipilimumab) management of IMARs.43
A number of tools to improve the flow of care for patients receiving ICIs have also been developed, including the tagging of electronic medical records. The electronic medical record environment alerts providers with popup flags when patients on ICIs first present to a new healthcare environment (ie, admitted to the hospital or the ED). These flags offer both an immediate alert regarding the unique IMAR spectrum experienced by the patient and links to additional information. ED teams may consider liaising with their local oncology team(s) to put similar measures in place. Standardised nursing assessment flow charts for IMAR assessment and documentation may also help healthcare providers in diagnosing IMARs, particularly because patients may not indicate that they are experiencing symptoms if they do not consider them relevant.44 Updating institutional telephone triage guidelines to better respond to patients receiving immunotherapy calling with concerns about side effects can aid optimal management of IMARs as well.24
Communication with the oncology team
It is extremely important for the patient’s oncology team to be informed about any new or worsening symptoms, ED visits, interventions or changes to their treatment. Encourage patients to report these to their oncology team as quickly as possible. Where possible, alert the oncology team directly in case the patient fails to do so, particularly for symptomatic IMARs, so that the patient’s oncologist can inform the patient about their diagnosis and potential need to discontinue ICI therapy. Moreover, relay any admission to the on-call oncologist for that patient. Institutions may want to consider a single institution-based telephone triage system providing support for non-specialist healthcare professionals such as the one currently being assessed at Smilow Cancer Hospital at Yale New Haven Hospital, New Haven, Connecticut, USA, for times when the patient’s usual team is not available and advice is required. However, even if immediate management advice is not required, follow-up and monitoring of the patient is crucial because toxicities may continue to evolve.
Conclusions
Early recognition and prompt, appropriate treatment of IMARs arising from ICIs increases the likelihood of resolving IMARs. This review increases ED providers’ awareness of potential IMARs related to ICI treatment. It also provides resources on how these IMARs differ from those related to chemotherapy, as well as a variety of tools and guidelines that can assist ED providers in effective assessment and management of IMARs. It is important to be aware that IMARs can develop or worsen at any time during treatment, even once ICI treatment has been discontinued. Most significantly, open communication between patients, ED providers and the patient’s oncologist is imperative to ensure optimal management of IMARs in the ED.
Acknowledgments
None
Footnotes
Contributors: All authors reviewed and approved the manuscript.
Funding: Medical writing support was provided by Jason Hoffman, and editorial support was provided by Jay Rathi, both of Spark Medica, supported by Bristol-Myers Squibb according to Good Publication Practice guidelines (no grant number).
Competing interests: JV-U reports consulting fees from Bristol-Myers Squibb. No other authors have commercial, financial or other relationships in any way related to the subject of this article to disclose, per ICMJE conflict of interest guidelines. Outside the submitted work, GD reports clinical trial support from Bristol-Myers Squibb, Nektar, Regeneron, Viralytics, Dynavax, OncoSec and Merck. DK reports personal fees and non-financial support from Bristol-Myers Squibb.
Provenance and peer review: Not commissioned; externally peer reviewed.
Press Release: Yes.
Patient consent for publication: Not required.
References
- 1. Harris TJ, Drake CG. Primer on tumor immunology and cancer immunotherapy. J Immunother Cancer 2013;1:12 10.1186/2051-1426-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haanen JB, Thienen H, Blank CU. Toxicity patterns with immunomodulating antibodies and their combinations. Semin Oncol 2015;42:423–8. 10.1053/j.seminoncol.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 3. He Y, Rivard CJ, Rozeboom L, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci 2016;107:1193–7. 10.1111/cas.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–9. 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- 5. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989–1004. 10.1016/j.immuni.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bristol-Myers Squibb. Highlights of prescribing information for YERVOY® (ipilimumab). 2018. https://packageinserts.bms.com/pi/pi_yervoy.pdf (Accessed 3 Oct 2018).
- 7. Bristol-Myers Squibb. Highlights of prescribing information for OPDIVO® (nivolumab). 2018. https://packageinserts.bms.com/pi/pi_opdivo.pdf (Accessed 3 Oct 2018).
- 8. Genentech, Inc. Highlights of prescribing information for TECENTRIQ® (atezolizumab). 2018. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf (Accessed 3 Oct 2018).
- 9. Merck KGaA. Highlights of prescribing information for BAVENCIO® (avelumab). 2017. http://www.emdserono.com/ms.country.us/en/images/Bavencio_PI_tcm115_161084.pdf?Version (Accessed 3 Oct 2018).
- 10. AstraZeneca UK Limited. Highlights of prescribing information for IMFINZI™ (durvalumab). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761069s000lbl.pdf (Accessed 3 Oct 2018).
- 11. Merck & Co., Inc. Highlights of prescribing information for KEYTRUDA® (pembrolizumab). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf (Accessed 3 Oct 2018).
- 12. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 15. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2:1346–53. 10.1001/jamaoncol.2016.1051 [DOI] [PubMed] [Google Scholar]
- 16. Lomax AJ, McNeil C. Acute management of autoimmune toxicity in cancer patients on immunotherapy: Common toxicities and the approach for the emergency physician. Emerg Med Australas 2017;29:245–51. 10.1111/1742-6723.12718 [DOI] [PubMed] [Google Scholar]
- 17. Chuzi S, Tavora F, Cruz M, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 2017;9:207–13. 10.2147/CMAR.S136818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 2017. https://evs.nci.nih.gov/ftp1/CTCAE/About.html (Accessed 3 Oct 2018).
- 19. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–91. 10.1093/annonc/mdv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018;36:773–9. 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 23. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 24. Fradkin M. Leveraging technology to optimize the care of patients treated with immunotherapy. Oncol Nurs Forum 2017;44:1. [Google Scholar]
- 25. Yasuda K, Tanaka T, Ishihara S, et al. Intestinal perforation after nivolumab immunotherapy for a malignant melanoma: a case report. Surg Case Rep 2017;3:94 10.1186/s40792-017-0370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med 2016;4:272 10.21037/atm.2016.07.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lahn M, Bijur P, Gallagher EJ. Randomized clinical trial of intramuscular vs oral methylprednisolone in the treatment of asthma exacerbations following discharge from an emergency department. Chest 2004;126:362–8. 10.1378/chest.126.2.362 [DOI] [PubMed] [Google Scholar]
- 28. Bristol-Myers Squibb. OPDIVO® (nivolumab) immune-mediated adverse reactions management guide. 2018. http://www.opdivohcp.com/servlet/servlet.FileDownload?file=00Pi000000onjAXEAY (Accessed 3 Oct 2018).
- 29. AstraZeneca. Immune-mediated adverse events management handbook. 2017. https://www.imfinzi.com/content/dam/website-services/us/498-rwd-imfinzi-com/pdf/imAE_management_handbook.pdf (Accessed 3 Oct 2018).
- 30. Merck Sharp & Dohme Corp. A treatment guide for Keytruda. 2018. https://www.keytruda.com/static/pdf/keytruda-treatment-guide.pdf (Accessed 3 Oct 2018).
- 31. Genentech, Inc. Managing select Tecentriq immune-related adverse events. 2017. https://www.tecentriq.com/content/dam/gene/tecentriq/Tecentriq-Adverse-Event-Management-Brochure.pdf (Accessed 3 Oct 2018).
- 32. Upton J. Creating guidelines for managing the side-effects of immunotherapy. Nursing Times 2017;114:24–5. [Google Scholar]
- 33. Association of Community Cancer Centers (ACCC). Real world experiences in immunotherapy delivery. 2017. https://www.accc-cancer.org/docs/Documents/oncology-issues/articles/JA17/ja17-real-world-experiences-in-immunotherapy-delivery (Accessed 3 Oct 2018).
- 34. Weber JS, Brock P, Kannan R, et al. The identification and management of immune-related adverse events in patients with cancer: practice essentials for emergency medicine providers. 2017. https://cme.healio.com/hematology-oncology/education-lab/2017/03_march/the-identification-and-management-of-immune-related-adverse-events-in-patients-with-cancer/cme-information (Accessed 3 Oct 2018).
- 35. Crawford J, Patel R. Mitigating the challenge of immune-related AEs in the emergency department. 2017. https://www.medscape.org/viewarticle/874003_2 (Accessed 3 Oct 2018).
- 36. Postow M, Wolchok J. Toxicities associated with checkpoint inhibitor immunotherapy. 2018. https://www.uptodate.com/contents/toxicities-associated-with-checkpoint-inhibitor-immunotherapy (Accessed 3 Oct 2018).
- 37. Reynolds K, Ananthakrishnan A, Dougan M, et al. Chapter 183: immune-related adverse events (irAEs) in cancer patients. 2017. https://accessmedicine.mhmedical.com/content.aspx?bookid=1872§ionid=146984610 (Accessed 3 Oct 2018).
- 38. Oncology Nursing Society. Immunotherapy resources. 2016. https://www.ons.org/practice-resources/cancer-therapies/immunotherapy-resources (Accessed 11 May 2018).
- 39. Oncology Nursing Society. Speed talk: checkpoint inhibitors in 5 minutes. 2017. https://www.youtube.com/watch?v=M2pTTDw0dtc&list=PLgND6mUAyF4GQq2xid3_B9SwfFv_QfwJv (Accessed 3 Oct 2018).
- 40. Weber JS. Managing immune-related adverse events: an interactive algorithm tool. 2017. https://www.clinicaloptions.com/Oncology/Treatment%20Updates/Merkel%20Cell%20Carcinoma/Interactive%20Decision%20Support%20Tool/Interactive_Tool.aspx (Accessed 3 Oct 2018).
- 41. EMD Serono, Inc. BAVENCIO important safety information and indications. 2017. https://www.bavencio.com/en_US/for-us-healthcare-professionals.html (Accessed 3 Oct 2018).
- 42. Bristol-Myers Squibb. OPDIVO® (nivolumab) safety tool. 2017. https://www.opdivosafetytool.com/#/superhome (Accessed 3 Oct 2018).
- 43. Bristol-Myers Squibb. YERVOY® (ipilimumab) immune-mediated side effects. 2018. http://www.hcp.yervoy.com/metastatic-melanoma/safety-immune-mediated-side-effects (Accessed 3 Oct 2018).
- 44. Madden KM, Hoffner B. Ipilimumab-based therapy: consensus statement from the faculty of the Melanoma Nursing Initiative on managing adverse events with ipilimumab monotherapy and combination therapy with nivolumab. Clin J Oncol Nurs 2017;21:30–41. 10.1188/17.CJON.S4.30-41 [DOI] [PubMed] [Google Scholar]
- 45. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691–7. 10.1200/JCO.2012.41.6750 [DOI] [PubMed] [Google Scholar]