Abstract
Introduction
Hantaviruses are maintained by mammalian hosts, such as rodents, and are shed in their excretions. Clinical disease can occur in humans from spillover infection. Brown rats (Rattus norvegicus) are the globally distributed reservoir host of Seoul virus (SEOV). Human cases of SEOV-associated haemorrhagic fever with renal syndrome (SEOV-HFRS)have been reported in Great Britain (GB) since 1977.
Methods
Brown rats (n=68) were trapped from a variety of peridomestic locations, with a focus on pig farms. Kidney and lung tissues were tested for viral RNA using a pan-hantavirus RT-PCR assay followed by Sanger sequencing and analysis.
Results
SEOV RNA was detected in 19 per cent (13/68, 95% CI 11 to 30) of rats and all sequences fell within SEOV lineage 9. Twelve sequences were highly similar to each other and to the previously reported GB Humber strain of SEOV (98 per cent). One rat SEOV sequence was more distant. The SEOV prevalence in rats from pig farms was significantly greater (p=0.047) than other sites sampled. No significant sex or age differences were observed among positive and negative rats.
Discussion
The results from this study suggest that SEOV could be widespread in wild rats in GB and therefore pose a potential risk to public health.
Keywords: disease surveillance, epidemiology, infectious diseases, virology, wildlife, zoonoses
Introduction
Hantaviruses are trisegmented RNA viruses that belong to the genus Orthohantavirus, which contains 35 recognised species.1 Hantaviruses establish persistent infections in their reservoir mammalian hosts, such as rodents, bats and insectivores.2 3 These reservoir hosts are capable of maintaining the infection without developing clinical signs or the immune-mediated pathology sometimes seen when these viruses infect humans.4 Hantaviruses replicate in the reservoir hosts’ cells and are subsequently shed in urine, faeces and saliva. It is through the inhalation of aerosolised viruses from these excretions that humans become infected.5
The severity of the hantavirus infection in humans can vary from asymptomatic to fatal, largely dependent on the virus species. Hantavirus infections were differentiated clinically and geographically into two syndromes. Hantavirus pulmonary syndrome (HPS), which has a mortality rate of 35–50 per cent,6 is mostly reported in the Americas and haemorrhagic fever with renal syndrome (HFRS), with a mortality rate of 1–15 per cent, predominates in Europe and Asia.7 However, due to the global distribution of Seoul virus (SEOV)-associated HFRS (SEOV-HFRS) and the overlap between the syndromes, such as pulmonary involvement in HFRS cases8 and acute kidney injury in HPS cases, it has more recently been recommended to describe the clinical syndromes as ‘hantavirus fever’ or ‘hantavirus disease’ to avoid misdiagnosis.9 10
SEOV-HFRS was first reported in Great Britain (GB) in 1977,11 and there have been several reports of human clinical disease and of hantavirus infection (RNA or seropositivity) in British brown rats (Rattus norvegicus).12 Pet rats (also R norvegicus),13 of which there are an estimated 100,000 in 24,000 GB households,14 have been confirmed as a source of SEOV-HFRS in their owners.13 SEOV may be widespread throughout the British pet rat community, as 34.1 per cent of pet rat owners have been shown to have hantavirus antibodies15 and a high hantavirus prevalence has been detected in breeding colonies of pet rats.12 Thus far, although not all identical, the UK strains of SEOV detected in pet, wild and laboratory rats have all belonged to lineage 9 of SEOV.12
In 2011, a 59-year-old man who worked on a rat-infested pig farm in Yorkshire, GB, was diagnosed with SEOV-HFRS, and SEOV was detected in wild rats from the same pig farm.16 However, very little is known about SEOV in British wild rats in terms of distribution or prevalence and therefore the public health risk is unclear. In this report, we describe a study of hantavirus surveillance in wild brown rats in Northern England and Wales in order to begin to better understand the epidemiology of this infection, and therefore its public health risk.
Materials and methods
Rats
Brown rats (n=68) were collected from various peridomestic locations across Northern England and Wales, which included pig farms, smallholdings and urban environments (figure 1). Rats were live trapped and humanely killed, donated from pest control programmes or collected as road kill. Each rat was examined postmortem and the kidney and lung tissues removed.
Figure 1.
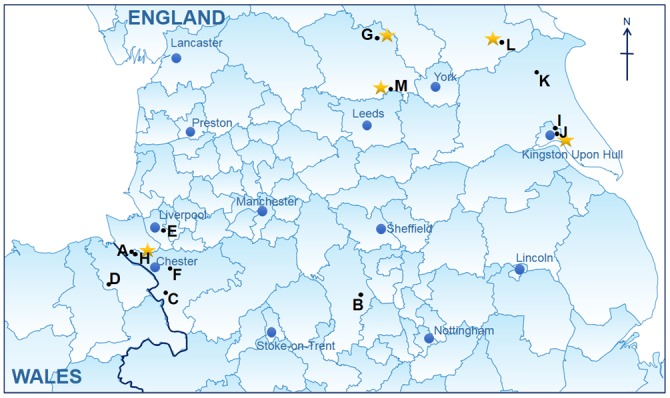
Locations of sites at which rat (Rattus norvegicus) samples were collected and correspond to table 1. Letters correspond to the sites which rats were collected and stars indicate the sites where hantavirus Seoul virus (SEOV)-positive rats were detected. This map was created using GQIS Desktop V.3.2.3 software.
RNA extraction
A 50–100 mg sample of tissue (kidney or lung) from each rat was homogenised using a motor pestle (Sigma-Aldrich, Dorset, UK) with 1 ml of TRIzol Reagent (Thermo Fisher Scientific, Leicestershire, UK). The extraction was performed according to the manufacturer’s instructions.
Nested RT-PCR
A pan-hantavirus reverse transcriptase (RT)-PCR assay was used to screen the kidney and lung tissues of the rats for viral RNA. A One-Step RT-PCR Kit (Qiagen, Manchester, UK) was used for the first round of the PCR with first-round primers17 and 1 µl of extracted RNA. In a thermocycler (BioRad, Watford, UK), a reverse transcriptase step was performed at 50°C for 30 minutes followed by 95°C for 15 minutes, then 45 cycles of 94°C for 30 seconds, 53°C for 30 seconds and 72°C for one minute followed by 72°C for seven minutes. In the second round, 1 µl of the first-round PCR products was added to a reaction mix containing the second-round primers17 and the HotStarTaq Plus Master Mix (Qiagen, Manchester, UK). Cycling parameters were 95°C for five minutes followed by 40 cycles at 94°C for 30 seconds, 53°C for 30 seconds then 72°C for one minute, then a final elongation step at 72°C for seven minutes. PCR products from both rounds (452 and 390 bp) were visualised under UV light after gel electrophoresis on a 1.8 per cent agarose gel at 120 V for 75 minutes.
Phylogenetic analysis
PCR products from the pan-hantavirus RT-PCR assay were Sanger sequenced and nucleotide sequences analysed using a DNAStar Lasergene software package. SeqMan Pro was used to assemble contiguous forward and reverse sequences and remove primer sequences. Consensus sequences were uploaded into MegAlign (DNAStar Lasergene software) and aligned using the ‘ClustalW’ method, and compared with published sequences using the nucleotide Basic Local Alignment Search Tool program produced by National Center for Biotechnology Information. Phylogenetic analysis was conducted in MEGA718 and the sequences from this study (GenBank accession numbers MK492669, MK492670, MK492671, MK492672 and MK492673) were aligned with other related hantavirus sequences. A phylogenetic tree of maximum likelihood was constructed using a best fit model19 and bootstrap analysis was performed with 1000 repeats.
Results
Using the RT pan-hantavirus assay (table 1), 19 per cent (13/68, 95% CI 11% to 30%) of brown rats were positive for hantavirus RNA. Sanger sequence analysis of amplicons confirmed the infection as being with SEOV. SEOV-infected rats were detected at 4/6 sites in the Yorkshire region (figure 1), and at another site in Cheshire at which 3/10 brown rats tested were SEOV positive (table 1). All but one positive rat was from a pig farm and the SEOV prevalence in rats from pig farms (26 per cent, 95% CI 16% to 40%) was significantly greater than from other site samples combined (5 per cent, 95% CI 0.8% to 21%) by Fisher’s exact test (two tailed P=0.047). There was no statistically significant association between infection and age or sex of the rats.
Table 1.
Hantavirus infection in brown rats using a pan-hantavirus RT-PCR assay
| Year | Site and map ID | Site type | Location | Rattus norvegicus collected | Hantavirus positive (%) |
| 2014 | A | Dairy farm | Cheshire | 5 | 0/5 (0)* |
| 2015 | B | Beef farm | Derbyshire | 1 | 0/1 (0)* |
| 2015 | C | Beef farm | Cheshire | 6 | 0/6 (0)* |
| 2015 | D | Smallholding | Denbighshire | 1 | 0/1 (0)* |
| 2015 | E | Urban | Merseyside | 4 | 0/4 (0)* |
| 2015 | F | Residential | Cheshire | 4 | 0/4 (0)* |
| 2015 | G | Pig Farm | North Yorkshire | 16 | 2/16 (12.5) |
| 2015–2016 | H | Pig farm | Cheshire | 10 | 3/10 (30) |
| 2015 | I | Pig farm | East Yorkshire | 1 | 0/1 (0) |
| 2015 | J | Road kill | East Yorkshire | 1 | 1/1 (100) |
| 2015 | K | Pig farm | East Yorkshire | 2 | 0/2 (0) |
| 2015 | L | Pig farm | North Yorkshire | 1 | 1/1 (100) |
| 2015 | M | Pig farm | West Yorkshire | 16 | 6/16 (37.5) |
| Total | 68 | 13/68 (19) |
PCR was performed on lung and kidney tissues except those marked by asterisks.
*Tested using only kidney tissue.
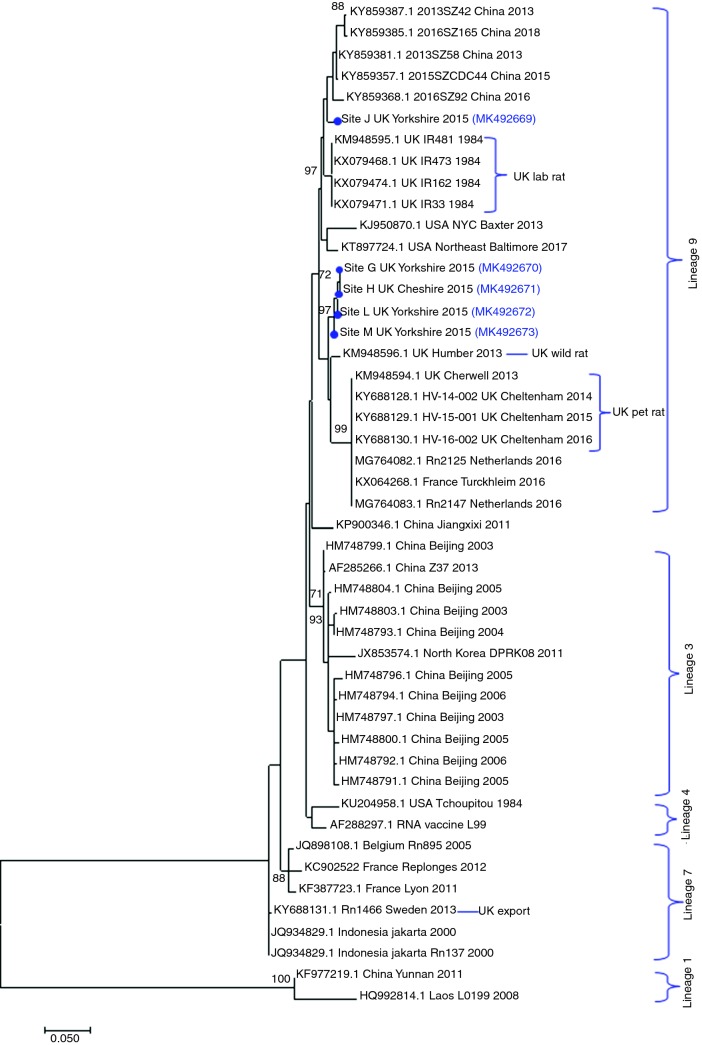
Phylogenetic analysis showed that all the SEOV sequences generated in this study belonged to lineage 9, as do all previous British SEOV and others from western Europe (figure 2).12 Furthermore, with one exception, all the rat SEOV sequences in this study were closely related (98 per cent identity at the nucleotide level) to the Humber strain, previously identified in a wild rat from Yorkshire in 2013.20 These wild rat strains differed from those detected in British pet rats12 and the 1984 laboratory rat strain.21 One wild rat SEOV strain in this study (R62, site J) was distinguishable from other UK wild rat SEOV sequences.
Figure 2.
Phylogenetic tree of Seoul virus (SEOV) sequences including those detected in this study and other strains detected in Great Britain (GB). The evolutionary history was inferred by using the maximum likelihood method based on the Tamura 3-parameter model plus gamma.19 The analysis involved 47 nucleotide sequences (published and sequences from this study) of a partial 329 nt fragment on the L-segment. Sequences from this study are shown with a blue dot with the corresponding GenBank accession numbers. Bootstrap values of at least 70 are shown. Evolutionary analyses were conducted in MEGA7.18
Discussion
The results of this study indicate that SEOV is geographically widespread, but not uniformly distributed in British wild rats. Transmission of hantaviruses within rodent populations is complex and varies not only with the virus-host combination but over time, with population density, behaviour and landscape or environment, all of which may be interlinked. These various factors cannot be distinguished in this study, but the geographic spread and finding of two strains suggest that SEOV is well established in British rats and not recently imported.
All but one of the SEOV-infected wild rats was collected from a pig farm and the remaining positive rat was collected as road kill adjacent to a pig farm. Most of the pig farms sampled were in Northern England, reflecting regional differences in farming in England that cannot be differentiated from simple geographic landscape differences that might also affect transmission among rats. Pounder22 found no SEOV RNA in 133 rats sampled in semirural and urban areas in Northwest England, and the only positive site in this region in this study was a pig farm. Furthermore, an unpublished study of 27 rats in rural Gloucestershire found none to be infected (Animal and Plant Health Agency unpublished data). It is not clear if pig farms really are more likely to harbour infected rats than other sites, and if so whether or not this reflects particularly suitable environments for sustaining large populations of brown rats and hantavirus transmission, or, indeed, if pig farms simply enable easier trapping, and therefore sampling, of rats.
There were six sites that had SEOV-infected rats, four of which were in the Yorkshire region, which raises the question of whether there might be a higher risk of SEOV infection in this region. The first GB wild rat SEOV strain to be reported, the Humber strain, originated from the Yorkshire region.20 Seroprevalence to SEOV has already been demonstrated in farmers from the Yorkshire region.23 The results from our study confirm that the 2013 detection of SEOV in a wild rat20 was not an isolated incident, as multiple pig farms across the Yorkshire region were shown to contain SEOV-positive rats. However, the results from this study are not sufficient to suggest a greater risk of SEOV transmission from wild rats in Yorkshire as the larger proportion of samples in this study (37/68, 54 per cent) were derived from this region, which could bias the results. Further surveillance in a wider study of multiple rat populations across GB would be needed to compare and statistically examine whether there is a greater public health risk of SEOV infection in the Yorkshire region. It may also be interesting to compare other UK regions with high numbers of pig farms, such as Norfolk or Wiltshire, to ascertain if there is an additional occupational risk associated with pig farming and SEOV infection.
SEOV transmission among rats can be direct or indirect, but has been associated in several studies with aggressive behaviour and wounding. As population pressure increases so does the aggressive behaviour towards members of the established colonies due to the increased competition for food and territory.24 Higher numbers of rats with more frequent aggressive biting encounters could lead to an increase in SEOV transmission in a population via infected saliva.25 This has also been proposed in infected pet rats that were housed with non-infected rats in close proximity, SEOV easily spreads throughout the entire colony12. Previous studies have also found male rats are more likely to be infected with SEOV than female rats,26 27 which has been linked to more aggressive encounters between males.24 25 28 Increased prevalence with age has also been reported.25 29 No obvious difference in prevalence between the sexes or with age was found in this study, however, probably owing to relatively small sample sizes.
All of the GB SEOV rat strains so far identified, including those from this study, are members of lineage 9 (figure 2), with the exception of one SEOV rat strain detected in a pet rat which was exported from GB to Sweden, which was lineage 7.12 Within lineage 9, two different SEOV clusters of strains were identified in rats in this study, one closely related to the Humber strain, previously has been identified in wild rats in Yorkshire,20 and one (R62, site J) that clustered with SEOV sequences from Chinese rats, laboratory rat SEOV strains and a Baxter SEOV strain identified in rats in New York City, USA.30 The patterns of genetic diversity observed likely reflect both the recent and longer term movement of the reservoir host. Brown rats are known to commonly travel 0.5 km within a day, with the longest overnight single journey recorded being 3.3 km.23 This movement might explain widespread dispersal SEOV strains across England. On the other hand, SEOV, like its host the brown rat, is believed to have originated from China. Brown rats first arrived in Europe only some 300 years ago. The greatest genetic diversity of both rats and SEOV is found in China, with particular lineages (in the case of SEOV phylogroup A) associated with migration westwards and, through shipping trade, globally.31 Sequences with high genetic similarity within lineage 9 of SEOV have been detected in farmed rats in mainland Europe, in countries such as the Netherlands32 and France,33 as well as identified in a human SEOV-HFRS case in Germany.34 The detection of highly similar, but distinguishable, sequences in England may reflect the multiple rat migration events that have occurred since rats arrived in GB. If so, then the increased detection of infected brown rats is likely not owing to SEOV being an emerging pathogen but ratheran increased interest, more surveillance studies and improved diagnostics.
The presence of SEOV in rat populations in different geographical locations of GB does raise the question of why there have not been more reported human cases linked to exposure to SEOV from wild rats as the majority of cases of confirmed UK SEOV-HFRS are as a result of exposure to infected pet rats.12 This could be due to the different exposure levels people have with pet and wild rats. Pet rat owners often have a close relationship with their pets and there is often close contact through handling and shared living quarters as well as frequent exposure to rat excretions through the cleaning of rat cages. There is also a high rate of transmission between rats which are kept in breeding colonies in confined spaces, as it has been shown that once the virus has been introduced it can easily spread throughout the entire colony.12 This could explain why there are more human cases due to exposure to SEOV relating to pet rats rather than wild rats. Although this study has detected multiple locations with SEOV-positive rats, the risk of exposure of SEOV from wild rats may be less than in pet rats as there is less direct interaction and exposure is likely to be coincidental when living, working or visiting an area with an infected rat population. However, SEOV has been shown to cause clinical human disease that can sometimes be severe, therefore the presence of this virus in the British wild rat population cannot be ignored as a potential public health risk. Further study would be required to determine if there are certain geographical areas or certain professions where the risk of infection could be higher.
Conclusion
The study has demonstrated that SEOV may be widespread in British rats, although this distribution is not uniform and the strains in wild rats are genetically distinguishable from those reported in UK pet and lab rats. SEOV can cause severe disease in humans, and wild rat populations are said to be increasing in GB, therefore this is clearly of public health concern. However, the transmission dynamics of SEOV among rats is likely to be complex, and transmission to humans even more so, therefore further work needs to be done to understand the epidemiology of this virus and the public health risk.
Footnotes
Contributors: Conceived and designed the experiments: EGM, MB, LM, NJW, JC. Collected samples: EGM. Performed the experiments: EGM, DJ. Collated, analysed and interpreted the data: EGM, DJ, LM, MB, NJW. All authors contributed to and approved the final manuscript.
Funding: This research was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Emerging and Zoonotic Infections at the University of Liverpool in partnership with Public Health England (PHE), in collaboration with Liverpool School of Tropical Medicine. EGM is based at the University of Liverpool. This work was produced in collaboration with the Animal and Plant Health Agency and was also supported by funding from Defra, the Scottish Government and the Welsh Government through grant SV3045 and the EU Horizon 2020 research and innovation programme under grant agreement number 653316 (EVAg).
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Competing interests: None declared.
Ethics approval: The study was approved by the University of Liverpool Veterinary Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.ICTV. International Committee on Taxonomy of Viruses (ICTV) [Internet]. Virus Taxonomy: 2018 Release, 2018. https://talk.ictvonline.org/taxonomy/ (cited 15 Jan 2019).
- 2.Meyer BJ, Schmaljohn C. Accumulation of terminally deleted RNAs may play a role in Seoul virus persistence. J Virol 2000;74:1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YZ. Discovery of hantaviruses in bats and insectivores and the evolution of the genus Hantavirus. Virus Res 2014;187:15–21. 10.1016/j.virusres.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 4.McCaughey C, Hart CA. Hantaviruses. J Med Microbiol 2000;49:587–99. 10.1099/0022-1317-49-7-587 [DOI] [PubMed] [Google Scholar]
- 5.Hansen A, Cameron S, Liu Q, et al. Transmission of haemorrhagic fever with renal syndrome in china and the role of climate factors: a review. Int J Infect Dis 2015;33:212–8. 10.1016/j.ijid.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 6.Kruger DH, Figueiredo LT, Song JW, et al. Hantaviruses—Globally emerging pathogens. J Clin Virol 2015;64:128–36. 10.1016/j.jcv.2014.08.033 [DOI] [PubMed] [Google Scholar]
- 7.CDC. CDC - Hemorrhagic Fever with Renal Syndrome (HFRS) - Hantavirus [Internet]. 2018https://www.cdc.gov/hantavirus/hfrs/index.html (cited 15 Jan 2019).
- 8.Connolly-Andersen AM, Ahlm K, Ahlm C, et al. Puumala virus infections associated with cardiovascular causes of death. Emerg Infect Dis 2013;19:126–8. 10.3201/eid1901.111587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement J, Maes P, Van Ranst M. Hemorrhagic Fever with Renal Syndrome in the New, and Hantavirus Pulmonary Syndrome in the old world: paradi(se)gm lost or regained? Virus Res 2014;187:55–8. 10.1016/j.virusres.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 10.Clement J, Maes P, Saegeman V, et al. Comment on “A Cluster of Three Cases of Hantavirus Pulmonary Syndrome among Canadian Military Personnel”. Can J Infect Dis Med Microbiol 2016;2016:1–3. 10.1155/2016/7458409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd G, Jones N. Infection of laboratory workers with hantavirus acquired from immunocytomas propagated in laboratory rats. J Infect 1986;12:117–25. [DOI] [PubMed] [Google Scholar]
- 12.McElhinney LM, Marston DA, Pounder KC, et al. High prevalence of Seoul hantavirus in a breeding colony of pet rats. Epidemiol Infect 2017;145:3115–24. 10.1017/S0950268817001819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElhinney L, Fooks AR, Featherstone C, et al. Hantavirus (Seoul virus) in pet rats: a zoonotic viral threat. Vet Rec 2016;178:171.3–2. 10.1136/vr.i817 [DOI] [PubMed] [Google Scholar]
- 14.PFMA. Pet Population Statistics [Internet]. 2018. https://www.pfma.org.uk/pet-population-2018 (cited 15 Jan 2019).
- 15.Duggan JM, Close R, McCann L, et al. A seroprevalence study to determine the frequency of hantavirus infection in people exposed to wild and pet fancy rats in England. Epidemiol Infect 2017;145:2458–65. 10.1017/S0950268817001480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams K, Jameson L, Meigh R, et al. Hantavirus: an infectious cause of acute kidney injury in the UK. BMJ Case Rep 2014;2014:bcr2014205529 10.1136/bcr-2014-205529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klempa B, Fichet-Calvet E, Lecompte E, et al. Hantavirus in African wood mouse, Guinea. Emerg Infect Dis 2006;12:838–40. 10.3201/eid1205.051487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 2016;33:1870–4. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 2004;101:11030–5. 10.1073/pnas.0404206101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jameson LJ, Logue CH, Atkinson B, et al. The continued emergence of hantaviruses: isolation of a Seoul virus implicated in human disease, United Kingdom, October 2012. Euro Surveill 2013;;18:4–7. [PubMed] [Google Scholar]
- 21.Shi X, McCaughey C, Elliott RM. Genetic characterisation of a Hantavirus isolated from a laboratory-acquired infection. J Med Virol 2003;71:105–9. 10.1002/jmv.10446 [DOI] [PubMed] [Google Scholar]
- 22.Pounder KC. Targeted surveillance for Ljungan virus and Hantaviruses in UK rodents. University of Liverpool 2013. [Google Scholar]
- 23.Jameson LJ, Newton A, Coole L, et al. Prevalence of antibodies against hantaviruses in serum and saliva of adults living or working on farms in Yorkshire, United Kingdom. Viruses 2014;6:524–34. 10.3390/v6020524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris S, Yalden D. Mammals of the British Isles: Handbook. The Mammal Society 2008. [Google Scholar]
- 25.Hinson ER, Shone SM, Zink MC, et al. Wounding: the primary mode of Seoul virus transmission among male Norway rats. Am J Trop Med Hyg 2004;70:310–7. [PubMed] [Google Scholar]
- 26.Escutenaire S, Chalon P, De Jaegere F, et al. Behavioral, physiologic, and habitat influences on the dynamics of Puumala virus infection in bank voles (Clethrionomys glareolus). Emerg Infect Dis 2002;8:930–6. 10.3201/eid0809.010537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein SL, Bird BH, Glass GE. Sex differences in immune responses and viral shedding following Seoul virus infection in Norway rats. Am J Trop Med Hyg 2001;65:57–63. [DOI] [PubMed] [Google Scholar]
- 28.Hinson ER, Hannah MF, Norris DE, et al. Social status does not predict responses to Seoul virus infection or reproductive success among male Norway rats. Brain Behav Immun 2006;20:182–90. 10.1016/j.bbi.2005.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dohmae K, Koshimizu U, Nishimune Y. In utero and mammary transfer of hantavirus antibody from dams to infant rats. Lab Anim Sci 1993;43:557–61. [PubMed] [Google Scholar]
- 30.Firth C, Bhat M, Firth MA, et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. MBio 2014;5:e01933–14. 10.1128/mBio.01933-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin XD, Guo WP, Wang W, et al. Migration of Norway rats resulted in the worldwide distribution of Seoul hantavirus today. J Virol 2012;86:972–81. 10.1128/JVI.00725-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanink C, Reimerink J, Gisolf J, et al. Autochthonous Human Case of Seoul Virus Infection, the Netherlands. Emerg Infect Dis 2018;24:2158–63. 10.3201/eid2412.180229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynes JM, Carli D, Bour JB, et al. Seoul Virus Infection in Humans, France, 2014–2016. Emerg Infect Dis 2017;23:973–7. 10.3201/eid2306.160927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann J, Weiss S, Kuhns M, et al. Importation of Human Seoul Virus Infection to Germany from Indonesia. Emerg Infect Dis 2018;24:1099–102. 10.3201/eid2406.172044 [DOI] [PMC free article] [PubMed] [Google Scholar]