Abstract
The human opportunistic fungal pathogen Candida albicans causes a severe health burden while the biofilms formed by C. albicans present a kind of infections that are hard to cure, highlighting the pressing need for new antifungal drugs against C. albicans. This study was to explore the antifungal activities of lycorine hydrochloride (LH) against C. albicans. The minimal inhibitory concentration (MIC) of LH against C. albicans SC5314 was 64 μM. Below its MIC, LH demonstrated antivirulence property by suppressing adhesion, filamentation, biofilm formation, and development, as well as the production of extracellular phospholipase and exopolymeric substances (EPS). The cytotoxicity of LH against mammalian cells was low, with half maximal inhibitory concentrations (IC50) above 256 μM. Moreover, LH showed a synergistic effect with AmB, although its interaction with fluconazole, as well as caspofungin, was indifferent. Thus, our study reports the potential use of LH, alone or in combination with current antifungal drugs, to fight C. albicans infections.
1. Introduction
The opportunistic fungal pathogen Candida albicans usually lives in oral cavity, gastrointestinal tract, and urogenital tract and on the skins as a commensal, but when the immune function was compromised, this fungus could cause a series of diseases such as oral thrush, vaginitis, and life-threatening bloodstream infections [1]. C. albicans cells can undergo the yeast-to-hyphal transition in response to the stimuli encountered in hosts, such as body temperature and epithelial cell contact, to facilitate its survival [2, 3]. The regulation of this transition involves multiple signaling pathways, including Ras1-cAMP-PKA signaling [4, 5]. Hyphae are also critical for tissue invasion and for maintaining the structures of C. albicans biofilms, the formation of which starts from adhesion to biotic or abiotic surfaces [6, 7]. Biofilms formed by C. albicans on the surfaces of medical devices, such as catheters and prosthetic joints, are often difficult to eradicate, because the condensed cell population and exopolymeric substances (EPS) in biofilms construct a physical barrier, in addition to its elevated expression of drug efflux pumps [7, 8]. Under these circumstances, replacing devices is often necessary, thus imposing a heavy burden on both public health systems and individual patients [8]. The currently used drugs (such as azoles, amphotericin B, and caspofungin) have been associated with resistance, side effects, or low oral bioavailability, while only caspofungin and the lipid formation of Amphotericin B are active against C. albicans biofilms, thus making developing new antifungal agents, as well as agents that can improve the efficacy of current antifungal drugs, a pressing mission [9, 10].
Lycorine hydrochloride (LH, Figure 1(a)) is the major active constituent isolated from the medicinal herb Lycoris radiata. It has suppressing effects on the biosynthesis of ascorbic acid in potato tubers [11]. This compound has shown potent antileukemia and antitumor activities against renal cell carcinoma (RCC), ovarian, lung, breast, glioblastoma, melanoma, and esophageal cancer cells at low concentrations through cell cycle arrest and apoptosis induction [12–15]. LH could synergize with cytotoxic T-lymphocyte associated protein 4 (CTLA-4, an immune checkpoint inhibitor) in suppressing RCC in a mouse model [15]. In addition, it could also inhibit the vasculogenic activity of melanoma cells in vitro and block the production of blood vessels in vivo [16]. What is more important, this compound owns very low toxicity in normal cell lines, as well as in the animal models [12, 13, 16], making it a very promising anticancer candidate.
Figure 1.
The chemical structure of lycorine hydrochloride (LH) and the Time-killing assay of LH against C. albicans SC5314. The initial inoculum of the assay was 106 cells/mL and the incubation was performed at 37°C. ∗ means p < 0.05 compared to drug-free controls.
However, the effects of LH against the human pathogenic fungus C. albicans have never been elucidated, although lycorine has been reported to have antifungal activities against C. albicans and C. dubliniensis [17, 18]. In this study, we first evaluated the antifungal activity of LH against the planktonic cells as well as the biofilms of C. albicans. The effects of LH on the virulence factors of C. albicans were also investigated.
2. Materials and Methods
2.1. Chemicals, Strains, and Growth Conditions
LH was bought from National Institutes of Food and Drug Control of China. RPMI-1640 medium powder, 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT), 2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), menadione, morpholinepropanesulfonic acid (MOPS), and dibutyryl-cAMP (db-cAMP) were bought from Sigma-Aldrich (Shanghai, China). LH was dissolved in DMSO and stored at -20°C.
C. albicans SC5314, C. albicans ATCC90028, Candida glabrata ATCC2001, Candida parapsilosis ATCC22019, and Candida tropicalis ATCC7349 bought from China General Microbiological Culture Collection Center (CGMCC) were maintained on yeast extract-peptone-dextrose (YPD) agar medium (1% yeast extract, 2% peptone, 2% dextrose, and 2% agar). Before each test, a colony was picked up and transferred into 5 mL YPD medium in a sterile tube and incubated overnight at 28°C with rotation (140 rpm).
2.2. Antifungal Susceptibility Assay
The minimal inhibitory concentrations (MICs) of LH against Candida species were determined following microdilution methods from Clinical and Laboratory Standard Institute (CLSI-M27-A3). Overnight grown fungal cultures in YPD medium were collected by centrifugation and diluted to 2 x 103 cells/mL in RPMI-1640 medium (without sodium carbonate, buffered to pH 7.0 with 0.165 M MOPS). 100 μL of such cell suspension was added into each well of 96-well plates. LH was added into each well through serial dilution to achieve various concentrations (4-256 μM). After incubation at 35°C for 24 h, the lowest concentration at which no visual growth was observed was defined as the MIC.
20 μL cell suspension from wells challenged with MIC, 2MIC, 4MIC, and 8MIC of LH was taken and smeared on YPD agar. After incubation at 37°C for 24 h, the colony forming units (CFU) of each well were counted. The minimum fungicidal concentration (MFC) was defined as the lowest concentration at which no colony of fungal strains was grown on the agar plate [19]. The value of MFC divided by MIC was used to judge whether LH had a fungistatic (MFC/MIC > 4) or fungicidal (MFC/MIC < 4) effect [20].
2.3. Time-Killing Kinetics
To further confirm the fungicidal or fungistatic effect of LH, time-killing assays were performed. Cell suspensions prepared from overnight YPD cultures were diluted to a density of 106 cells/mL in 1640 medium and 5 mL of such suspension was transferred into each testing tube where C. albicans cells were treated with different concentrations of LH. The fungal cells in tubes were grown at 28°C with a rotation of 140 rpm. At 2, 4, 6, 8, 12, and 24 h after the addition of LH, 100 μL cells suspension from each tube was taken out, diluted, and plated on YPD agars. These agars were incubated for 24 h at 37°C before the numbers of CFUs were counted. This assay was performed in triplicate and repeated for three times.
2.4. Adhesion Assay
The influence of LH on the adhesion capacity of C. albicans on polystyrene surfaces was evaluated by XTT reduction assay, as we described elsewhere [21]. In brief, 100 μL of fungal cell suspension (106 cells/mL in 1640 medium) diluted from overnight YPD culture was transferred into each well of 96-well plates and incubated with 0, 16, 32, and 64 μM of LH at 37°C for 1.5 h. Then, wells were washed with PBS and subjected to XTT assay.
2.5. Antibiofilm Assay
The antibiofilm activity of LH against C. albicans SC5314 was assessed in 96-well plates [22]. In brief, overnight grown fungal cells in YPD medium were collected by centrifugation (3000g, 5 minutes) and resuspended in RPMI-1640 medium at a density of 106 cells/mL. 100 μL of such suspension was added into wells of 96-well plate. After incubation at 37°C for 24 h, biofilms were formed in each well. Wells containing only medium serve as blank controls.
To test the activity of LH on the biofilm formation, different concentrations of LH were added into wells with cell suspension and plates were incubated for 24 h at 37°C. Free-floating cells in each well were removed by washing with PBS for three times and later the biofilm viability in each well was quantified by XTT reduction assay.
To assess the activity of LH on the preformed biofilms, fresh 1640 medium containing different concentrations of LH was added into each well after 24 h biofilms were washed with PBS. After another incubation for 24 h and PBS washing, XTT reduction assay was performed. These assays were performed in triplicate and repeated for three times.
2.6. XTT Reduction Assay
Sterile XTT solution (50 g/L in PBS) was mixed with menadione (final concentration: 1μM) before 100 μL of the mixture was added into each well and incubated in dark for 120 minutes at 37°C. Then, 70 μL of supernatant from each well was transferred into a new 96-well plate and the optical density (OD) at 490 nm of the solution in each well was determined by a microplate reader (VarioSkan, Thermo). The viability of biofilm formed in each well was calculated as viability % = (OD490nm treatment − OD490nm blank)/(OD490nm control − OD490nm blank) × 100%, where OD490nm treatment and OD490nm control mean OD490nm values of treated biofilms and untreated biofilms while OD490nm blank is OD490nm values of wells containing only medium without fungal cells [22].
2.7. Morphological Transition
To test the influence of LH on the morphological transition of C. albicans cells, overnight grown fungal cells were collected and resuspended in three kinds of hyphal-inducing media, namely, RPMI-1640 medium, Spider medium (1% mannitol, 1% nutrient broth, 0.2% K2HPO4, pH 7.2), and Sabouraud dextrose (SD) medium (4% dextrose, 1% peptone) plus 10% fetal bovine serum (FBS), to achieve a density of 106 cells/mL. The cell suspensions were supplemented with different final concentrations of LH and incubated at 37°C for 4 h statically. Morphological changes in fungal cells were recorded by an inverted microscope (Olympus IX81, Japan).
On the Spider agar (1% mannitol, 1% nutrient broth, 0.2% K2HPO4, 1.8% agar, pH 7.2), C. albicans colonies employ the filamentous growth, and therefore it was used to assess the effects of LH on the morphological changes of fungal colonies [23]. Overnight C. albicans cells in YPD medium were collected and resuspended in PBS to get a density of 500 cells/mL. 100 μL of such suspension was plated on Spider agars supplemented with different concentrations of LH and plates were incubated at 37°C for 96 h. Morphologies of colonies were photographed by an anatomical microscope (Olympus SZX16, Japan).
2.8. cAMP Rescue Experiment
Db-cAMP was used to investigate the involvement of cAMP in the inhibitory effect of LH on morphological changes. In this assay [23], C. albicans cells in 1640 medium (106 cells/mL) were transferred into 96-well plates and cells were exposed to 32 μM LH. Db-cAMP was added into cell suspensions immediately after the addition of LH to achieve a final concentration of 5 mM. Cells treated with the same volume of DMSO or db-cAMP were set as negative controls. After incubation for 4 h at 37°C, morphological changes of cells were recorded by a microscope. These four groups (DMSO, LH, db-cAMP, and LH + db-cAMP) were also used for biofilm formation assay as mentioned above to evaluate the involvement of cAMP in the antibiofilm activity of LH. After treatment for 24 h, the viability of biofilms in each group was determined by XTT reduction assay. This assay was performed in triplicate and repeated for three times.
2.9. Phospholipase Production
The influence of LH on the extracellular phospholipase production was evaluated as described elsewhere [24]. In brief, 1 μL of cell suspension (106 cells/mL in 1640 medium) was spotted onto the center of egg yolk emulsion agar supplemented with different concentrations of LH. After 4 days' incubation at 37°C, diameters of colonies (d1) and precipitation zones (d2) surrounding the colonies were measured. The production of phospholipase was expressed in Pz: Pz=d1/d2. This assay was performed in triplicate and repeated for three times.
2.10. EPS Production of C. albicans Biofilms
The EPS production in preformed biofilms was determined by colorimetry [24]. Preformed biofilms in 24-well plates were challenged by different concentrations of LH for 24 h before biofilms were washed with 0.9% NaCl solution for three times. 0.2 mL NaCl solution and 0.2 mL 0.5% phenol solution were added into each well and mixed well. Then, 2 mL 0.2% hydrazine sulfate solution (in H2SO4) was slowly added and a two-hour incubation was followed. The OD490nm of the reaction products in each well was measured by a microplate reader.
2.11. Checkerboard Assays with Antifungal Drugs
To determine whether LH can synergize with current available antifungal drugs, checkerboard assays were performed as described elsewhere [25]. Fungal cell suspensions were prepared and cultured as described above in antifungal susceptibility tests. The concentrations for AmB and caspofungin acetate were 10 ~ 0.039 μg/mL, and for fluconazole it was 20 ~ 0.078 μg/mL. The concentration for LH was 256 ~ 4 μM. After 24 h incubation at 37°C, MIC of each drug was recorded to calculate the fractional inhibitory concentration (FIC). FICI = FICIA + FICIB = MICA combination/MICA alone + MICB combination/MICB alone. The combination is considered synergistic when FICI ≤ 0.5, indifferent when FICI > 0.5 and ≤ 4, and antagonist when FICI > 4.
2.12. Cytotoxicity against Human Cells
MTT assays performed on HUVEC and Chang's liver cells were used to assess the cytotoxicity of LH, as previously described elsewhere [26].
2.13. Statistical Analysis
Each assay was performed in triplicate and repeated for at least three times. Graphs shown were produced by GraphPad Prism Software while data were presented as means with standard deviation (SD). Student's t-tests were performed to calculate the differences between treatment and drug-free controls. ∗, p<0.05.
3. Results
In the present study, we first examined the antifungal activity of LH against the five Candida strains belonging to four species according to CLSI guidelines. The MICs and MFCs were listed in Table 1. The MICs of LH against two C. albicans strains were 64 μM while the MFCs were above 256 μM (4MIC), as there were still colonies grown on the solid agar after fungal cells were exposed to this concentration for 24 h. Therefore, the MFC/MIC value is above 4, indicating a fungistatic effect. Due to the prevalence of C. albicans and the well-known genetic background of C. albicans SC5314, this strain was selected for further research. To further confirm the fungistatic effect of LH, the time-killing assay was performed. As shown in Figure 1(b), the concentration used (16-64 μM) did not decrease the viable cells in this assay, consistent with the fungistatic effect of LH revealed by the high value of MFC/MIC.
Table 1.
The antifungal activities of LH against Candida species.
| Fungal strains | MIC (μM) | MFC (μM) |
|---|---|---|
| C. albicans SC5314 | 64 | >512 |
| C. albicans ATCC10231 | 64 | 512 |
| C. glabrata ATCC2001 | 64 | 512 |
| C. parapsilosis ATCC22019 | 64 | >512 |
| C. tropicalis ATCC7349 | 128 | >512 |
As shown in Figure 2, 16-64 μM of LH significantly decreased the adhesion of C. albicans cells to polystyrene surfaces of microplates. At the highest concentration used (64 μM), LH could decrease about 80% of adhesion, as compared to drug-free controls.
Figure 2.
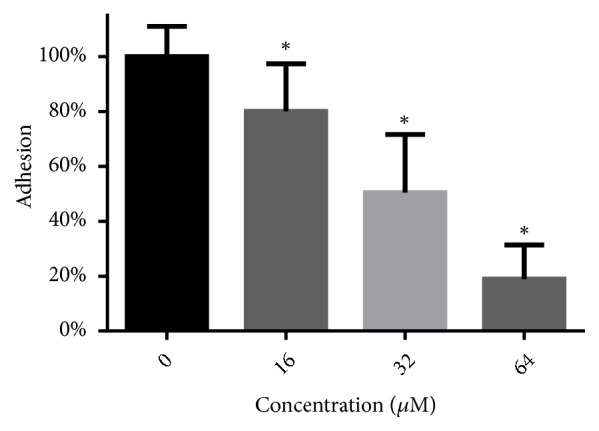
LH decreases the adhesion capacity of C. albicans to polystyrene surfaces. After treatment with LH for 1.5 hours at 37°C, XTT assays were performed to calculate the viability of cells left on polystyrene surfaces after PBS washing. ∗ means p < 0.05 compared to drug-free controls.
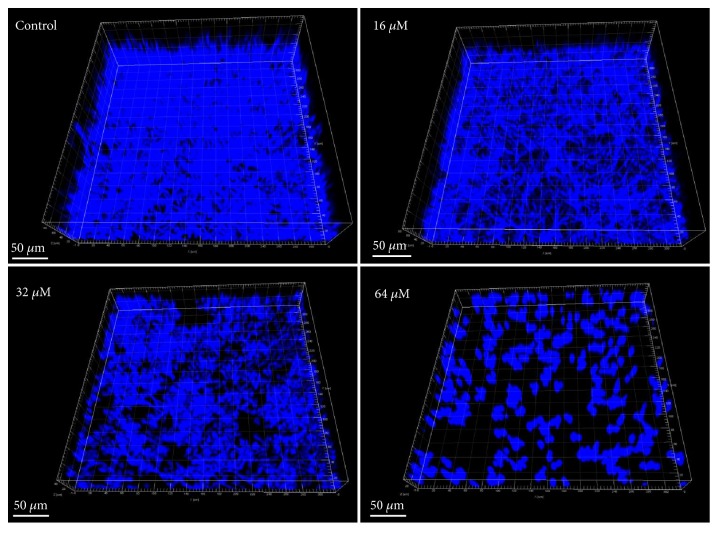
The antibiofilm activity of LH was quantified through XTT reduction assay. In the biofilm formation assay (Figure 3(a)), treatment with 16-64 μM of LH could significantly reduce the metabolic viability of C. albicans cells in biofilms. The antibiofilm activity of LH could also be seen in Figure 4, where the 3D structures of C. albicans biofilms were visualized by Imaris software using z-axis photographs recorded by confocal microscope. As shown in Figure 4, increasing the LH concentration would result in fewer hyphae and total C. albicans cells. As for preformed biofilms, exposure to 16-64 μM of LH could only reduce the viability of mature biofilms by 20%-30% (Figure 3(b)), as compared to drug-free controls.
Figure 3.
The effects of LH on the formation and development of C. albicans biofilm. (a) After incubation with different concentrations of LH under biofilm forming conditions for 24 h, the viability of biofilms in 96-well plates was determined by XTT reduction assay. (b) Preformed biofilms were further cultured for 24 h in the presence of LH, followed by XTT assay. ∗ means p < 0.05 compared to drug-free controls.
Figure 4.
The effects of LH on the formation of C. albicans biofilms. The 3D structures of C. albicans biofilms were reconstructed by Imaris 7.02 using z-axis photos recorded by confocal microscope.
To test the inhibitory effects of LH on filamentation in response to different stimuli (neutral pH and nutrition limitation), 1640 medium and Spider medium were used to induce hyphal formation. As expected, LH could suppress the hyphal formation in a concentration-dependent manner in both media tested (Figure 5). We also investigated the inhibitory capacity of LH on filamentation in the presence of serum, which is a strong inducer and is a key requirement for filamentation in the host. Although a little weaker inhibitory activity was displayed, LH did suppress the filamentation of C. albicans induced by serum (Figure 5). The filamentous growth on Spider agar could also be suppressed by LH, evidenced by shortened peripheral filaments around the central smooth colony in the presence of LH.
Figure 5.
LH inhibits the yeast-to-hyphal transition of C. albicans. 106 cells/mL C. albicans with various concentrations (0, 16, 32, and 64 μM) of LH in RPMI-1640 medium, spider medium, or SD broth supplemented with 10% FBS were incubated at 37°C for 4 h and recorded by an inverted microscope. Magnification, 40×. Morphologies of colonies on Spider agars were photographed by an anatomical microscope, after incubation at 37°C for 96 h.
In the hyphal growth, cAMP plays an important role. Thus, we tested whether cAMP was involved in the hyphal inhibition by LH. As shown in Figure 6(a), the addition of exogenous cAMP analog, namely, db-cAMP, could rescue the hyphal formation inhibition induced by LH exposure. This indicated that LH inhibits hyphal formation through decreasing cAMP in C. albicans cells. The effects of exogenous cAMP on the biofilm inhibition caused by LH were also tested, through XTT assay. As revealed by Figure 6(b), although the addition of 5 mM db-cAMP could rescue part of biofilm viability, the differences were not statistically significant.
Figure 6.
cAMP is involved in the inhibitory effects of LH on hyphal induction and biofilm formation of C. albicans. (a) The inhibitory effect of LH on hyphal formation could be rescued by addition of cAMP analog. (b) Treatment with db-cAMP saved part of cell viability of cells in biofilms challenged by LH.
Extracellular phospholipase production of C. albicans could also be decreased by treatment with LH, as evaluated by the egg yolk emulsion method. As shown in Figure 7, 16-64 μM of LH increased the Pz value significantly, in a concentration-dependent way.
Figure 7.
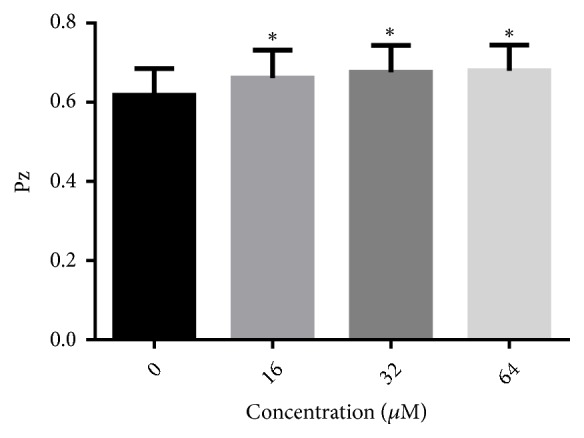
The effect of LH on the production of extracellular phospholipase of C. albicans. 1 μL of cell suspension (1x106 cells/mL) was spotted onto phospholipase agar and incubated at 37°C for 4 days. Pz value means the ratio of the diameter of colony to the diameter of colony plus the precipitation zone. The smaller Pz value indicates the stronger phospholipase activity. ∗ means p < 0.05 compared to drug-free controls.
EPS of preformed biofilm represents a physical barrier that prevents the access of antifungal drugs into cells within biofilms. The influence of LH on the EPS production in preformed C. albicans biofilms was evaluated by determining the OD490nm of the reaction products of EPS and hydrazine sulfate/phenol. As shown in Figure 8, preformed biofilms treated with 16-64 μM of LH produced less EPS, as compared to drug-free controls. This inhibition was also dose-dependent.
Figure 8.

LH inhibits the production of EPS in C. albicans biofilms. EPS production in preformed biofilms was determined by the phenol-sulfuric acid method. ∗ means p < 0.05 compared to drug-free controls.
To test whether LH can synergize with current available antifungal drugs, checkerboard assays were performed. As shown in Table 2, CAS and FLZ have no interactions with LH; that is to say, at least LH cannot impair or attenuate the efficacy of CAS and FLZ, if used together. A synergistic effect was observed between the combinations of LH and AmB.
Table 2.
Interaction of LH with antifungal drugs.
| Drug A | MIC of drug A (μg/mL) | MIC of LH (μM) | FICI | Interaction | ||
|---|---|---|---|---|---|---|
| Alone | Combined | Alone | Combined | |||
| AmB | 1.25 | 0.3125 | 64 | 16 | 0.5 | Synergistic |
| CAS | 0.625 | 0.3125 | 64 | 64 | 1.5 | Indifferent |
| FLZ | 1.25 | 0.625 | 64 | 64 | 1.5 | Indifferent |
The cytotoxicity of LH against mammalian cells was evaluated through proliferation inhibition assays. The half maximal inhibitory concentrations (IC50) of LH against both Chang's liver cells and HUVEC cells were above 256 μM (Table 3), indicative of its low cytotoxicity.
Table 3.
Cytotoxicity of LH against mammalian cell lines.
| Cell lines | IC50 (μM) |
|---|---|
| Chang's liver cells | >256 |
| HUVEC | >256 |
4. Discussion
C. albicans represents a major fungal pathogen, causing several kinds of infections including oral thrush, vaginitis, and candidemia [1]. The imperfect pharmacological properties of current antifungal drugs, along with resistance, make it a necessary and pressing mission to develop new antifungal agents, especially those effective against C. albicans biofilms, which are notorious in clinic context for its resistance and recalcitrance, and cause a heavy burden on patients with catheters [27]. Natural products, especially those from traditional medicinal herbs, represent a potential reservoir for mining antifungal agents [26–28]. LH, separated from the traditional Chinese herb Lycoris radiata which has been used for hundreds of years, has been reported to have antitumor activity against multiple kinds of cancer cell lines [12, 14–16, 29]. In this study, we for the first time report the in vitro antifungal activity of LH against the human fungal pathogen C. albicans.
The MICs of LH against C. albicans SC5314 and ATCC 10231 were both 64 μM, which was much lower than that of lycorine against C. albicans (about 200 μM) and C. dublinensis (about 100 μM) [17, 18]. The fungistatic effect of LH could be inferred from high MFC/MIC ratio (above 4), as well as nonreduction curves in the time-kill assays where a reduction of more than 3 log10 (CFU/mL) relative to initial inoculum was considered as fungicidal activity [24, 26]. Therefore, LH did exert a fungistatic effect.
Biofilms of C. albicans often represent a notorious life style that undermines the therapeutic efficacy of current antifungal drugs. Thus, we assessed the activity of LH against C. albicans biofilm, in both formation and development phases. As revealed by XTT assays, LH could inhibit the formation and development of C. albicans biofilms. The inhibition on biofilm formation could also be confirmed by CLSI images. The inhibition on preformed biofilms was weaker than that on biofilm formation, which can also be seen with many other antifungal agents [21, 24, 26]. This may be due to the condensed networks of intertwined hyphae and the existence of extracellular matrix, which compromise the access of drugs into cells within biofilms. As for the EPS, one of the major components of the extracellular matrix, LH could also decrease its production in preformed biofilms, and the extent of inhibition was similar to and consistent with its inhibition on the viability of preformed biofilms. This further indicates the potential of LH to combat C. albicans infections. In addition, adhesion, as the first move of C. albicans to form biofilm on biotic or abiotic surfaces, was also suppressed by treatment with LH. This suppression may be associated with decreased expression of adhesins, such as Hwp1 and Als3, which need to be further confirmed [30, 31].
Hyphal formation in C. albicans is closely associated with biofilm formation and other virulence factors, and mutants defective in hyphal formation simultaneously demonstrated defects in biofilm formation [32]. Hyphal cells can produce the cytolytic peptide toxin, candidalysin, to cause damage on mucosa [33]. Moreover, hyphal-specific Als3 of C. albicans can promote the iron acquisition from ferritin, thus compromising host nutritional immunity [3, 34]. Also, during hyphal growth, the expressions of PRA1 and ZRT1 genes are increased to promote zinc acquisition in milieu [35]. Therefore, the hyphal growth of C. albicans may represent a novel target to develop antivirulence strategies to fight C. albicans infections [36, 37]. Hyphal formation in different media is induced by different stimuli that were encountered by C. albicans and is mediated by multiple filamentation-inducing pathways. For example, hyphal growth in Spider medium is mediated by cAMP, GlcNAc medium by Efg1, and 1640 medium by neutral pH [36, 38]. Our data showing that LH inhibited hyphal growth in different media indicated its inhibition on diverse hyphal-inducing signaling pathways.
Extracellular phospholipase of C. albicans, the expression of which was increased during C. albicans infections, can break down lipids of membrane for tissue invasion, while C. albicans mutants with phospholipase defects showed attenuated virulence in murine infection models [21, 24, 39, 40]. Our results showed that LH could inhibit the production of extracellular phospholipase. This inhibition on phospholipase production, which could also be demonstrated by other antifungal agents [41, 42], may further contribute to its antifungal effects.
Fungal cells and human cells are both eukaryotes, resulting in the paucity of targets that could be employed for treating fungal infections. The limited antifungal drugs were impeded by unwanted side effects and the development of drug resistance, which further contributes to the severe status of fungal infections. With the deeper understanding of the pathogenesis of Candida infections, it was increasingly accepted that targeting virulence factors without causing cell death might be an attractive strategy [36, 39, 43]. There are already published researches on compounds which inhibit virulence factors rather than induce death of fungal cells [36]. These compounds have demonstrated therapeutic efficacy in vivo, corroborating the plausibility of this antivirulence strategy.
Combination therapy can lower the side effects associated with high doses of antifungal drugs and impose less selection pressure for the antifungal drug resistance [44, 45]. Although LH did not show synergistic effects with FLZ and CAS, it did show synergistic activity when combined with AmB. In other words, the use of LH could help to lower the dose of AmB, thus mitigating the toxicity of AmB.
Although LH has demonstrated potent activities of growth inhibition against ovarian cancer cells (IC50 = 1.2 μM) [12], the inhibitory effects against Chang's liver cells and HUVEC cells were weak, with IC50 higher than 256 μM. These results confirmed the selectivity of LH, and so was the safety, which could also be seen in mice and dogs receiving daily injection: LH did not cause obvious effects, such as loss of body weight [12, 13]. The low toxicity, combined with its inhibitory activity against virulence factors, suggests that LH is a promising candidate for anti-Candida therapy development, although the in vivo antifungal activity of LH and the underlying mechanisms remain to be elucidated.
5. Conclusion
In summary, the fungistatic LH is effective against C. albicans, in both planktonic and biofilm forms. LH could also inhibit the virulence traits such as filamentous growth, adhesion, production of extracellular degrading enzymes, and EPS, which play important roles in the pathogenicity of C. albicans. Moreover, LH could synergize with AmB in inhibiting C. albicans. Since LH did not exert fungicidal activity against C. albicans, it may confer less selective pressure for incurring resistance [46]. Our current study may provide a promising candidate for developing antifungal therapies, although much more research on the in vivo activity and pharmacokinetics of LH is needed before its entry into clinic use.
Acknowledgments
This work was supported by the Natural Science Foundation of Jilin Province [20180520118JH], Financial Foundation for Medicine of Jilin Province [No. 201817421534] and National Natural Science Foundation of China [No. 81800849].
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors confirm that there are no conflicts of interest.
Authors' Contributions
Longfei Yang and Xin Liu contributed equally.
References
- 1.Kim J., Sudbery P. Candida albicans, a major human fungal pathogen. Journal of Microbiology. 2011;49(2):171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert A. S., Wheeler R. T., May R. C. Fungal pathogens: survival and replication within macrophages. Cold Spring Harbor Perspectives in Medicine. 2015;5(7, article a019661) doi: 10.1101/cshperspect.a019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva Dantas A., Lee K. K., Raziunaite I., et al. Cell biology of Candida albicans–host interactions. Current Opinion in Microbiology. 2016;34:111–118. doi: 10.1016/j.mib.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang G., Huang Q., Wei Y., Wang Y., Du H. Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans. Molecular Microbiology. 2019;111(1):6–16. doi: 10.1111/mmi.14148. [DOI] [PubMed] [Google Scholar]
- 5.Huang G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence. 2012;3(3):251–261. doi: 10.4161/viru.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noble S. M., Gianetti B. A., Witchley J. N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nature Reviews Microbiology. 2017;15(2):96–108. doi: 10.1038/nrmicro.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohse M. B., Gulati M., Johnson A. D., Nobile C. J. Development and regulation of single-and multi-species Candida albicans biofilms. Nature Reviews Microbiology. 2018;16(1):19–31. doi: 10.1038/nrmicro.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulati M., Nobile C. J. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes and Infection. 2016;18(5):310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins N., Wright G. D., Cowen L. E. Antifungal drugs: the current armamentarium and development of new agents. Microbiology Spectrum. 2016;4(5) doi: 10.1128/microbiolspec.FUNK-0002-2016. [DOI] [PubMed] [Google Scholar]
- 10.Vila T. V. M., Chaturvedi A. K., Rozental S., Lopez-Ribot J. L. In vitro activity of miltefosine against Candida albicans under planktonic and biofilm growth conditions and in vivo efficacy in a murine model of oral candidiasis. Antimicrobial Agents and Chemotherapy. 2015;59(12):7611–7620. doi: 10.1128/AAC.01890-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evidente A., Cicala M. R., Randazzo G., et al. Lycorine structure-activity relationships. Phytochemistry. 1983;22(10):2193–2196. doi: 10.1016/S0031-9422(00)80145-4. [DOI] [Google Scholar]
- 12.Cao Z., Yu D., Fu S., et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicology Letters. 2013;218(2):174–185. doi: 10.1016/j.toxlet.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Lamoral-Theys D., Andolfi A., Van Goietsenoven G., et al. Lycorine, the main phenanthridine amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. Journal of Medicinal Chemistry. 2009;52(20):6244–6256. doi: 10.1021/jm901031h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y., Yu M., Qi Z., et al. Study on apoptosis effect of human breast cancer cell MCF-7 induced by lycorine hydrochloride via death receptor pathway. Saudi Pharmaceutical Journal. 2017;25(4):633–637. doi: 10.1016/j.jsps.2017.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Xu P., Wang C., et al. Synergistic effects of the immune checkpoint inhibitor CTLA-4 combined with the growth inhibitor lycorine in a mouse model of renal cell carcinoma. Oncotarget. 2017;8(13):21177–21186. doi: 10.18632/oncotarget.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R., Cao Z., Tu J., et al. Lycorine hydrochloride inhibits metastatic melanoma cell-dominant vasculogenic mimicry. Pigment Cell & Melanoma Research. 2012;25(5):630–638. doi: 10.1111/j.1755-148X.2012.01036.x. [DOI] [PubMed] [Google Scholar]
- 17.Ločárek M., Nováková J., Klouček P., et al. Antifungal and antibacterial activity of extracts and alkaloids of selected amaryllidaceae species. Natural Product Communications (NPC) 2015;10(9):1537–1540. doi: 10.1177/1934578X1501000912. [DOI] [PubMed] [Google Scholar]
- 18.Bonvicini F., Antognoni F., Iannello C., Maxia A., Poli F., Gentilomi G. A. N. Relevant and selective activity of Pancratium illyricum L. against Candida albicans clinical isolates: a combined effect on yeast growth and virulence. BMC Complementary and Alternative Medicine. 2014;14, article 409 doi: 10.1186/1472-6882-14-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shokri H., Sharifzadeh A. Fungicidal efficacy of various honeys against fluconazole-resistant Candida species isolated from HIV(+) patients with candidiasis. Journal de Mycologie Médicale. 2017;27(2):159–165. doi: 10.1016/j.mycmed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Barhouchi B., Aouadi S., Abdi A. Preparations based on minerals extracts of Calicotome villosa roots and bovine butyrate matter: Evaluation in vitro of their antifungal activity. Journal de Mycologie Médicale. 2017;27(2):210–219. doi: 10.1016/j.mycmed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Yang L., Liu X., Zhong L., et al. Dioscin inhibits virulence factors of Candida albicans. BioMed Research International. 2018;2018:9. doi: 10.1155/2018/4651726.4651726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siles S. A., Srinivasan A., Pierce C. G., Lopez-Ribot J. L., Ramasubramanian A. K. High-throughput screening of a collection of known pharmacologically active small compounds for identification of candida albicans biofilm inhibitors. Antimicrobial Agents and Chemotherapy. 2013;57(8):3681–3687. doi: 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Chang W., Zhang M., Ying Z., Lou H. Natural product solasodine-3-O-ß-D-glucopyranoside inhibits the virulence factors of Candida albicans. FEMS Yeast Research. 2015;15(6) doi: 10.1093/femsyr/fov060. [DOI] [PubMed] [Google Scholar]
- 24.Yang L., Liu X., Lv L., Ma Z., Feng X., Ma T. Dracorhodin perchlorate inhibits biofilm formation and virulence factors of Candida albicans. Journal de Mycologie Médicale. 2018;28(1):36–44. doi: 10.1016/j.mycmed.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Turecka K., Chylewska A., Kawiak A., Waleron K. F. Antifungal activity and mechanism of action of the Co(III) coordination complexes with diamine chelate ligands against reference and clinical strains of Candida spp. Frontiers in Microbiology. 2018;9, article 1594 doi: 10.3389/fmicb.2018.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L., Liu X., Zhuang X., Feng X., Zhong L., Ma T. Antifungal effects of saponin extract from rhizomes of dioscorea panthaica prain et burk against Candida albicans. Evidence-Based Complementary and Alternative Medicine. 2018;2018:13. doi: 10.1155/2018/6095307.6095307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Ma Z., Zhang J., Yang L. Antifungal compounds against candida infections from traditional Chinese medicine. BioMed Research International. 2017;2017:12. doi: 10.1155/2017/4614183.4614183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roemer T., Xu D., Singh S. B., et al. Confronting the challenges of natural product-based antifungal discovery. Chemistry & Biology. 2011;18(2):148–164. doi: 10.1016/j.chembiol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Chen G. L., Tian Y. Q., Wu J. L., Li N., Guo M. Q. Antiproliferative activities of Amaryllidaceae alkaloids from Lycoris radiata targeting DNA topoisomerase I. Scientific Reports. 2016;6(1, article 38284) doi: 10.1038/srep38284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su C., Yu J., Sun Q., Liu Q., Lu Y. Hyphal induction under the condition without inoculation in Candida albicans is triggered by Brg1-mediated removal of NRG1 inhibition. Molecular Microbiology. 2018;108(4):410–423. doi: 10.1111/mmi.13944. [DOI] [PubMed] [Google Scholar]
- 31.Chen H., Lan C., Coste A. T. Role of SFP1 in the regulation of Candida albicans biofilm formation. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0129903.e0129903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konstantinidou N., Morrissey J. P. Co-occurence of filamentation defects and impaired biofilms in Candida albicans protein kinase mutants. FEMS Yeast Research. 2015;15(8) doi: 10.1093/femsyr/fov092.fov092 [DOI] [PubMed] [Google Scholar]
- 33.Moyes D. L., Wilson D., Richardson J. P., et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida R. S., Brunke S., Albrecht A., et al. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathogens. 2008;4(11) doi: 10.1371/journal.ppat.1000217.e1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citiulo F., Jacobsen I. D., Miramón P., et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathogens. 2012;8(6) doi: 10.1371/journal.ppat.1002777.e1002777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romo J. A., Pierce C. G., Chaturvedi A. K., et al. Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of Candida albicans filamentation. mBio. 2017;8(6) doi: 10.1128/mBio.01991-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vila T., Romo J. A., Pierce C. G., McHardy S. F., Saville S. P., Lopez-Ribot J. L. Targeting Candida albicans filamentation for antifungal drug development. Virulence. 2017;8(2):150–158. doi: 10.1080/21505594.2016.1197444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudbery P. E. Growth of Candida albicans hyphae. Nature Reviews Microbiology. 2011;9(10):737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 39.Mayer F. L., Wilson D., Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samaranayake Y. H., Dassanayake R. S., Cheung B. P. K., et al. Differential phospholipase gene expression by Candida albicans in artificial media and cultured human oral epithelium. APMIS-Acta Pathologica, Microbiologica et Immunologica Scandinavica. 2006;114(12):857–866. doi: 10.1111/j.1600-0463.2006.apm_479.x. [DOI] [PubMed] [Google Scholar]
- 41.Padmavathi A. R., Bakkiyaraj D., Thajuddin N., Pandian S. K. Effect of 2, 4-di-tert-butylphenol on growth and biofilm formation by an opportunistic fungus Candida albicans. Biofouling. 2015;31(7):565–574. doi: 10.1080/08927014.2015.1077383. [DOI] [PubMed] [Google Scholar]
- 42.Singh B. N., Upreti D. K., Singh B. R., et al. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrobial Agents and Chemotherapy. 2015;59(4):2153–2168. doi: 10.1128/AAC.03599-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierce C. G., Lopez-Ribot J. L. Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs. Expert Opinion on Drug Discovery. 2013;8(9):1117–1126. doi: 10.1517/17460441.2013.807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haque F., Alfatah M., Ganesan K., Bhattacharyya M. S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Scientific Reports. 2016;6 doi: 10.1038/srep23575.23575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui J., Ren B., Tong Y., Dai H., Zhang L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence. 2015;6(4):362–371. doi: 10.1080/21505594.2015.1039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clatworthy A. E., Pierson E., Hung D. T. Targeting virulence: a new paradigm for antimicrobial therapy. Nature Chemical Biology. 2007;3(9):541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.