Abstract
Nonalcoholic fatty liver disease (NAFLD) is a rapidly emerging hepatic manifestation of metabolic syndrome. However, its unrevealed mechanism and complicated comorbidities have led to no specific medication, except for weight loss and lifestyle modification. Alisma orientale (Sam.) Juzep (A. orientale, Alismataceae) has been increasingly reported on therapeutic effects of A. orientale against NAFLD and metabolic syndrome such as insulin resistance, hyperlipidemia, and obesity. Therefore, this study aimed to review the preclinical efficacy of A. orientale and its chemical constituents including Alisol A 24-acetate, Alisol B 23-acetate, Alisol F, and Alismol against NAFLD and metabolic syndrome. A. orientale prevented hepatic triglyceride accumulation through suppressing de novo lipogenesis and increasing lipid export. In addition, it controlled oxidative stress markers, lipoapoptosis, liver injury panels, and inflammatory and fibrotic mediators, eventually influencing steatohepatitis and liver fibrosis. Moreover, it exhibited pharmacological activities against hyperlipidemia, obesity, and hyperglycemia as well as appetite. These biological actions of A. orientale might contribute to adiponectin activation or a role as a farnesoid X receptor agonist. In particular, Alisol A 24-acetate and Alisol B 23-acetate could be expected as main compounds. Taken together, A. orientale might be an effective candidate agent for the treatment of NAFLD and its comorbidities, although further assessment of its standardization, safety test, and clinical trials is consistently required.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD), a new challenge of chronic liver disease in the 21st century, includes simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. A recent meta-analysis reported that global prevalence of NAFLD was assessed to be 25.24% [1], and its prevalence is likely to increase up to 33.5% in adults by 2030 [2]. As the prevalence of NAFLD constantly grows, economic burden is also predicted to consistently increase [3]. Most NAFLD patients have a high risk of cardiovascular disease-related mortality rather than liver-related death. Hence, NAFLD is not only a type of chronic liver diseases but also an independent risk factor of metabolic syndrome such as obesity, hypertension, type II diabetes mellitus (T2DM), and hyperlipidemia.
Unfortunately, there is still no gold standard medication to treat NAFLD. Pharmacological therapies for NAFLD currently depend on various options such as insulin sensitizing agents, antioxidants, incretin-based therapy, lipid lowering agents, and weight loss drugs other than lifestyle modification [4]. However, unfavorable side effects such as gastrointestinal upset, hemorrhagic stroke, myopathy, pruritus, osteoporosis, and transient increase in serum creatinine have hampered the authority approval as standard medication to treat NAFLD [5].
In the absence of optimal therapeutic strategies to approach NAFLD, herbal medicines containing abundant active substances could be an alternative and innovative therapeutic solution. Previous studies have demonstrated the brief but encouraging results on a total of 24 herbal plants against NASH [6, 7]. Among those plants, Alisma orientale (Sam.) Juz. (A. orientale) is worthy of notice. A. orientale is a synonym of Alisma plantago-aquatica subsp. orientale (Sam.) and belongs to the Alisma genus of the family of Alismataceae in the major group of Angiosperms. The tuber part of A. orientale contains various phytochemical constituents such as terpenoids, flavonoids, polysaccharides, phytosterols, and amino acids. Terpenoids including triterpenes, sesquiterpenes, and diterpenes are key compound classes of A. orientale contributing to its bioactive effects [8]. It has been mainly used for over 2000 years in Asian countries exhibiting diverse effects such as diuretic, hypolipidemic, hypoglycemic, antiallergic, and anti-inflammatory actions with no toxicity [8]. Numerous recent experimental studies suggested that A. orientale and its compounds exhibit therapeutic activities against NAFLD and its related comorbidities. Although A. orientale was introduced in the above two reviews, they were based on only three articles about the efficacy of A. orientale against NAFLD and its accompanied pathological diseases.
Therefore, this review summarizes preclinical evidence for A. orientale and its four constituents for the treatment of NAFLD and metabolic syndrome.
2. Pharmacological Effects of A. orientale
The pathological mechanism underlying the development and progression of NAFLD is complex and multifactorial. Thus, a ‘multiple hit' hypothesis provides a relatively accurate explanation about NAFLD pathogenesis. Such hits include insulin resistance, hormones secreted from the adipose tissue, oxidative stress, and inflammatory cytokines [9], which play important roles in the development of NAFLD and its progression. In addition, there exists an increasing evidence linking NAFLD with metabolic syndrome such as hyperlipidemia, obesity, and T2DM. Therefore, the pharmacological effects of A. orientale against NAFLD and metabolic syndrome could be discussed according to the following eight subthemes as antisteatotic, antioxidant, antilipoapoptotic, hepatoprotective, anti-inflammatory and antifibrotic, hypolipidemic, antiobesity, and hypoglycemic effects (Table 1).
Table 1.
Pharmacological properties of A. orientale.
| Extraction solvent | Country | Type | Model | Efficient doses | Results | References |
|---|---|---|---|---|---|---|
| Antisteatotic activity | ||||||
|
| ||||||
| Water | China | In vitro | DL-ethionine-treated rat hepatocytes | 1, 5, 10, 20, and 50 μg/ml | Apolipoprotein B ↑ liver TG ↓ lipid droplet ↓ | [13] |
| In vivo | High-fat diet rats | 2.26 g/kg | Liver TG↓ | [17] | ||
|
| ||||||
| Ethanol | South Korea | In vitro | FFA-treated HepG2 | 100 μg/ml | Lipid droplet ↓ | [11] |
| In vitro | NEFAs-treated HepG2 | 300 μg/ml | Lipid droplet ↓ FAS mRNA & protein ↓ ACC mRNA & protein ↓ | [12] | ||
|
| ||||||
| Methanol | China | In vivo | High-fat diet rats | 300, 600 mg/kg | Liver weight↓ Liver weight/body weight ratio↓ Liver TG ↓ lipid droplet ↓ | [16] |
| South Korea | In vivo | Benzo(a)pyrene-injected rats | 0.15 g/kg | Liver TG↓ | [14] | |
| South Korea | In vitro | Tunicamycin-treated HepG2 | 10, 50, and 100 μg/ml | TG ↓ VLDL receptor ↓ Apolipoprotein B ↑ | [15] | |
| In vitro | Palmitate -treated HepG2 | 10, 50, and 100 μg/ml | Hepatic lipogenic genes (FAS, ACC, and GPAT) ↓ TG ↓ VLDL receptor ↓Apolipoprotein B ↑ | |||
| In vivo | Tunicamycin-injected mice | 50, 100 mg/kg | Liver TG ↓VLDL receptor ↓Apolipoprotein B ↑ | |||
| In vivo | High-fat diet mice | 100, 300 mg/kg | Hepatic lipogenic genes ↓liver TG ↓VLDL receptor↓ Apolipoprotein B ↑ | |||
|
| ||||||
| Antioxidant activity | ||||||
|
| ||||||
| Water | South Korea | In vitro | Palmitate -treated HepG2 | 100 μg/ml | ROS ↓ TBARS ↓ | [21] |
|
| ||||||
| Ethanol | South Korea | In vitro | tert-Butyl hydroperoxide-induced HepG2 | 0.05, 0.1 mg/ml | Free radicals ↓ Superoxide anions ↓ MDA ↓ | [20] |
| In vivo | tert-Butyl hydroperoxide-induced rats | 1 g/kg | Liver MDA ↓ | |||
|
| ||||||
| Methanol | China | In vivo | High-fat diet rats | 300, 600 mg/kg | Serum MDA ↓ Serum SOD ↑ | [16] |
| South Korea | In vivo | High-fat diet rats | 100, 200, and 300 mg/kg | Serum MDA ↓ | [19] | |
|
| ||||||
| Antilipoapoptotic activity | ||||||
|
| ||||||
| Water | South Korea | In vitro | Palmitate -treated HepG2 | 100 μg/ml | Apoptotic cells ↓ sub-G1 cells ↓ BAX ↓ Bcl-2 ↑ pJNK ↓ | [21] |
| South Korea | In vitro | Palmitate -treated HepG2 | 10, 100 μg/ml | Sub-G1 cells ↓ | [23] | |
|
| ||||||
| Ethanol | South Korea | In vitro | NEFAs-treated HepG2 | 300 μg/ml | MAPK8 mRNA ↓ p-JNK ↓ BAX ↓ Bcl-2 ↑ Cleaved caspase-9 ↓ Cleaved caspase-3↓ |
[12] |
| South Korea | In vitro | FFA-treated HepG2 | 100 μg/ml | p-JNK ↓ PUMA mRNA & protein ↓ BAX ↓ Bcl-2 ↑ Cleaved caspase-3 ↓ Cleaved caspase-9 ↓ | [11] | |
|
| ||||||
| Hepatoprotective activity | ||||||
|
| ||||||
| Water | South Korea | In vivo | High-fat diet mice | 100, 300 mg/kg | Serum AST ↓ Serum ALT ↓ | [26] |
| China | In vivo | High-fat diet mice | 2.26 g/kg | Serum AST ↓ Serum ALT ↓ | [17] | |
| South Korea | In vivo | Benzo(a)pyrene-injected rats | 9 g/kg | Serum AST ↓ Serum ALT ↓ Liver AST ↓ Liver ALT ↓ | [27] | |
|
| ||||||
| Ethanol | South Korea | In vivo | tert-Butyl hydroperoxide-induced rats | 1 g/kg | Serum AST ↓ Serum ALT ↓ | [20] |
|
| ||||||
| Methanol | China | In vivo | High-fat diet rats | 150, 300, and 600 mg/kg | Serum AST ↓ Serum ALT ↓ | [16] |
| South Korea | In vivo | High-fat diet rats | 100, 200, and 300 mg/kg | Serum AST ↓ Serum ALT ↓ | [19] | |
| South Korea | In vivo | Acetaminophen-injected rats | 250, 500 mg/kg | Serum AST ↓ Serum ALT ↓ | [28] | |
|
| ||||||
| Anti-inflammatory and antifibrotic activity | ||||||
|
| ||||||
| Water | South Korea | In vivo | High-fat diet mice | 100 mg/kg | Serum adiponectin↑ | [26] |
|
| ||||||
| Ethanol | South Korea | In vitro | NEFAs-treated HepG2 | 300 μg/ml | NF-κB p65(p65) ↓ p-p65 ↓ COX-2 ↓ iNOS ↓ | [12] |
| South Korea | In vitro | Human hepatic stellate cells | 0.02, 0.1 mg/ml | TIMP-1↓ | [29] | |
|
| ||||||
| Methanol | South Korea | In vitro | Tunicamycin-treated HepG2 | 10, 50, and 100 μg/ml | GRP78 mRNA↓ CHOP mRNA↓ XBP-1 mRNA↓ IL-6 mRNA ↓ TNF-α mRNA↓ MCP-1 mRNA ↑ | [15] |
| In vitro | Palmitate -treated HepG2 | 10, 50, and 100 μg/ml | ||||
| In vivo | Tunicamycin-injected mice | 50, 100 mg/kg | Liver GRP78 mRNA↓ liver CHOP mRNA↓ liver XBP-1 mRNA↓ liver IL-6 mRNA ↓ Liver TNF-α mRNA↓ liver MCP-1 mRNA ↑ |
|||
| In vivo | High-fat diet mice | 100, 300 mg/kg | ||||
| China | In vivo | High-fat diet rats | 150, 300, and 600 mg/kg | Liver collagen deposition↓ | [16] | |
|
| ||||||
| Hypolipidemic activity | ||||||
|
| ||||||
| Water | South Korea | In vitro | Microsome from rat liver | 10 μl | Liver ACAT↓ Liver HMA-CoA reductase↓ | [30] |
| South Korea | In vivo | High-fat diet mice | 100 mg/kg | Serum TG ↓ Serum TC ↓ Serum LDL ↓ Serum HDL ↑ Serum HDL/LDL ↑ | [26] | |
| China | In vivo | High-fat diet rats | 2.26 g/kg | Serum TC ↓ Serum TG ↓ Liver TC ↓ Serum HDL ↑ Liver HMG-CoA reductase ↓ |
[17] | |
|
| ||||||
| Methanol | China | In vivo | High-fat diet rats | 300, 600 mg/kg | Serum TC ↓ Serum TG ↓ Liver TC ↓ | [16] |
| South Korea | In vivo | Benzo(a)pyrene-injected rats | 0.15 g/kg | Serum TG↓ Serum TC↓ liver TC↓ | [14] | |
| South Korea | In vivo | High-fat diet rats | 100, 200, and 300 mg/kg | Serum LDL ↓ | [19] | |
|
| ||||||
| Antiobesity activity | ||||||
|
| ||||||
| Water | China | In vivo | Goto-Kakizaki rats | 3 mg/g | Body weight↓ | [31] |
| South Korea | In vivo | High-fat diet mice | 100 mg/kg | Body weight↓ Total fat weight/ Body weight↓ Adipocyte size↓ Serum Adiponectin↑ |
[32] | |
| South Korea | In vitro | 3T3-L1 cells | 10 mg/ml | Proliferation↓ Differentiation↓ | [33] | |
| China | In vitro | Caco-2/TC7 transfected with human ApoA-IV promoter | 1 mg/ml | ApoA-IV promoter activity↑ ApoA-IV mRNA↑ | [34] | |
| 3T3-L1 cells | 1, 10 mg/ml | TG↓ | ||||
|
| ||||||
| Ethanol | South Korea | In vitro | OP9 cells | 20, 40 μg/ml | PPARγ protein↓ PPARγ mRNA↓ C/EBPα mRNA↓ C/EBPβ protein↓ |
[35] |
|
| ||||||
| Methanol | China | In vivo | High-fat diet rats | 300, 600 mg/kg | Epididymal fat weight ↓ Epididymal fat weight/body weight↓ | [16] |
|
| ||||||
| Hypoglycemic activity | ||||||
|
| ||||||
| Water | China | In vitro | BBMV | 1 mg/ml | Intestinal glucose absorption ↓ | [36] |
| Hs68 cells | 0.01, 0.1, and 1 mg/ml | Fibroblast glucose uptake ↑ | ||||
| 3T3-L1 cells | 0.01, 0.1, and 1 mg/ml | Adipocyte glucose uptake ↑ | ||||
| China | In vivo | Streptozotocin-induced mice | 1.5, 3 g/kg | Serum glucose ↓ Serum insulin ↑ | [37] | |
| South Korea | In vivo | Streptozotocin-induced rats | 61.25 mg/kg | Serum glucose ↓ | [38] | |
| China | In vivo | Goto-Kakizaki rats | 3 mg/g | Fasting serum glucose ↓ glucose tolerance ↑ | [31] | |
| South Korea | In vivo | High-fat diet mice | 100 mg/kg | Serum adiponectin↑ Liver PGC-1α, ERRγ, G6Pase, PEPCK mRNA↓ | [26] | |
|
| ||||||
| Ethanol | South Korea | In vitro | 3T3-L1 cells | 50 μg/ml | PPARγ agonist activity ↑ | [39] |
|
| ||||||
| Methanol | China | In vivo | High-fat diet rats | 300, 600 mg/kg | Fasting serum glucose ↓ insulin sensitivity index ↑ Insulin resistance index ↓ |
[16] |
|
| ||||||
| Alcohol | China | In vitro | 3T3-L1 cells | 25, 50, and 100 μg/ml | Adipocyte glucose uptake ↑ | [40] |
| α-Glucosidase assay | 25 μg/ml | α-Glucosidase activity ↓ | ||||
|
| ||||||
| Ethyl acetate | China | In vivo | High-fat diet and streptozotocin-induced mice | 100 mg/kg | Fasting serum glucose ↓ serum insulin ↑ serum HbA1c ↓ IRS-1 protein ↑ Akt protein ↑ |
[41] |
2.1. Antisteatotic Activity
Hepatic steatosis, the hallmark of NAFLD, is characterized by TG accumulation in the hepatocyte cytoplasm, and regulating hepatic steatosis is an essential strategy to treat NAFLD and prevent its progression to NASH and hepatic inflammation. Currently, Aramchol™ (arachidyl-amino cholanoic acid) is an investigational anti-NASH drug suppressing TG levels and the activity of Stearoyl Coenzyme A Desaturase 1 (SCD1) involved in hepatic lipid accumulation [10].
Similarly, A. orientale inhibited overaccumulation of TG induced by free fatty acid (FFA) [11, 12], DL-ethionine [13], benzo(a)pyrene [14], high-fat diet [15–17], and tunicamycin [15] with accompanied lipid droplet decrease [11–13, 16]. Interestingly, A. orientale repressed the mRNA levels of Very Low-Density Lipoprotein (VLDL) receptor which accelerated hepatic TG overload in tunicamycin-treated HepG2 cells [15] and enhanced apoprotein B secretary protein supporting the excretion TG from hepatic cells in experimental models induced by DL-ethionine [13], tunicamycin, palmitate, and high-fat diet [15]. In addition, A. orientale could block hepatic lipid production by regulating hepatic lipogenic genes including fatty acid synthase (FAS), acetyl-coenzyme A carboxylase (ACC), and glycerol-3-phosphate acyltransferase (GPAT) [12, 15]. Taken together, these experimental results suggest that A. orientale could alleviate simple fatty hepatocytes via ER stress inhibition, hepatic lipogenesis suppression, and transfer of lipids out of liver.
2.2. Antioxidant Activity
Oxidative stress contributes to the pathological transition of simple hepatic steatosis to steatohepatitis and fibrosis. Particularly, oxidative stress markers such as thiobarbituric acid reactive substances (TBARS) or malondialdehyde (MDA) decrease beneficial antioxidant enzymes like superoxide dismutase (SOD) and cause overproduction of reactive oxygen species (ROS), secretion of proinflammatory cytokines, and influx of inflammatory monocytes into liver [18]. Consequently, ROS toxicity may activate Kupffer and hepatic stellate cells, leading to inflammation and fibrosis. Therefore, reducing oxidative stress and improving antioxidant defense system are essential to regulate the progression of NAFLD.
A. orientale administration elevated serum SOD activities [16] and reduced serum MDA levels in high-fat diet rats [16, 19]. In addition, A. orientale pretreatment significantly suppressed hepatic MDA formation induced by tert-butyl hydroperoxide in both HepG2 cells and rats [20]. Levels of ROS, TBARS, free radicals, and peroxides were also significantly reduced by A. orientale treatment in oxidative stress experimental models induced by palmitate [21] and tert-butyl hydroperoxide [20]. These results suggest that A. orientale has antioxidant effects to protect against liver damage initiated by oxidative stressors and it could be clinically applied as a therapeutic option to treat NAFLD patients.
2.3. Antilipoapoptotic Activity
Excessive nonesterified fatty acids (NEFAs) may induce lipotoxicity including the promotion of apoptosis. Thus, this lipid-induced apoptotic phenomenon is termed lipoapoptosis. Lipoapoptosis is a prominent feature of NASH and is associated with the severity and progression of NASH. In particular, c-Jun N-terminal kinase (JNK) phosphorylation is an important cause and mediator of lipoapoptosis in fibrosis as well as inflammation in the NASH liver [22]. Hence, blocking the JNK signaling pathway might be a beneficial strategy to treat NASH and prevent this progression.
In HepG2 cell models, free fatty acids treatment induced JNK-dependent lipoapoptosis by activating proapoptotic family including Bcl-2-associated X protein (Bax), caspase-3, and caspase-9 [11, 12, 21, 23]. Upon treatment of A. orientale, apoptotic mediators (Bax, Bcl-2, caspase-3, and caspase-9) and JNK activation were regulated resulting in a reduced number of apoptotic cells quantitatively [11, 12, 21, 23]. It is noteworthy that the mRNA and protein levels of p53 upregulated modulator of apoptosis (PUMA), a proapoptotic protein contributing to lipoapoptosis in hepatocytes, were suppressed by 100 μg/ml of A. orientale treatment [11]. As JNK activation in NASH, PUMA is also overexpressed in the liver of NASH patients. In addition, PUMA is higher in liver tumor patients because it contributes to hepatocarcinogenesis [24] as well as lipoapoptosis [25]. JNK signaling is known to affect hepatic steatosis, insulin resistance, inflammation, fibrosis, and even cancer. Therefore, A. orientale could inhibit JNK-PUMA-mediated lipoapoptosis, which consequently might improve NASH and prevent its progression to fibrosis or HCC.
2.4. Hepatoprotective Activity
Simple hepatic steatosis is benign, but NASH is closely related to liver injury. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are important biochemistry markers associated with liver injury. A large prospective UK cohort study suggested that NAFLD was the most prevalent cause of patients with abnormal AST and ALT levels [57]. In addition, fibrosis-4 index (age, platelet, ALT, and AST) and NAFLD fibrosis score (age, hyperglycemia, BMI, platelet, albumin, and AST/ALT ratio) including AST and ALT levels are calculated to predict the NASH progression and severity in clinical settings. Hence, normalizing AST and ALT levels is useful to treat NAFLD patients.
A. orientale exhibited hepatoprotective effects by lowering serum AST and ALT levels increased by high-fat diet in in vivo models [16, 17, 19, 26]. Moreover, the relatively high levels of AST and ALT in fatal liver injury rat models by benzo(a)pyrene [27], acetaminophen [28], and tert-butyl hydroperoxide [20] were significantly decreased after the administration of A. orientale. Consequently, it indicates that A. orientale could improve AST and ALT levels which are predictive of the presence of NAFLD or NASH and can be developed as a hepatoprotective agent like ursodeoxycholic acid to prevent its development and progression.
2.5. Anti-Inflammatory and Antifibrotic Activity
Inflammation and fibrosis are closely associated with the progress of hepatic simple steatosis to steatohepatitis and liver cirrhosis. In recent times, inflammatory and fibrotic mediators to treat NAFLD and prevent its progression are gaining attention for new therapeutic targets. Cenicriviroc, the dual antagonist of C-C chemokine receptor (CCR)2/CCR5 pathways in NASH-mediated inflammation and fibrosis, is currently being under phase III clinical trials for NASH patients with fibrosis (ClinicalTrials.gov Identifier: NCT03028740) [58].
As therapeutic targets for hepatic inflammation or fibrosis, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1 are representative cytokines known to correlate positively with severe NASH and advanced fibrosis [59, 60]. A. orientale extract reduced the mRNA levels of TNF-α, IL-6, and MCP-1 increased in tunicamycin or palmitate-treated HepG2 cells and livers of high-fat diet or tunicamycin-injected mice [15]. Regarding proinflammatory cytokines, the protein expressions of cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) were suppressed through the inhibition of phosphorylation of p65 subunit NF-κB in NEFAs-treated HepG2 cells [12]. In addition, ER stress aggravates the inflammatory response through NF-κB activation, but A. orientale prevented ER stress response by suppressing the mRNA expression of ER stress markers such as C/EBP homologous protein (CHOP), glucose-regulated protein 78 (GRP78), and X-box Binding Protein-1 (XBP-1) [15]. This activity of A. orientale against inflammation mediators could be explained by enhanced secretion of serum adiponectin by A. orientale administration in high-fat diet mice [26]. Adiponectin is one of important proteins involved in NAFLD pathogenesis and it exhibits anti-inflammatory actions by blocking NF-κB and lowering the release of TNF-α, IL-6, COX-2, and iNOS. Additionally, adiponectin is known as an antifibrotic adipokine in the liver [61]. Consistently, A. orientale attenuated collagen deposition near the central veins and portal tracts in the liver in high-fat diet rats and tissue inhibitors of metalloproteinases (TIMP)-1 expression in hepatic stellate cells (HSC) [16, 29]. Collectively, although the specific mechanisms underlying these activities of A. orientale remain unclear, it likely influences the inflammatory and fibrogenic response of NAFLD and prevents the progression to NASH and fibrosis by regulating NF-κB, adiponectin, and related markers.
2.6. Hypolipidemic Activity
Hypercholesterolemia results in hepatic cholesterol overload, in which cholesterol burden in liver brings about fatty liver. Moreover, cholesterol deposits activate resident macrophage, Kupffer cells, and subsequently lead to steatohepatitis. In particular, hydroxy-methylglutaryl CoA (HMG-CoA) reductase and acyl-CoA:cholesterol acyltransferase (ACAT) are two important enzymes affecting cholesterol synthesis and storage in liver. Hence, HMG-CoA inhibitor such as statin [62] or ACAT inhibitor like avasimibe [63] is possible in NAFLD patients for achieving cholesterol homeostasis.
A. orientale water extract showed comparatively high inhibition rates 18% and 13.08% against ACAT and HMG-CoA reductase activities in rat livers, respectively [30]. In addition, A. orientale lowered serum LDL levels in high-fat diet rats; increased LDL occurs due to overexpression of HMG-CoA reductase in NAFLD patients and it is a proatherosclerotic factor and a risk factor of NASH [19, 32]. Besides, A. orientale decreased serum TC and TG levels increased by high-fat diet intake as well as TC levels in the liver [14, 16, 17, 26]. A recent study reported that HDL and TG levels were more important causative factors of NASH than LDL or VLDL level [64]. The remarkable thing is that A. orientale increased the HDL serum levels and HDL/LDL ratio in high-fat diet rats [17, 26]. Elevated HDL is inversely proportional to cardiovascular disease which is the leading cause of the death in NAFLD patients. Collectively, A. orientale might regulate abnormal cholesterol-related markers which were indicative of NAFLD severity and progression and help prevent the development of cardiovascular diseases.
2.7. Antiobesity Activity
Obesity (BMI ≥ 30 kg/m2 in adults) is one of the risk factors resulting in the development and severity of NAFLD. Weight and BMI reduction could be one efficient intervention to treat obese NAFLD patients. However, around 8-19% of lean Asian (BMI < 25 kg/m2) are reported to have NAFLD [65] and this shows that BMI is an imperfect tool because it does not calculate muscle and fat mass separately. To approach the pharmacological treatment of obese and nonobese NAFLD patients, adipogenesis could be another therapeutic target as well as simple weight loss.
Administration of A. orientale extract markedly decreased not only body weight but also fat mass (abdominal subcutaneous fat, perirenal fat, and epididymal fat), which were increased by high-fat diet intake in in vivo models [16, 26, 31]. A histological finding showed that A. orientale water extract of 100 and 300 mg/kg reduced the size of adipocytes in fat tissue as compared with that in the normal diet group [26]. Consistently, A. orientale prevented adipocytes such as OP9 and 3T3-L1 cells from proliferating in number and differentiating into mature cells, leading to the inhibition of TG formation in differentiated 3T3-L1 adipocytes [33–35]. Concerning OP9 adipocyte differentiation, the expression of adipocyte-specific genes such as CCAAT enhancer binding protein (C/EBP)β (very early initiator of adipogenesis), C/EBPα, and peroxisome proliferator-activated receptor (PPAR)γ (essential transcriptional factors of adipogenesis) are suppressed by A. orientale treatment, suggesting that A. orientale might regulate adipogenic inducers [35]. In addition, A. orientale elevated the level of serum adiponectin [26], an important peptide hormone reduced in obese NAFLD patients [66], in mouse models, and ApoA-IV mRNA levels in intestinal cells. Importantly, elevated levels of ApoA-IV in the blood may reduce appetite for food by mediating hypothalamic melanocortin system [67]. These findings demonstrate that A. orientale may serve as an efficient drug to control food intake, reduce hyperplasia, hypertrophy, and differentiation of adipocytes and lose fat weight of obese and nonobese NAFLD patients. Currently, obese adults with NAFLD are more likely to increase the risk of drug-induced hepatotoxicity [68] and a randomized controlled trial identified that orlistat did not influence weight loss in NAFLD patients [69]. To overcome the limitation of oral medications for obese NAFLD patients, A. orientale extract and its chemical constituents could be applied to treat them because it has hepatoprotective effects as well as antiobesity effects.
2.8. Hypoglycemic Activity
Hepatic TG accumulation leads to insulin resistance and T2DM. Conversely, insulin resistance aggravates hepatic steatosis or inflammation. Most patients with NAFLD are at risk of the presence of insulin resistance. Although NAFLD patients with T2DM are exposed to increased risk of poor prognosis such as cardiovascular disease, liver cirrhosis, and HCC compared to those without T2DM [70], to date there is no specific drug approved to manage NAFLD and T2DM simultaneously. Currently, metformin is recommended as a safe drug in NASH and T2DM because it is excreted by renal clearance and not by liver metabolism. However, several meta-analyses showed that it is not efficient to improve NASH. Pioglitazone improved hepatic histological findings in necroinflammation and fibrosis but had some side effects such as weight gain or fluid retention [71]. This means that new therapeutic approaches to improve not only hyperglycemia but also NAFLD are required for NAFLD patients with T2DM.
A. orientale administration reduced the levels of serum glucose and HbA1c in in vivo diabetic models [16, 31, 37, 38, 41]. Suppressed brush border membrane vesicles (BBMV) (rabbit small intestine) intestinal glucose absorption and stimulated Hs68 (fibroblasts) and 3T3-L1 (adipocytes) glucose uptake by A. orientale treatment might be responsible for lowering glucose [36, 40]. In particular, the reduction of intestinal glucose absorption by A. orientale was supported by its inhibitory effect on α-glucosidase activity [40] which plays a key role in postprandial glycemic level through gastrointestinal absorption. Additionally, A. orientale, like TZD, increased insulin secretion and sensitivity through PPARγ activation, contributing to its actions against hyperglycemia [16, 37, 39, 41]. Moreover, A. orientale enhanced the protein levels of insulin receptor substrate (IRS)-1 and protein kinase B (Akt) which are decreased in insulin resistant mice induced by high-fat diet and streptozotocin [41]. Subsequently, A. orientale suppressed the mRNA levels of hepatic PPARγ coactivator (PGC)-1α, estrogen-related receptor (ERR)γ, and PGC-1α-dependent enzymes (G6Pase, phosphoenolpyruvate carboxykinase (PEPCK)) which are involved in gluconeogenesis in the liver tissue [26]. Furthermore, A. orientale elevated the serum adiponectin levels in high-fat diet mice [26] which is an important adipokine maintaining body and hepatic glucose homeostasis and preventing from progressing into inflammation and fibrosis. As mentioned above, A. orientale exhibited potential antidiabetic activity by regulating serum glucose, adiponectin, and insulin levels, hepatic/body insulin resistance, and excessive glucose production in the liver.
3. Pharmacological Effects of Active Constituents of A. orientale
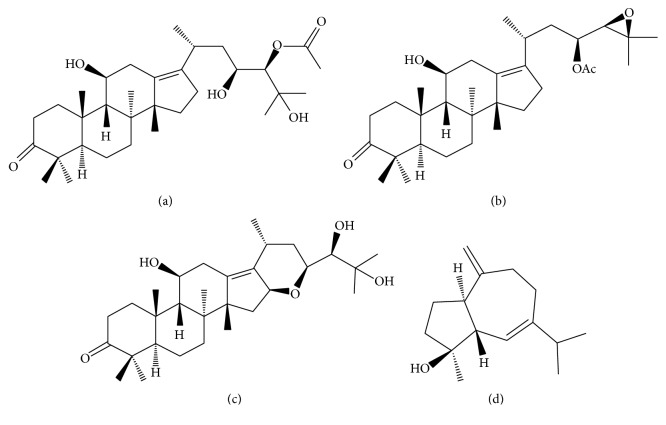
Chemical constituents of A. orientale are identified as about 120 compounds including guaiane-type sesquiterpenes, protostane-type triterpenes, guaiane-type and kaurane-type diterpenes [8], and small amounts of flavonoids, alkaloids, asparagine, phytosterols, fatty acids, and resins [72]. Protostane-type triterpenoids mainly include Alisols A–I and their derivatives while guaiane-type sesquiterpenoids include Alismol, Alismoxide, Orientalols A–F, and Orientalols sulphate [72]. In particular, experimental studies regarding the pharmacological activities of four compounds of Alisol A 24-acetate, Alisol B 23-acetate, Alisol F, and Alismol (Figure 1) among these various constituents of A. orientale have been increasingly reported. Hence, these four constituents were reviewed in this study in terms of their medicinal effects against NAFLD and its pathological process (Table 2).
Figure 1.
Chemical structures of constituents from A. orientale. (a) Alisol A 24-acetate (C32H52O6, molecular weight (MW) of 532.75 g/mol), (b) Alisol B 23-acetate (C32H50O5, MW of 514.8 g/mol), (c) Alisol F (C30H48O5, MW of 488.7 g/mol), and (d) Alismol (C15H24O, MW of 220.356 g/mol).
Table 2.
Pharmacological activities of Alisol A 24-acetate, Alisol B 23-acetate, Alisol F, and Alismol.
| Pharmacological effects | Country | Type | Model | Doses | Results | References |
|---|---|---|---|---|---|---|
| Alisol A 24-acetate | ||||||
|
| ||||||
| Antisteatotic | China | In vitro | FFA-treated HepG2 | 10 μM | Lipid droplet↓ FAS, ACC, AMPK, SREBP-1c mRNA & protein ↓ CPT1, ACOX1 mRNA & protein ↑ |
[42] |
| China | In vitro | MCD-treated WRL-68 | 1, 2, 4, 8, and 16 μM | TG↓ | [43] | |
| In vivo | MCD diet mice | 60 mg/kg | Lipid droplet↓ Liver TG↓ Liver FFA↓ | |||
| Antioxidant | China | In vivo | MCD diet mice | 60 mg/kg | Liver ROS, MDA, MPO↓ | |
| In vitro | LX-2 | 4, 8 μM | ROS↓ | |||
| Hepatoprotective | China | In vivo | MCD diet mice | 30, 60 mg/kg | Serum AST↓ Serum ALT↓ | |
| Anti-inflammatory | China | In vivo | MCD diet mice | 60 mg/kg | Liver inflammatory foci↓ IL-6↓ IL-1β↓ MCP-1↓ | |
| In vitro | LX-2 | 4, 8 μM | IL-6, IL-1β, MCP-1 mRNA↓ | |||
| In vitro | FFA-treated HepG2 | 10 μM | TNF-α ↓ IL-6 ↓ Adiponectin ↑ | [42] | ||
| Antifibrotic | China | In vivo | MCD diet mice | 60 mg/kg | Liver extracellular matrix↓ α-SMA↓ TGF-β↓ TIMP↓ | [43] |
| In vitro | LX-2 | 8, 16 μM | α-SMA, TGF-β mRNA&protein↓ TIMP mRNA↓ | |||
| Hypolipidemic | China | In vivo | Lipid emulsion diet mice | 0.64, 1.28, and 2.56 mg/kg | Serum TC, TG, LDL, HDL↓ liver HMG-CoA reductase ↓ | [44] |
| Japan | In vivo | Atherogenic diet rats | 97.5 mg/kg | Serum TC↓ Liver fat↓ Liver TC↓ | [45] | |
| Antiobesity | China | In vitro | FFA-treated HepG2 | 10 μM | Adiponectin ↑ | [42] |
| Hypoglycemic | China | In vitro | FFA-treated HepG2 | 10 μM | Adiponectin ↑ | |
|
| ||||||
| Alisol B 23-acetate | ||||||
|
| ||||||
| Antisteatotic | China | In vivo | MCD diet mice | 15, 30, and 60 mg/kg | Lipid droplet↓ liver TG, FFA↓ FAS, ACC, SCD1 protein↓ CPT1, ACOX1 mRNA↑ PPARα mRNA↑ |
[46] |
| Antioxidant | South Korea | In vivo | Bromobenzene -injected rats | 10, 20 mg/kg | Liver MDA, glutathione↓liver Glutathione↓ | [47] |
| Hepatoprotective | China | In vivo | MCD diet mice | 15, 30, and 60 mg/kg | Serum AST↓ Serum ALT↓ | [46] |
| Anti-inflammatory | China | In vivo | MCD diet mice | 30, 60 mg/kg | Serum MCP-1↓ mouse keratinocyte-derived chemokine↓ | |
| 60 mg/kg | liver MCP-1, VCAM-1 mRNA↓ | |||||
| Antifibrotic | China | In vivo | MCD diet mice | 60 mg/kg | a1(I), a2(I) collagen mRNA↓ α-SMA, TGF-β, MMP-2, TIMP-1 mRNA↓ |
|
| Hypolipidemic | China | In vivo | Lipid emulsion diet mice | 0.64, 1.28, 2.56 mg/kg | Serum TC, TG, LDL, HDL↓ liver HMG-CoA reductase ↓ | [44] |
| China | In vivo | MCD diet mice | 30, 60 mg/kg | Serum TG, FFA, TC↓ liver TC↓ LPL mRNA↑ ApoC-II mRNA↑ ApoC-III mRNA↓ ANGPTL3 mRNA↓ | [46] | |
|
| ||||||
| Alisol F | ||||||
|
| ||||||
| Hepatoprotective | China | In vivo | LPS/D-gal-induced mice | 20 mg/kg | Serum AST↓ Serum ALT↓ | [48] |
| Anti-inflammatory | China | In vitro | LPS-treated RAW264.7 | 3.7, 11, and 33 μM | iNOS, COX-2 mRNA & protein↓ TNF-α, IL-6, IL-1β mRNA & protein↓ NF-κB↓ MAPKs(ERK, p38, JNK)↓ STAT3↓ |
|
| In vivo | LPS/D-gal-induced mice | 20 mg/kg | Serum TNF-α, IL-6, IL-1β ↓ liver MAPKs(ERK, JNK)↓ | |||
| China | In vitro | LPS-treated RAW264.7 | 3.7, 11, and 33 μM | NO↓ | [49] | |
| Japan | In vitro | LPS-treated macrophages | 50, 100 μM | NO↓ iNOS↓ | [50] | |
| Hypoglycemic | China | In vitro | 3T3-L1 cells | 10 μM | Cell differentiation↓ | [40] |
| α-Glucosidase assay | 0.125, 0.25, 0.5, 1, and 2.5 mM | α-Glucosidase activity↓ | ||||
|
| ||||||
| Alismol | ||||||
|
| ||||||
| Anti-inflammatory | South Korea | In vitro | Tunicamycin-treated HepG2 | 100 μM | GRP78 mRNA↓ | [15] |
| China | In vitro | LPS-treated RAW264.7 | 0.39, 1.56, 6.25, 25, and 100 μM | NO↓ | [51] | |
| Japan | In vitro | LPS-treated macrophages | 50, 100 μM | NO↓ iNOS↓ | [50] | |
| Antihypertensive | Japan | In vivo | Hypertensive rats | 100 mg/kg | Blood pressure↓ | [52] |
| Japan | In vivo | Heparin-treated rats | 10 mM | Cardiac output↓ Heart rate↓ Left ventricular pressure↓ Coronary flow↑ | [53] | |
| Japan | In vitro | Ca2+-treated rabbit thoracic aorta tissue | 10, 300 mM | Contractile response↓ | [54] | |
| Japan | In vitro | Angiotensin I-treated rabbit thoracic aorta tissue | 10 mM | Contractile response↓ | [55] | |
| Japan | In vitro | Noradrenaline | 10 mM | Contractile response↓ | [56] | |
3.1. Alisol A 24-Acetate
Triterpenes are currently regarded as one of the attractive phytochemical groups due to their therapeutic potential of anti-inflammatory, antiviral, antimicrobial, immunomodulatory, and antitumor actions [73]. Alisol A 24-acetate (Figure 1(a), C32H52O6, 532.75 g/mol) is one of the major active protostane-type triterpenes isolated from A. orientale. Alisol A 24-acetate decreased the lipid droplet, intracellular/hepatic TG and liver FFA contents accumulated by FFA or methionine and choline-deficient (MCD) in HepG2, WRL-68 human liver embryonic cell, and mouse models [42, 43, 45]. Simple hepatic steatosis is caused by excessive expression of TG synthetic genes (ACC and FAS) and reduced expression of carnitine palmitoyltransferase (CPT)1 and acyl-coA oxidase (ACOX)1 activating fatty acid oxidation. AMP-activated protein kinase (AMPK) activation suppressed TG accumulation in liver via inhibiting the activation of the sterol regulatory element-binding protein (SREBP)-1c transcriptional factor. The possible mechanism of Alisol A 24-acetate is likely through AMPK-SREBP-1c-FAS-ACC-CPT1-ACOX1 pathways against simple hepatic steatosis, and its intrinsic signaling may contribute to the antisteatotic effects of A. orientale [42]. In addition, adiponectin activation in the liver is known to be associated with the AMPK pathways [74]. Alisol A 24-acetate enhanced the adiponectin level in FFA-treated HepG2 cells and suppressed inflammatory cytokines (IL-6, IL-1β, MCP-1, and TNF-α) and fibrogenic factors (α-smooth muscle actin (SMA), transforming growth factor (TGF)-β, and TIMP) in NASH and fibrosis experimental models [42, 43]). Furthermore, Alisol A 24-acetate improved serum/liver lipid profile in hyperlipidemic mice induced by lipid emulsion diet and atherogenic diet [44, 45]. Collectively, Alisol A 24-acetate could be a key compound of A. orientale to contribute to its efficacy against NAFLD in the perspective of inhibiting the fundamental pathological process such as steatosis, inflammation, and fibrosis. Besides, a recent study reported that it effectively reversed the atherosclerotic markers, in particular, matrix metalloproteinase (MMP)-2/MMP-9 in smooth muscle cells [43]. Hence, Alisol A 24-acetate might be applied to manage a cardiovascular disease, a representative risk factor of NAFLD-related mortality.
3.2. Alisol B 23-Acetate
Alisol B 23-acetate (Figure 1(b), C32H50O5, 514.8 g/mol) is a major protostane triterpene which exhibits potent bioactivity. It is currently regarded as the official indicator for the quality control of medicinal herb A. orientale in the Pharmacopoeia of the People's Republic of China [72]. To date, emerging evidence demonstrates that Alisol B 23-acetate has a variety of therapeutic effects. In particular, its involvement with FXR deserves important results. Alisol B 23-acetate enhanced liver regeneration after partial resection [75] and maintained bile-acid homeostasis [76], both via FXR activation. Its role as FXR agonist is expanded to its protective activities against NASH in mice. Indeed, it played a critical action in TG and fatty acid synthesis and metabolism, thus preventing inflammation and fibrogenesis in MCD-diet mice via FXR stimulation [46]. Its antioxidant [47, 77] and hepatoprotective [46] effects are also likely associated with downstream target genes of FXR. Hence, Alisol B 23-acetate is anticipated to show pharmacological effects similar to FXR agonists such as obeticholic acid and ursodeoxycholic acid in NAFLD treatment. In addition, it exhibited strong hypolipidemic effects by inhibiting the activity of HMG-CoA reductase and activating lipoprotein lipase (LPL) activity. In particular, its binding interaction with HMG-CoA reductase is even more potent than Alisol A 24-acetate [44]. Taken together, Alisol B 23-acetate might substantially contribute to the biological actions of A. orientale against NAFLD based on its effect of downregulating hepatic lipid genesis, increasing lipid output, regulating inflammation and fibrosis, and exerting hepatoprotective effects via FXR activation.
3.3. Alisol F
Alisol F (Figure 1(c), C30H48O5, 488.7 g/mol) is one of protostane-type triterpenes isolated from A. orientale like above two compounds. A previous study reported that it exhibited antiviral activity against hepatitis B virus in HepG2.2.15 cells with the inhibitory concentration (IC)50 of 0.6 μM and 8.5 μM on HBsAg and HBeAg secretion, respectively [78]. Apart from its antiviral effect, Alisol F has been mainly investigated to check its pharmacological activities against inflammation. Alisol F suppressed a variety of powerful inflammatory mediators such as iNOS, NO, COX-2, TNF-α, IL-6, and IL-1β elevated by lipopolysaccharide (LPS) in macrophages and LPS or D-gal injection in mice [48–50]. The molecular mechanism of Alisol F against LPS-induced inflammation is reported to involve activation of NF-κB and phosphorylation of its upstream molecules mitogen-activated protein kinase (MAPK)s (extracellular-signal-regulated kinase (ERK), p38, JNK) [48]. Additionally, Alisol F alleviated acute hepatic failure induced by LPS and D-gal injection by lowering AST and ALT levels in mice [48]. Besides, Alisol F could regulate hyperglycemia via α-glucosidase activity inhibition without adipose cell differentiation and lipogenesis unlike thiazolidinedione (TZD)s [40]. However, evidence on the effects of Alisol F against NAFLD is lacking and further study is needed to elucidate its pharmacological activities.
3.4. Alismol
Alismol (Figure 1(d), C15H24O, 220.356 g/mol) is one of guaiane-type sesquiterpenes isolated from A. orientale that possesses anti-inflammatory activity by inhibiting NO production and iNOS synthesis in RAW 264.7 cells induced by LPS [50, 51]. In addition, Alismol showed significant blocking effects against the GRP78 expression, a ER stress marker, in tunicamycin-treated HepG2 cells [15]. Tunicamycin is a sort of ER stress inducer by causing the unfolded protein response (UPR) in cells. UPR and ER stress are often observed in hepatic cells of NAFLD or obesity patients and may play a pivotal role in the progression to NASH or cirrhosis. Since GRP78 leads to the UPR survival, the pharmacological effects suppressing the GRP78 expression of Alismol suggest that it might be one of representative compounds of A. orientale contributing to anti-ER stress and hepatic steatosis [15]. Interestingly, Alisol B 23-acetate, a triterpenoid of A. orientale did not show protective effects against ER stress marker proteins. Therefore, further study of therapeutic and preventive effects of A. orientale against NAFLD and its progression needs to be implemented using Alismol as well as Alisol B 23-acetate. Furthermore, Alismol was found to exhibit antihypertensive effects via the inhibition of sympathetic neuron and Ca2+ influx [50, 52–55], which might be interconnected with the diuretic activities of A. orientale. Since hypertension is a risk factor of cardiovascular disease which increases the mortality of NAFLD patients, Alismol is worthy of attention for the treatment of NAFLD.
4. Discussion
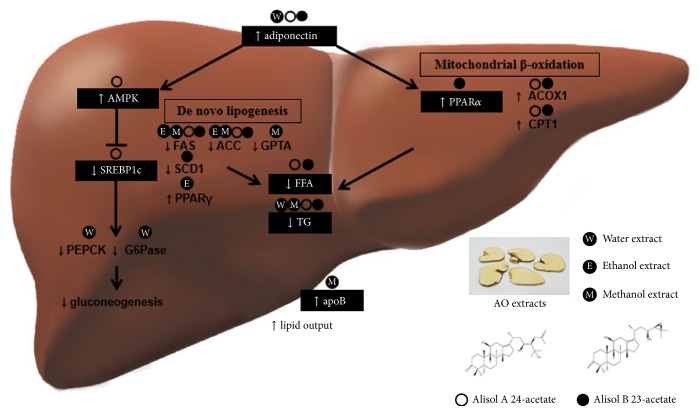
This is the first review of A. orientale actions and its molecular mechanisms against NAFLD and metabolic syndrome. First, A. orientale including Alisol A 24-acetate and Alisol A 23-acetate hindered hepatic de novo lipogenesis and accelerated β-oxidation via AMPK and PPARα activation by adiponectin, leading to the inhibition of hepatic TG accumulation and increase of lipid output from liver. In addition, A. orientale suppressed hepatic gluconeogenesis by regulating hepatic expression of glucogenic genes like PEPCK and G6Pase via AMPK-SREBP1c signaling (Figure 2). This antidiabetic effect of A. orientale could be influenced by its actions elevating adiponectin. Adiponectin not only is a key cytokine for NAFLD but also is involved in obesity, T2DM, inflammation, apoptosis, fibrosis, and even cancer. Adiponectin has been described as an ideal target against NAFLD. TZDs, approved T2DM drugs, are currently regarded as replaceable agents for targeting adiponectin in NAFLD patients, but there exist some limitations of weight gain or insignificant lobular inflammation, ballooning, and fibrosis improvement [79]. These results stimulate the further investigation of A. orientale.
Figure 2.
Molecular mechanism related to pharmacological effects of A. orientale regulating lipid and glucose metabolism in liver. A. orientale stimulated adiponectin and subsequently suppressed hepatic de novo lipogenesis and accelerated fatty acid oxidation via AMPK and PPARα activation, resulting in decreased hepatic TG contents and lipid output acceleration from liver. In addition, A. orientale regulated hepatic gluconeogenesis by lowering PEPCK and G6Pase mRNA via AMPK-SREBP1c signaling.
Second, A. orientale is expected to exhibit pharmacological effects against NAFLD and metabolic syndrome similar to an FXR agonist. Alisol B 23-acetate intervened the downstream regulators of FXR such as SREBP1c, PPARα, and genes involved in triglyceride metabolism (ApoC-II, ApoC-III, and angiopoietin like ANGPTL3), contributing to the improvement of hyperlipidemia as well as hepatic steatosis. In addition, A. orientale reversed cholestasis, AST, and ALT levels by activating FXR. FXR is a transcriptional factor mainly expressed in the liver, intestine, and kidney. Since patients with NAFLD have decreased hepatic expression of protein and mRNA of FXR and it is associated with hepatic steatosis, inflammation, fibrosis, injury, and even cancer [80], FXR appears to gain increasing interest as a promising target to treat NAFLD. Although obeticholic acid is a representative FXR agonist, hyperlipidemia and hyperglycemia are well-known unfavorable effects [81]. Hence, it needs further study about AO to elucidate its role as a FXR agonist and check its possible side effects for the treatment of NAFLD and metabolic syndrome.
Based on most studies, Alisol A 24-acetate and Alisol B 23-acetate could be key bioactive components of A. orientale against NAFLD and metabolic syndrome. However, these compounds were not obtained from the water extract of A. orientale which is prevalent in making herbal decoctions, and it is difficult to use methanol extract of A. orientale in practical use because of safety concerns from methanol extraction process. Meanwhile, water extract of A. orientale exhibited antisteatotic, antioxidant, antilipoapoptotic, hepatoprotective, anti-inflammatory and antifibrotic, hypolipidemic, antiobesity, and hypoglycemic effects. Therefore, identification for efficacy component from water extract of A. orientale needs to be established using systematic novel analysis to show the correlation between component contents and bioactivity for the quality control of A. orientale.
5. Conclusion
Despite ongoing studies, there still exist difficulties in identifying specific pathophysiological mechanism underlying NAFLD and metabolic syndrome. This is the first review to demonstrate in detail that A. orientale and its chemical substances can contribute to the treatment of NAFLD and metabolic syndrome based on their pharmacological activities such as antisteatotic, antioxidant, antilipoapoptotic, hepatoprotective, anti-inflammatory, antifibrotic, hypolipidemic, antiobesity, and hypoglycemic effects. In particular, A. orientale regulated effectively lipid and glucose metabolism in the liver and controlled liver injury like oxidative stress, inflammation, and fibrosis. Moreover, A. orientale was involved in hyperglycemia, obesity, or hyperlipidemia, representative comorbidities of NAFLD. The underlying mechanism of A. orientale is partly revealed to be linked to adiponectin, AMPK, SREBP1c, or FXR. In particular, Alisol A 24-acetate and Alisol B 23-acetate are considered main effective compounds of A. orientale. In addition, since A. orientale strengthened the ApoA-IV promoter activity and elevated its mRNA level in intestinal cells, more supplementary data and efforts to identify effective compounds on the control of food intake are required to support the potential of A. orientale. With a comprehensive approach, this review is anticipated to support pharmacological activities of A. orientale and its compounds and serve as a stimulus to develop novel therapeutic and preventive drugs against NAFLD and metabolic syndrome. Further studies are needed to verify valid ingredients, efficient dosage, and extraction procedures of A. orientale to maximize its therapeutic potential. Moreover, a variety of preclinical results in this review are required to be driven to more reasonable conclusion through a well-designed and appropriate clinical trials for potential clinical application of A. orientale.
Acknowledgments
This research was supported by a Grant (HI14C0955) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Eunsol Choi and Eungyeong Jang equally contributed to this work.
References
- 1.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Estes C., Razavi H., Loomba R., Younossi Z., Sanyal A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumida Y., Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. Journal of Gastroenterology. 2017;53(3):1–15. doi: 10.1007/s00535-017-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geier A., Rau M. Emerging therapies for NASH - the future is now. Expert Review of Clinical Pharmacology. 2017;10(5):467–469. doi: 10.1080/17512433.2017.1305269. [DOI] [PubMed] [Google Scholar]
- 5.Pappachan J. M., Babu S., Krishnan B., Ravindran N. C. Non-alcoholic fatty liver disease: a clinical update. Journal of Clinical and Translational Hepatology. 2018;5(4):384–393. doi: 10.14218/JCTH.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thounaojam M. C., Navalsinh Jadeja R., Vijaysinh Devkar R., Vadathala Ramachandran A. Non-alcoholic steatohepatitis: an overview including treatments with herbals as alternative therapeutics. Journal of Applied Biomedicine. 2012;10(3):119–136. doi: 10.2478/v10136-012-0008-9. [DOI] [Google Scholar]
- 7.Jadeja R., Devkar R. V., Nammi S. Herbal medicines for the treatment of nonalcoholic steatohepatitis: current scenario and future prospects. Evidence-Based Complementary and Alternative Medicine. 2014;2014:18. doi: 10.1155/2014/648308.648308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian T., Chen H., Zhao Y.-Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: a review. Journal of Ethnopharmacology. 2014;158(1):373–387. doi: 10.1016/j.jep.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Buzzetti E., Pinzani M., Tsochatzis E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease. Metabolism - Clinical and Experimental. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Iruarrizaga-Lejarreta M., Varela-Rey M., Fernández-Ramos D., et al. Role of aramchol in steatohepatitis and fibrosis in mice. Hepatology Communications. 2017;1(9):911–927. doi: 10.1002/hep4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim E. Y., Lee J. H. The effect of alisma orientale extract on free fatty acid-induced lipoapoptosis in HepG2 cells. Journal of Internal Korean Medicine. 2014;35(2):184–194. [Google Scholar]
- 12.Jeong H.-S., Cho Y.-H., Kim K.-H., et al. Anti-lipoapoptotic effects of Alisma orientalis extract on non-esterified fatty acid-induced HepG2 cells. BMC Complementary and Alternative Medicine. 2016;16(1):1–11. doi: 10.1186/s12906-016-1181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying G. Effection of rhizoma alismatis on adipose degeneration hepatocytes. Chinese Journal of Veterinary Medicine. 2016;52(9):38–40. [Google Scholar]
- 14.Seo M. J., Ha H., Yoon S. H. Effects of alismatis rhizoma extracts on lipid components in experimental liver injury. Korean Journal of Environmental Toxicology. 1995;10(3-4):15–20. [Google Scholar]
- 15.Jang M.-K., Han Y.-R., Nam J. S., et al. Protective effects of Alisma orientale extract against hepatic steatosis via inhibition of endoplasmic reticulum stress. International Journal of Molecular Sciences. 2015;16(11):26151–26165. doi: 10.3390/ijms161125944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong X., Tang H., Wu L., Li A. Protective effects of the Alisma orientalis extract on the experimental nonalcoholic fatty liver disease. Journal of Pharmacy and Pharmacology. 2006;58(10):1391–1398. doi: 10.1211/jpp.57.10.0013. [DOI] [PubMed] [Google Scholar]
- 17.Dan H., Wu J., Peng M., et al. Hypolipidemic effects of alismatis rhizome on lipid profile in mice fed high-fat diet. Saudi Medical Journal. 2011;32(7):701–707. [PubMed] [Google Scholar]
- 18.Sumida Y., Niki E., Naito Y., Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radical Research. 2013;47(11):869–880. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 19.Lee J. C., Lee E., Lee Y. C. Effects of rhizoma alismatis on lipid composition and TBARS concentration in rat fed high fat diet. The Korea Journal of Herbology. 2008;23(3):113–117. [Google Scholar]
- 20.Rhew K. Y., Choi H. J., Kim N. J., Lee J. H. Hepatoprotective and antioxidative effects of Alisma orientale. Natural Product Sciences. 2011;17(4):285–290. [Google Scholar]
- 21.Han C. W., Kang E. S., Ham S. A., Woo H. J., Lee J. H., Seo H. G. Antioxidative effects of Alisma orientale extract in palmitate-induced cellular injury. Pharmaceutical Biology. 2012;50(10):1281–1288. doi: 10.3109/13880209.2012.673629. [DOI] [PubMed] [Google Scholar]
- 22.Seki E., Brenner D. A., Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143(2):307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han C. W., Joo M. S., Lee J. H. Comparison of the therapeutic efficacy of rhizoma alismatis, fructus crataegi, fructus lycii, radix curcumae, radix salviae miltiorrhizae, herba artemisiae scopariae on the experimental cellular model of nonalcoholic fatty liver disease. China Journal of Chinese Materia Medica. 2012;33(4):533–542. doi: 10.4268/cjcmm20110629. [DOI] [Google Scholar]
- 24.Qiu W., Wang X., Leibowitz B., Yang W., Zhang L., Yu J. PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology. 2011;54(4):1249–1258. doi: 10.1002/hep.24516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazanave S. C., Mott J. L., Elmi N. A., et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. The Journal of Biological Chemistry. 2009;284(39):26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong H. S. Efficacy of alismatis orientale rhizoma on obesity induced by high fat diet. The Korea Journal of Herbology. 2013;28(3):95–106. doi: 10.6116/kjh.2013.28.3.95. [DOI] [Google Scholar]
- 27.Yoon S. H., Seo M. J., Cjang Y. H., Kwon O. J. The effect of Alismatis extract on benzo(a)pyrene induced hepatotoxicity. Journal of Korean Environmental and Hygienic Science. 1994;2(1):109–114. doi: 10.1126/science.7912449. [DOI] [Google Scholar]
- 28.Yang K. H., Choi S. H., Park J. C. Protective effects of methanol extract and alisol B 23-acetate of Alisma orientale on acetaminophen-induced hepatotoxicity in rats. Natural Product Sciences. 2012;18(2):121–129. [Google Scholar]
- 29.Rhew K. Antioxidative effects of dichloromethane extract of Alisma orientale on non-alcoholic fatty liver disease induced by methionine and choline-deficient(MCD) diet [Thesis for the Degree of Doctor of Philosophy at Kyung Hee University in South Korea] South Korea: Kyung Hee University; 2012. [Google Scholar]
- 30.Lee H.-J., Choi M.-S. Measurement of inhibitory activities on 3-hydroxy-3-methylglutaryl CoA reductase and acyl-CoA: cholesterol acyltransferase by various plant extracts in vitro. Journal of the Korean Society of Food Science and Nutrition. 1999;28(4):958–962. doi: 10.1126/science.286.5441.958. [DOI] [Google Scholar]
- 31.Ding C.-Y., Tan Q.-Y., Shi N.-C. Alisma versus Gliclazide in the Treatment of Primary Diabetes in Goto-Kakizaki Rats. Acta Academiae Medicinae Sinicae. 2015;37(4):451–455. doi: 10.3881/j.issn.1000-503X.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Jeong E. H., Jun D. W., Cho Y. K., et al. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clinical and Molecular Hepatology. 2013;19(3):p. 266. doi: 10.3350/cmh.2013.19.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eun J. S., Hong J. S., So J. N. Effects of the extracts from hoelen alba, alismatis rhizoma and atractylodes rhizoma on proliferation and differentiation of 3T3-L1 cells. Korean Journal of Pharmacognosy. 1993;24(2):131–139. [Google Scholar]
- 34.Guo A. J., Choi R. C.-Y., Cheung A. W.-H., et al. Stimulation of Apolipoprotein A-IV expression in Caco-2/TC7 enterocytes and reduction of triglyceride formation in 3T3-L1 adipocytes by potential anti-obesity Chinese herbal medicines. Chinese Medicine. 2009;4(1):1–8. doi: 10.1186/1749-8546-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park Y.-J., Kim M.-S., Kim H.-R., et al. Ethanol extract of Alismatis rhizome inhibits adipocyte differentiation of OP9 cells. Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/415097.415097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau C. H., Chan C. M., Chan Y. W., et al. In vitro antidiabetic activities of five medicinal herbs used in Chinese medicinal formulae. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2008;22(10):1384–1388. doi: 10.1002/ptr.2513. [DOI] [PubMed] [Google Scholar]
- 37.Bo Y. X., Ming H. Z., Bin C. W., Yan C. H., Hua W. J., Le X. Therapeutic and protective effects of water-ethanolic extract from rhizoma alismatis on streptozotocin-induced diabetic mice. Pharmaceutical Journal of Chinese People's Liberation Army. 2002;6(18):336–339. [Google Scholar]
- 38.Nam J., Rhee S., Kang M. Effects of different lengths of treatment with rhizoma alismatis on diabetic mellitus of streptozotocin-induced hyperglycemic rats. The Journal of Internal Korean Medicine. 2006;27(4):791–796. [Google Scholar]
- 39.Shin S. M., Jeong Y. J., Park D. W., et al. Screening for anti-diabetic effects of prescribed korean traditional medicines. Korean Journal of Plant Resources. 2012;25(6):670–681. doi: 10.7732/kjpr.2012.25.6.670. [DOI] [Google Scholar]
- 40.Li Q., Qu H. Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis Rhizoma. Fitoterapia. 2012;83(6):1046–1053. doi: 10.1016/j.fitote.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Cao L., Zhu K., Jiang C., et al. Intervention effects of the extract of alismatis rhizoma on streptozotocin-induced type 2 diabetic rats. Journal of China Pharmaceutical University. 2017;48(5):601–608. [Google Scholar]
- 42.Zeng L., Tang W., Yin J., et al. Alisol A 24-acetate prevents hepatic steatosis and metabolic disorders in HepG2 cells. Cellular Physiology and Biochemistry. 2016;40(3-4):453–464. doi: 10.1159/000452560. [DOI] [PubMed] [Google Scholar]
- 43.Wu C., Jing M., Yang L., et al. Alisol A 24-acetate ameliorates nonalcoholic steatohepatitis by inhibiting oxidative stress and stimulating autophagy through the AMPK/mTOR pathway. Chemico-Biological Interactions. 2018;291:111–119. doi: 10.1016/j.cbi.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Xu F., Yu H., Lu C., Chen J., Gu W. The cholesterol-lowering effect of alisol acetates based on HMG-CoA reductase and its molecular mechanism. Evidence-Based Complementary and Alternative Medicine. 2016;2016:11. doi: 10.1155/2016/4753852.4753852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai Y., Matsumura H., Aramaki Y. Hypocholesterolemic effect of alisol a-24-mono-acetate and its related compounds in rats. The Japanese Journal of Pharmacology. 1970;20(2):222–228. doi: 10.1254/jjp.20.222. [DOI] [PubMed] [Google Scholar]
- 46.Meng Q., Duan X.-P., Wang C.-Y., et al. Alisol B 23-acetate protects against non-alcoholic steatohepatitis in mice via farnesoid X receptor activation. Acta Pharmacologica Sinica. 2017;38(1):69–79. doi: 10.1038/aps.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jong M. H., Jong W. C., Jong C. P. Effects of methanol extract of Alisma orientale rhizome and its major component, alisol B 23-acetate, on hepatic drug metabolizing enzymes in rats treated with bromobenzene. Archives of Pharmacal Research. 2007;30(12):1543–1549. doi: 10.1007/BF02977323. [DOI] [PubMed] [Google Scholar]
- 48.Bi X., Wang P., Ma Q., et al. Anti-Inflammatory activities and liver protection of alisol F and 25-anhydroalisol F through the inhibition of MAPK, STAT3, and NF-κB activation in vitro and in vivo. Molecules. 2017;22(6):1–13. doi: 10.3390/molecules22060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Q., Han L., Bi X., et al. Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale. Phytochemistry. 2016;131:150–157. doi: 10.1016/j.phytochem.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda H., Kageura T., Toguchida I., Murakami T., Kishi A., Yoshikawa M. Effects of sesquiterpenes and triterpenes from the rhizome of Alisma orientale on nitric oxide production in lipopolysaccharide-activated macrophages: Absolute stereostructures of alismaketones-B 23-acetate and -C 23-acetate. Bioorganic & Medicinal Chemistry Letters. 1999;9(21):3081–3086. doi: 10.1016/S0960-894X(99)00536-3. [DOI] [PubMed] [Google Scholar]
- 51.Li H.-M., Fan M., Xue Y., et al. Guaiane-type sesquiterpenoids from alismatis rhizoma and their anti-inflammatory activity. Chemical & Pharmaceutical Bulletin. 2017;65(4):403–407. doi: 10.1248/cpb.c16-00798. [DOI] [PubMed] [Google Scholar]
- 52.Yamahara J., Kobayashi G., Iwamoto M., Matsuda H., Fujimura H. The effect of alismol isolated from alismatis rhizoma on experimental hypertensive models in rats. Phytotherapy Research. 1989;3(2):57–60. doi: 10.1002/ptr.2650030205. [DOI] [Google Scholar]
- 53.Yamahara J., Matsuda H., Kobayashi G., Katayama T., Fujimura H. Effect of alismol isolated from Alismatis Rhizoma on working heart perfusion in rat. Phytotherapy Research. 1989;3(2):72–74. doi: 10.1002/ptr.2650030209. [DOI] [Google Scholar]
- 54.Matsuda H., Kobayashi G., Yamahara J., Fujimura H., Kurahashi K., Fujiwara M. Effects of alismol isolated from Alismatis Rhizoma on calcium-induced contraction in the rabbit thoracic aorta. Life Sciences. 1987;41(15):1845–1852. doi: 10.1016/0024-3205(87)90704-1. [DOI] [PubMed] [Google Scholar]
- 55.Yamahara J., Matsuda H., Murakami H., Fujimura H. The active principle of alismatis rhizoma which inhibits contractile responses in aorta. Chemical & Pharmaceutical Bulletin. 1986;34(10):4422–4424. doi: 10.1248/cpb.34.4422. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda H., Yamahara J., Kobayashi G., Fujimura H., Kurahashi K., Fujiwara M. Effect of alismol on adrenergic mechanism in isolated rabbit ear artery. The Japanese Journal of Pharmacology. 1988;46(4):331–335. doi: 10.1254/jjp.46.331. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong M. J., Houlihan D. D., Bentham L., et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. Journal of Hepatology. 2012;56(1):234–240. doi: 10.1016/j.jhep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Kruger A. J., Fuchs B. C., Masia R., et al. Prolonged cenicriviroc therapy reduces hepatic fibrosis despite steatohepatitis in a diet‐induced mouse model of nonalcoholic steatohepatitis. Hepatology Communications. 2018;2(5):529–545. doi: 10.1002/hep4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berlanga A., Guiu-Jurado E., Porras J. A., Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clinical and Experimental Gastroenterology. 2014;7(1):221–239. doi: 10.2147/CEG.S62831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirovski G., Dorn C., Huber H., et al. Elevated systemic monocyte chemoattractrant protein-1 in hepatic steatosis without significant hepatic inflammation. Experimental and Molecular Pathology. 2011;91(3):780–783. doi: 10.1016/j.yexmp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Park P.-H, Sanz-Garcia C., Nagy L. E. Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in the liver. Current Pathobiology Reports. 2015;3(4):243–252. doi: 10.1007/s40139-015-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pastori D., Polimeni L., Baratta F., Pani A., Del Ben M., Angelico F. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Digestive and Liver Disease. 2015;47(1):4–11. doi: 10.1016/j.dld.2014.07.170. [DOI] [PubMed] [Google Scholar]
- 63.Martins I. J. Avasimibe and sirt 1 activators reverse NAFLD and obesity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;17(1):211–216. doi: 10.1161/ATV.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 64.Corey K. E., Vuppalanchi R., Wilson L. A., Cummings O. W., Chalasani N. NASH resolution is associated with improvements in HDL and triglyceride levels but not improvement in LDL or non-HDL-C levels. Alimentary Pharmacology & Therapeutics. 2015;41(3):301–309. doi: 10.1111/apt.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan J., Kim S., Wong V. W. New trends on obesity and NAFLD in Asia. Journal of Hepatology. 2017;67(4):862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Buechler C., Wanninger J., Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World Journal of Gastroenterology. 2011;17(23):2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M., Doi T., Shen L., et al. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2001;280(5):R1382–R1387. doi: 10.1152/ajpregu.2001.280.5.R1382. [DOI] [PubMed] [Google Scholar]
- 68.Aubert J., Begriche K., Knockaert L., Robin M. A., Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clinics and Research in Hepatology and Gastroenterology. 2011;35(10):630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Zelber-Sagi S., Kessler A., Brazowsky E., et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2006;4(5):639–644. doi: 10.1016/j.cgh.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Tilg H., Moschen A. R., Roden M. NAFLD and diabetes mellitus. Nature Reviews Gastroenterology & Hepatology. 2017;14(1):32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 71.Younossi Z. M., Gramlich T., Matteoni C. A., Boparai N., McCullough A. J. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clinical Gastroenterology and Hepatology. 2004;2(3):262–265. doi: 10.1016/S1542-3565(04)00014-X. [DOI] [PubMed] [Google Scholar]
- 72.Shu Z., Pu J., Chen L., et al. Alisma orientale: ethnopharmacology, phytochemistry and pharmacology of an important traditional chinese medicine. American Journal of Chinese Medicine. 2016;44(2):227–251. doi: 10.1142/S0192415X16500142. [DOI] [PubMed] [Google Scholar]
- 73.Ríos J.-L. Effects of triterpenes on the immune system. Journal of Ethnopharmacology. 2010;128(1):1–14. doi: 10.1016/j.jep.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 74.Combs T. P., Marliss E. B. Adiponectin signaling in the liver. Reviews in Endocrine and Metabolic Disorders. 2014;15(2):137–147. doi: 10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng Q., Chen X., Wang C., et al. Alisol B 23-acetate promotes liver regeneration in mice after partial hepatectomy via activating farnesoid X receptor. Biochemical Pharmacology. 2014;92(2):289–298. doi: 10.1016/j.bcp.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Meng Q., Chen X.-L., Wang C.-Y., et al. Alisol B 23-acetate protects against ANIT-induced hepatotoxity and cholestasis, due to FXR-mediated regulation of transporters and enzymes involved in bile acid homeostasis. Toxicology and Applied Pharmacology. 2015;283(3):178–186. doi: 10.1016/j.taap.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 77.Kim S. E., Rhyu D. Y., Yokozawa T., Park J. C. Antioxidant effect of Alisma plantago-aquatica var. orientale and its main component. Korean Journal of Pharmacognosy. 2007;38(4):372–375. [Google Scholar]
- 78.Jiang Z. Y., Zhang X. M., Zhang F. X., et al. A new triterpene and anti-hepatitis B virus active compounds from Alisma orientalis. Planta Medica. 2006;72(10):951–954. doi: 10.1055/s-2006-947178. [DOI] [PubMed] [Google Scholar]
- 79.Polyzos S. A., Mantzoros C. S. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: a systematic review. Metabolism - Clinical and Experimental. 2016;65(9):1297–1306. doi: 10.1016/j.metabol.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 80.Huang X., Zhao W., Huang W. FXR and liver carcinogenesis. Acta Pharmacologica Sinica. 2015;36(1):37–43. doi: 10.1038/aps.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makri E., Cholongitas E., Tziomalos K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2016;22(41):9039–9043. doi: 10.3748/wjg.v22.i41.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]