Abstract
Sleep disturbance and respiratory sinus arrhythmia (RSA) are well-known to be independently associated with depression. Yet, it remains unclear how sleep disturbance and impaired physiological regulation (indexed by RSA) may synergistically contribute to depression risk. The current study examined the relationship between sleep disturbance (duration, insomnia) on daily depressive symptoms, and whether RSA moderated this relationship in a sample of young adults with a history of depression. To examine hypotheses, participants (N = 102, ages 18–22) completed a laboratory socio-evaluative stressor task to assess RSA at rest and reactivity. Participants then completed daily measures of sleep duration, insomnia symptoms, and depressive symptoms for two weeks. For main effects, multilevel modeling indicated that shorter overall sleep duration (but not insomnia) predicted higher depressive symptoms, and individual fluctuations in insomnia symptoms (but not sleep duration) predicted higher levels of next-day depressive symptoms. Lower resting RSA, but not reactivity, potentiated these relationships. Individual differences in sleep disturbance (duration and insomnia) predicted prospective levels of depressive symptoms among individuals with lower physiological regulation (indexed by lower RSA), who were particularly vulnerable to the daily effects of sleep disturbance on depressed mood. These results suggest the need to examine both daily sleep disturbance and physiological regulation to understand who may be at greatest risk for depression.
Keywords: depression, sleep disturbance, insomnia, sleep duration, physiological regulation, respiratory sinus arrhythmia
Depression is one of the most prevalent mental health conditions world-wide (World Health Organization, 2017), with considerable personal and societal costs (Greenberg et al., 2015). High rates of depression recurrence enhance its debilitating effects, with over 50% of individuals with prior depression likely to have a depression recurrence (Burcusa and Iacono, 2007; Kessler et al., 1997). These alarming statistics underscore the importance of identifying individual differences in risk among individuals with a prior depression history, which may provide clinical targets for prevention and early intervention for depression.
Sleep disturbance, including insufficient sleep duration and insomnia symptoms (i.e., difficulty initiating and/or maintaining sleep) are consistently noted among individuals with current, past, and at risk for depression (Franzen and Buysse, 2008; Zhai et al., 2015). Over 90% of individuals with depression report sleep complaints (Geoffroy et al., 2018), which are also the most persistent symptoms of depression and predictive of recurrence (Buysse et al., 2008; Judd et al., 1994; van Mill et al., 2014). To date, most research on sleep and depression have focused on these relationships using global, retrospective measures, which may obscure differences within the individual and day-to-day. Experience sampling methods are well-suited to capture daily fluctuations in sleep both within and across people to better evaluate the day-to-day temporal impact on depressed mood. Indeed, a recent systematic review of daily sleep and mood indicates that poor sleep quality is associated with higher next-day negative affect (Konjarski et al., 2018). Although findings with sleep duration were mixed, younger individuals were more likely to have increased next-day negative affect after shorter sleep duration compared to their norm (Konjarski et al., 2018). However, all studies included in the review examined daily sleep and mood among healthy or currently depressed individuals. Thus, our study will be the first to examine the effect of both habitual and individual fluctuations of sleep disturbance (sleep duration and insomnia symptoms) on daily depressive symptoms among individuals with prior depression. This approach may illuminate important targets for early detection and intervention by identifying which individuals are at elevated risk for depressed mood (i.e., those with higher sleep disturbance) and when (i.e., when exposed to sleep disturbance the prior night).
Despite the robust associations between sleep disturbance and depression, not all individuals are equally affected by poor sleep. This highlights the need to identify vulnerability factors that exacerbate the effects of sleep disturbance on depression. One candidate process is heart rate variability that occurs in the range of respiration (i.e., respiratory sinus arrhythmia (RSA), which has been consistently implicated in regulatory processes and psychopathology (Beauchaine and Thayer, 2015; Stange et al., 2017b; Thayer and Lane, 2000), including depression (for reviews, see Hamilton and Alloy, 2016; Rottenberg, 2007). Adaptive physiological regulation is typically marked by higher levels of RSA at rest (reflecting appropriate physiological flexibility) and RSA reactivity (i.e., decrease in RSA from rest during challenge), reflecting the efficiency of physiological regulation to environmental challenge. In contrast, lower RSA and blunted RSA reactivity reflect more impaired physiological regulation, with research and theory demonstrating its associations to poor emotion regulatory responses and depression (Rottenberg et al., 2007b; Yaroslavsky et al., 2013a; Yaroslavsky et al., 2013b). Though most studies have examined differences between individuals with and without current or past depression (Bylsma et al., 2014), several studies demonstrate that individual differences in resting RSA predict depressive symptoms among individuals with current or past depression (major and minor) (Hamilton and Alloy, 2017; Rottenberg et al., 2007a).
Extending this research to sleep and depression, it is possible that individuals with impaired physiological regulation (indexed by lower resting RSA and blunted RSA reactivity) may be more vulnerable to the cognitive and depressogenic effects of poor sleep. Though research indicates that lower RSA and poor sleep are bi-directionally associated (Dodds et al., 2017; Werner et al., 2015), fewer studies have examined both RSA and sleep in relation to depression. These studies demonstrate that poor sleep quality is associated with lower resting RSA and blunted RSA reactivity in healthy individuals and those with current depression (Bylsma et al., 2014; Rottenberg et al., 2007a; Yang et al., 2011). Only two studies have investigated their joint associations in depressed mood among younger populations, including toddlers (Cho et al., 2017) and elementary-aged children (El-Sheikh et al., 2007). El-Sheikh and colleagues (2007) found that shorter sleep duration and lower resting RSA predicted elevated depressive and externalizing symptoms (El-Sheikh et al., 2007). No known study has evaluated RSA (resting or stress-reactive) and sleep disturbance in depressed mood among individuals with prior depression, who are vulnerable to sleep disturbance and impaired physiological regulation (lower resting RSA; blunted RSA reactivity) (Li et al., 2012; Visted et al., 2018). Given that both RSA and sleep disturbance are linked with neurocognitive and affective impairments, particularly emotion dysregulation (Beauchaine, 2015; Palmer and Alfano, 2017; Palmer et al., 2018; Thayer et al., 2009; Walker, 2009; Watling et al., 2017), it is possible that individuals with atypical RSA– both at rest and in response to environmental demands–have particular difficulty regulating emotions following sleep disturbance. Among individuals with prior depression, individual differences in RSA may serve as a vulnerability factor that exacerbates the effects of daily sleep on depression. Thus, identifying an objective index of physiological regulation may provide more precise targets for identifying which individuals are most affected by poor sleep and therefore at heightened risk for future depression.
The Current Study
The present study sought to examine the daily effects of sleep duration and insomnia symptoms in depressed mood among young adults with a depression history, and RSA as a potential moderator of this relationship. The current study utilized a nuanced approach to sleep disturbance by examining both between-person (mean-level or habitual) and daily fluctuations in sleep (within-person). Further, we included a heterogenous sample of individuals with past major or minor depression, given prior research demonstrating similar levels of RSA, emotional reactivity, and functional impairment (Bylsma et al., 2011; Hamilton and Alloy, 2017; O’Leary et al., 2017). We hypothesized that individuals reporting more overall and daily fluctuations in sleep disturbance (shorter sleep duration, insomnia symptoms) would exhibit higher levels of overall and next-day depressive symptoms. We also expected individuals with poorer physiological regulation (indexed by lower resting RSA and blunted RSA reactivity) to have more depression following sleep disturbance. Understanding the potential interactive effects of impaired physiological regulation in this vulnerable population may better inform depression intervention and prevention efforts targeting sleep and depression.
Method
Procedure
Participants included 102 late adolescents and emerging adults (ages 18–22) in the Stress and Emotion study at a northeastern university. Participants first completed an online screener to determine eligibility, which required individuals to be 1) ages 18–22, 2) fluent in written and spoken English, and 3) have a history of major depression (MDD) or minor depression (mD), but not current depression or bipolar disorder. Eligible participants completed a baseline laboratory assessment and two weeks of daily surveys. During baseline, participants completed questionnaires (e.g., depression symptoms), diagnostic interview to confirm past depression diagnoses, and a well-validated laboratory-based socio-evaluative stressor during which participants were connected to an electrocardiogram (ECG) from which RSA was derived. Participants then remotely completed 14 days of electronic diary assessments of sleep and mood using an internet-capable device at night (between the hours of 6PM-12AM), designed to accommodate school and work schedules (see below for more details). Overall, participants completed 12.20 diaries (87.14%; range = 6–14; SD = .03) over the 14-day period. Thus, all participants were included in analytic sample.
Participants
Participants (N = 102) in the present study were 19.86 years old (SD = 1.17 years), 78% were female, and 70% self-identified as Caucasian, 7% as African American, 6% as Biracial, 15% as Asian, 2% as ‘Other,’ and 7% as Hispanic/Latino. Twenty percent of our sample identified as lesbian, gay, or bisexual, or ‘Something else/Other’. Diagnostic screening via clinical interview indicated that 76 met criteria for past MDD and 26 for mD depression (see Table 1). On average, first onset of depression (MDD or mD) was 15.77 years (SD = 3.32). About half (N = 50; 49%) of participants reported one depression episode, 39 (38%) reported two, and 13 (13%) reported three or more. More detailed information about recruitment and screening procedures are reported elsewhere (Hamilton and Alloy, 2017).
Table 1.
Descriptive statistics of study variables for overall sample and by major and minor depression
| Overall (N = 102) | MDD (N = 76) | mD (N = 26) | |
|---|---|---|---|
| Measure | M (SD) or N (%) | M (SD) or N (%) | M (SD) or N (%) |
| Age | 19.86 (1.17) | 19.91 (1.16) | 19.73 (1.22) |
| Sex (% female) | 79 (77%) | 61 (80%) | 18 (69%) |
| Resting RSA | 6.49 (1.14) | 6.57 (1.10) | 6.28 (1.22) |
| RSA Reactivity | .57 (1.04) | .64 (1.01) | .36 (1.11) |
| Dep Sx | 10.89 (2.95) | 11.28 (3.20) | 9.77 (1.61)** |
| Sleep Duration | 7.79 (.86) | 7.77 (.84) | 7.85 (.94) |
| Insomnia Sx | 1.59 (1.32) | 1.71 (1.32) | 1.21 (1.28) |
Note: RSA = Respiratory Sinus Arrhythmia; Dep = Depression; Sx = Symptoms. There was only a significant difference between individuals with a history of major and minor depression on current depressive symptoms (t = 3.12, p < .01).
Baseline Assessment
Depression Diagnoses.
To confirm past depression diagnosis, participants completed the mood portions of a semi-structured diagnostic interview (expanded Schedule for Affective Disorders and Schizophrenia – Lifetime; SADS-L (Alloy et al., 2008; Endicott and Spitzer, 1978). Depressive disorders were defined as DSM-IV major depression or minor depression (2 or more clinically significant symptoms for 2 weeks or more, or 5 or more symptoms for at least a week, with functional impairment). The SADS-L has demonstrated excellent inter-rater reliability in prior studies, with κ > .90 for depression diagnoses based on 80 jointly rated interviews (Alloy et al., 2000). Interviews were audio-recorded, and interviewer agreement was examined in 20 randomly-selected interviews. Interviewers demonstrated excellent agreement in the study (κ = .95, p < .001).
Respiratory Sinus Arrhythmia (RSA) and Trier Social Stress Task (TSST).
Heart rate variability was measured using electrocardiogram (ECG) data from disposable Ag/AgCl electrodes placed in a modified Lead-II configuration on the chest, sampled at 1000 Hz using the BioPac BioHarness MP150 and AcqKnowledge v. 4.2 software. To assess resting RSA, heart rate was monitored continuously for three minutes while participants sat quietly. Participants then completed the Trier Social Stress Task (TSST), which is a well-validated socio-evaluative stress task. Our version included a 3-minute speech preparatory phase, 3-minute speech about themselves in front of a neutral experimenter and camera and 1-minute surprise calculation task [full stressor task], and three-minute recovery period. ECG data were imported into Kubios HRV and QRS detection using the piecewise cubic spline interpolation with the default rate of 4 Hz for RR interpolation rate (version 2.2)(Tarvainen et al., 2014). ECG data was pre-processed in Kubios and visually inspected and manually corrected for artifacts (< 1% required adjustment); no artifact correction algorithms or detrending methods were applied to the data. We used fast Fourier transformation with a 256-s window with 50% overlap (Welch’s periodogram) for each phase of the TSST. Consistent with the recommendations by the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology (1996), the absolute power density (ms2) for each band of high frequency (HF; .15–.40 Hz), low frequency (LF; .04–.15), and very low frequency (VLF; 0–.04) was calculated and the average for each period was used. Only HF HRV was included in analyses, subsequently referred to as RSA. Due to the continuous nature of the stressor and immediacy of the math task following speech, the full stressor portion [speech and math] were combined and analyzed. Anticipatory stress was similarly analyzed separately. 1 Due to violations of normality, the data were log-transformed to normalize the distribution (Bylsma et al., 2014) and we controlled for respiration by regressing RSA on average RR for each phase using the unstandardized residuals (Bylsma et al., 2014; Hamilton and Alloy, 2017). RSA reactivity to the stressor task was calculated by subtracting the residualized RSA value during the full stressor [speech and math tasks] from baseline (resting) RSA. Residualized RSA variables were used in study analyses, and unresidualized log-transformed (ms2) RSA is presented in Table 1.
Depression Symptoms.
At baseline, participants also completed a 28-item measure of depressive symptoms over the past two weeks: Patient Reported Outcomes Measurement Information System-Depression Long Form (PROMIS-Depression- LF; (Pilkonis et al., 2011)). The PROMIS-Depression-LF was used as a covariate in analyses. In the current study, internal consistency was α = .97.
Daily Diary
Daily Depressive Symptoms over the past day were measured with the 8-item PROMIS-Depression-Short Form (SF) during the two-week diary (Pilkonis et al., 2011). Of note, the PROMIS-Depression-SF does not include any items assessing sleep. The PROMIS-Depression-SF also has been found to have sound psychometrics similar to the long form version (Olino et al., 2012; Olino et al., 2013; Pilkonis et al., 2011). In the present study, the internal reliability of the PROMIS-Depression-SF was α = .90.
Sleep Domains.
On each daily diary, participants reported the time (hour and minutes) that they went to bed and wakeup time (hour and minutes). The total time in bed (waketime minus bedtime) was used as an estimate of sleep duration. Participants also completed three items of insomnia (i.e., difficulty falling and staying asleep in the night and early morning) from the Insomnia Severity Index (ISI) (Bastien et al., 2001; Morin, 1993). These items are on a 5-point scale, ranging from 0 (none) to 4 (very severe), with a higher sum reflecting more insomnia symptoms (ranging from 0–12). For mean-level (habitual) sleep, we calculated each person’s mean across the 14-day period for each sleep domain. Using this mean, we calculated individual daily fluctuations around each person’s own mean by subtracting daily sleep values (e.g., person-centered). Thus, higher daily values reflect individual increases in insomnia and lower daily values in duration reflect individual decreases in duration compared to each person’s average or mean-level sleep duration and insomnia symptoms.
Statistical Analyses
Descriptive statistics and bivariate correlations between the primary variables were examined. T-tests were conducted to examine potential race and gender differences in primary study variables (RSA, sleep, depressive symptoms). We also conducted t-tests to evaluate differences on sleep characteristics by depression history (MDD vs. sub-threshold).
To test our hypotheses, we conducted two-level multilevel modeling in Mplus 7.0 (Muthén and Muthén, 2013). Multilevel modeling is a statistical technique well-suited to examine within-person daily observations collected using experience sampling methods. There were no significant patterns of missing data, and data was considered to be missing at random. Thus, we used Maximum Likelihood to estimate parameters for missing data (e.g., daily items) within individuals to maximize data use. This approach is consistent with guidelines for missing data that use all available observations without removing data (Schafer and Graham, 2002). For all analyses, Level 1 predictors are the daily sleep domains. To parse out the effects of daily sleep on depressed mood at the within-person level, sleep duration and insomnia symptoms were person mean-centered. Specifically, we subtracted each person’s individual mean of sleep duration or insomnia symptoms from their daily sleep values (for each domain, respectively). Thus, in the current analyses, daily values of sleep duration and insomnia represent individual (within-person) fluctuations in sleep above or below each person’s own average sleep duration or insomnia. Level 2 predictors represent each person’s individual mean of sleep duration and insomnia over the two-week period (between-person), which allows for comparison between individuals. RSA was grand-mean centered prior to conducting interaction analyses. Fixed effects were estimated with Level 1 (within-person sleep) and Level 2 predictors (mean-level sleep). We also estimated a random intercept and random slope of daily sleep on depressive symptoms to allow for individual variability. For the main effects of sleep disturbance on depression, we covaried MDD history, baseline depressive symptoms, gender, and age, as well as prior-day depressive symptoms. We also covaried for body mass index (BMI) and depression medication use, which are linked to RSA (Kemp et al., 2010).
First, to examine the main effects of sleep duration and insomnia symptoms, we examined both between and within-person sleep (mean-level) and individual fluctuations of each sleep domain as a predictor of next-day depressive symptoms. This allowed us to compare the effects of individual sleep disturbance on depressive symptoms both within and across individuals.2 We also tested one full model with both sleep duration and insomnia symptoms to determine the relative strength on depressive symptoms. To examine whether RSA (resting or stress-reactive) moderated the effects of sleep disturbance on depressed mood, we conducted a cross-level interaction between RSA (resting and reactivity) and sleep (average and individual fluctuations of sleep duration and insomnia symptoms), such that the slope of sleep on depressive symptoms was modeled and RSA was entered as a predictor of slope. For these models, as in above, the random intercept and random slope of individual sleep fluctuations on depressive symptoms were estimated. Resting and RSA reactivity moderation analyses were conducted separately. A total of six moderation models were conducted. When there was evidence of a significant interaction, we probed the interaction at high and low levels (plus or minus 1 SD) of RSA.
Results
Preliminary Analyses
Table 1 presents demographic characteristics and decriptives of primary study variables for the overall sample and by past depression diagnosis (MDD vs. mD), and Table 2 presents bivariate associations. On average, participants had 7.79 hours of sleep per night. However, almost all participants (92%) reported less than 7 hours of sleep on at least one night, which is the minimum amount of sleep recommended for young adults (Hirshkowitz et al., 2015), and 72% and 39% reported less than six and five hours on at least one night, respectively. Of note, there were no significant differences by sex (ts < 1.80, ps > .08) or race (ts < 1.43, ps > .16) of primary study variables.
Table 2.
Descriptive statistics and bivariate correlations of primary study variables
| Measure | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1 | Resting RSA | - | ||||
| 2 | RSA Reactivity | .57 | - | |||
| 3 | Dep Sx | −.07 | −.03 | - | ||
| 4 | Sleep Duration | .01 | −.09 | −.23 | - | |
| 5 | Insomnia Sx | .09 | −.05 | .16 | .07 | - |
Note. Correlations ≥ .17 are statistically significant (p < .05). Mean levels of daily depressive symptoms and sleep are reported. RSA = Respiratory Sinus Arrhythmia (log-transformed ms2 unadjusted for respiration rates); Dep = Depression; Sx = Symptoms.
Main Effects of Sleep Domains on Prospective Daily Depressive Symptoms
Consistent with our hypotheses, we found main effects of sleep disturbance on depressed mood, controlling for depression history (MDD vs. mD), baseline depressive symptoms, age, gender, BMI, and depression medication. For sleep duration (Table 3), we found that shorter average sleep duration over the two-week period predicted higher depressive symptoms. However, at the individual level, we found that only marginally significant effects of daily shorter sleep duration on depressive symptoms, controlling for prior-day depressive symptoms.
Table 3.
Effect of Sleep Duration on Depression Symptoms
| Variable | Depression Symptoms | ||
|---|---|---|---|
| Fixed Effects | B | SE | z-score (B/SE) |
| Between-person | |||
| Intercept (Dep Sx) | 8.83 | 3.82 | 2.31* |
| MDD | .59 | .39 | 1.50 |
| Sex | .69 | .45 | 1.55 |
| Dep Med | .76 | .44 | 1.71 |
| BMI | −.01 | .05 | −.28 |
| Age | .11 | .15 | .72 |
| Baseline Dep Sx | .04 | .01 | 4.63*** |
| Sleep Duration | −.49 | .21 | −2.30* |
| Within-person | |||
| Prior Day Dep Sx | −.25 | .04 | 6.97*** |
| Sleep Duration | −.13 | .07 | −1.82 |
| Random Effects | |||
| Intercept | 2.32 | .57 | 4.07*** |
| Slope of Sleep Duration | .10 | .06 | 1.71 |
Note.
p <. 05
p < .01
p < .001.
B = unstandardized parameter estimate; SE = Standard Error. Dep Sx = Depression Symptoms; MDD = Major Depressive Disorder (Coded as 0 = None; 1 = Present); Dep Med = Depression Medication; BMI = Body Mass Index.
For insomnia symptoms (Table 4), there were no main effects of overall mean-level insomnia symptoms on depressive symptoms. There was, however, a main effect of individual fluctuations of insomnia symptoms on next-day depressive symptoms. Specifically, individual increases in insomnia were associated with more depressive symptoms the next day, even after accounting for depressive symptoms the prior day. Add random interpretation. Of note, for the full model containing all sleep disturbance measures (average and individual fluctuations) was consistent with these findings, demonstrating that only shorter average sleep duration (B= −.58, SE = .24, p = .02, CI [95] = −1.07– −.03) and individual increases in insomnia (B= .28, SE = .07, p < .001; CI [95] = .12–.41) predicted next-day depression symptoms.
Table 4.
Effect of Insomnia Symptoms on Depression Symptoms
| Variable | Depression Symptoms | ||
|---|---|---|---|
| Fixed Effects | B | SE | z-score (B/SE) |
| Between-person | |||
| Intercept (Dep Sx) | 4.53 | 3.48 | 1.30 |
| MDD | .59 | .41 | 1.44 |
| Sex | .61 | .45 | 1.34 |
| Dep Med | .67 | .45 | 1.48 |
| BMI | −.001 | .05 | −.04 |
| Age | .10 | .16 | .64 |
| Baseline Dep Sx | .04 | .01 | 4.77*** |
| Insomnia Sx | .09 | .15 | .61 |
| Within-person | |||
| Prior Day Dep Sx | .20 | .04 | 5.63*** |
| Insomnia Sx | .30 | .07 | 4.11*** |
| Random Effects | |||
| Intercept | 2.50 | .60 | 4.15*** |
| Slope of Insomnia Sx | .01 | .04 | .33 |
Note.
p <. 05
p < .01
p < .001.
B = unstandardized parameter estimate; SE = Standard Error. Dep = Depression; Sx = Symptoms; MDD = Major Depressive Disorder (Coded as 0 = None; 1 = Present); Dep Med = Depression Medication; BMI = Body Mass Index.
Interactive Effects of RSA and Sleep Domains on Depression Symptoms
Consistent with our hypotheses, there were significant interactions between resting RSA and individual fluctuations in sleep duration (B = .15; SE = .07; p = .04; CI [95] = .001–.29; Figure 1) and insomnia symptoms (B = −.15; SE = .07; p = .04; CI [95] = −.29 – −.01; Figure 2) on depressive symptoms. Specifically, we found that individual decreases in sleep duration and increases in insomnia symptoms were associated with more next-day depressive symptoms, but only for individuals with lower resting RSA (sleep duration: B = −.28, SE = .06, p < .01, CI [95] = −.49– −.08; insomnia symptoms: B = .40, SE = .10, p < .001, CI [95] = .20 – .60). These associations were attenuated among individuals with higher resting RSA (sleep duration: B = .09, SE = .06, p = .81, CI [95] = −.08 – .12; insomnia: B = .09, SE = .11, p = .41, CI [95] = −.13 – .31). In addition, our findings with sleep duration and insomnia symptoms were specific to individual fluctuations of sleep disturbance, and there were no significant interactions with average sleep duration (B = −.31, SE = .36, p =.24; CI [95] = −.28 –.57, or insomnia (B = .04, SE = .08, p =.83; CI [95] = −.32 – .41), or with stress-reactive RSA for daily sleep duration (B = .07, SE = .08, p = .36),) or insomnia symptoms (B = −.13, SE = .07, p = .06), or habitual sleep duration (B = .33, SE = .26, p = .21), or insomnia (B = −.10, SE = .21, p = .63).
Figure 1.
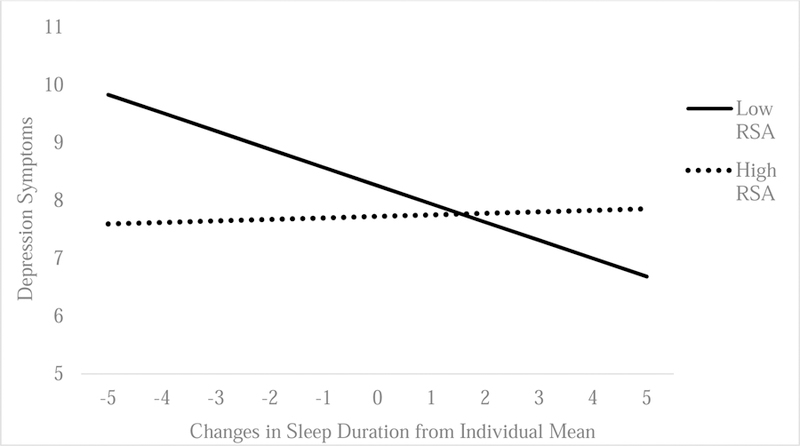
Sleep Duration and Resting RSA Predicting Next-Day Depression Symptoms
Note. Horizontal axis reflects changes in hours of sleep duration from the individual mean. Thus, negative numbers reflect the number of hours of sleep lost (decreases in sleep duration) compared to individual’s mean, whereas positive numbers reflect sleep gained (increases in sleep duration) from individual mean.
Figure 2.
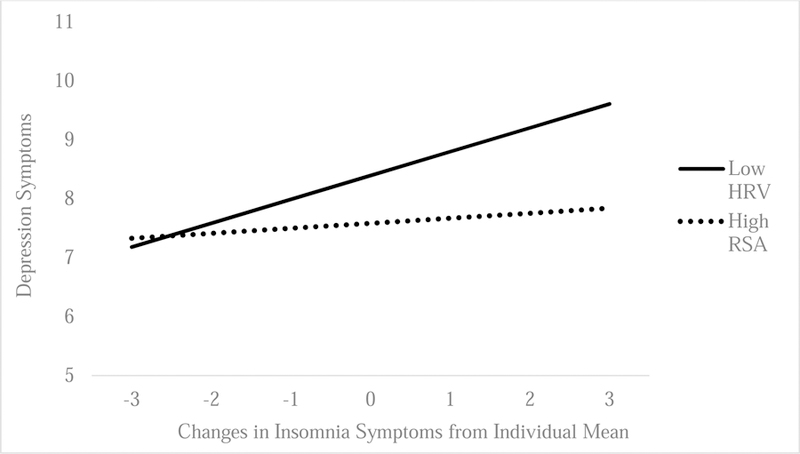
Insomnia Symptoms and Resting RSA Predicting Next-Day Depression Symptoms
Note. Horizontal axis reflects changes in quantity of insomnia symptoms (reported on the Insomnia Severity Index; raw range 0–12, with higher numbers indicating more difficulty initiating and maintain sleep). Thus, negative numbers reflect decreases in reported insomnia symptoms from the individual mean, whereas positive numbers reflect increases in insomnia symptoms from individual mean.
Discussion
Extending prior research using experience sampling methods to examine sleep and mood (Konjarski et al., 2018), our study found differential effects of habitual and individual fluctuations of sleep disturbance on depressive symptoms. First, we found that individuals with shorter habitual sleep, but not insomnia symptoms, experienced elevated depressive symptoms over the two-week period among young adults with prior depression. Second, examining the day-to-day associations of sleep disturbance indicated that individual fluctuations in insomnia symptoms (but not sleep duration) predicted next-day depressive symptoms, such that daily increases in insomnia symptoms compared to an individuals’ mean predicted next-day depressive symptoms. These findings support past research that chronic or habitual short sleep duration contributes to elevated depressive symptoms (Franzen and Buysse, 2008), and daily increases in insomnia contribute to higher levels of depressed mood the next day (Konjarski et al., 2018). However, our study is the first to demonstrate these individual differences in sleep disturbance among individuals with prior depression, who are vulnerable to future depression and sleep problems (Burcusa and Iacono, 2007). These findings may highlight both who is at risk for elevated depression (i.e., those with chronic sleep deprivation) and when certain individuals are most at risk for depressive symptoms (i.e., worsening insomnia compared to one’s norm).
Importantly, our results indicate that acute individual fluctuations in sleep disturbance (shorter duration and increased insomnia symptoms compared to one’s mean) may be most detrimental for those with lower resting RSA. These findings build upon prior research with youth (El-Sheikh et al., 2007), and extend results to an at-risk sample for future depression. These findings suggest that sleep disturbance may be more depressogenic for individuals with already impaired self-regulatory capacity to manage cognitive and emotional resources due to lower resting RSA (Thayer and Lane, 2000)— as reflected by resting RSA. For instance, sleep disturbance contributes to heightened affective arousal and diminished cognitive regulatory abilities, particularly in the context of emotion (Baum et al., 2014; Palmer and Alfano, 2017). Individuals with lower resting RSA often exhibit poorer emotion regulation (Beauchaine, 2015). In combination with sleep loss, these individuals may be more compromised and have more difficulty navigating emotional states and external demands. Thus, individuals with lower RSA levels and sleep disturbance and may be doubly at risk for symptoms of depression, thereby heightening risk for depression recurrence. Importantly, these findings are also in the context of those with a history of depression who typically exhibit impaired emotion regulation and sleep disturbance (Visted et al., 2018). Examining sleep disturbance and indices of physiological regulation among at-risk individuals may reveal those at greatest risk for depression recurrence.
Surprisingly, we did not find interactive effects between sleep and stress-reactive RSA, which we expected given research indicating that blunted RSA reactivity predicts depression (Hamilton and Alloy, 2016; Stange et al., 2017a). However, it is possible that our stress task did not map onto RSA fluctuations during more naturalistic events or stressors (Hedge et al., 2018). Future studies should use ambulatory methods to assess RSA reactivity in a real-world setting, which may better capture the context in which individuals with poor physiological regulation are vulnerable to depression following sleep disturbance. Future research should take advantage of the technological advances in ambulatory assessment to evaluate sleep and RSA in an ecologically-valid context using behavioral indices (Mohr et al., 2017). In addition, our study did not find that habitual sleep disturbance interacted with RSA to predict higher levels of depressive symptoms, which suggests that self-regulatory capacity may have stronger influences on the sleep-depression relationship for acute sleep fluctuations rather than on overall sleep levels. Thus, physiological regulation (RSA) may be particularly important when internal cognitive and emotional resources are taxed due to shorter sleep or insomnia symptoms. However, it is also possible that a longer follow-up may reveal a continued impact of habitual sleep disturbance and lower resting RSA, which would also allow for a more nuanced temporal examination between sleep and RSA. Specifically, given prior research that sleep and RSA have direct associations at rest (Werner et al., 2015) and during challenge (Mezick et al., 2014), it is possible that lower RSA contributes to poor sleep, which further impacts physiological regulation, thereby contributing to a vicious cycle of risk for depression.
Our study should be considered within the context of several notable limitations. First, self-reported measures were used for sleep duration and insomnia symptoms, and future studies should utilize objective measures of sleep via actigraphy to identify behavioral patterns of sleep. In addition, diaries of wake- and bed- times were used to estimate sleep duration, and we did not assess sleep onset latency or nocturnal awakenings. Thus, our measure of sleep duration reflects ‘time in bed,’ and may be overestimated and/or biased by the individuals’ perceptions of bed and wake times. Second, our study did not measure all sleep domains that predict depression, such as nightmares and sleep variability (Bei et al., 2017; Bei et al., 2016), which is important for future research. We also did not examine sleep at baseline, which limits our ability to control for prior sleep disturbances or identify the temporal patterns with depression. Further, although we covaried for respiration, speaking during the stressor tasks (speech and math calculations) may confound respiration rate estimates and impact RSA. Although it is important to assess RSA during a naturalistic stress conditions, future studies should consider a social stressor that does not require speech components and may better estimate RSA.
Overall, the present study indicates the importance of a personalized approach to evaluating sleep disturbance, and the moderating influence of physiological regulation among young adults with a depression history. Importantly, our study used both laboratory-based tasks and daily measurements to assess the relationships between RSA, sleep, and depression, which allowed for the simultaneous examination of both habitual and individual fluctuations sleep on depression and interactive effects of laboratory-assessed RSA. This is one of the first studies to use a fine-grained, idiographic approach to examine sleep disturbance on depressed mood and moderating effects of RSA among a high-risk sample for future depression, which provides valuable insight into for who and when sleep disturbance may contribute to depressed mood among at-risk individuals. Although preliminary, this work may have clinical implications by highlighting when certain individuals may be at risk. It also demonstrates the importance of objectively-indexed regulatory function (RSA) as a potential marker of which individuals are vulnerable to depression following sleep disturbance, which can be a therapeutic target (Lehrer and Gevirtz, 2014). Finally, it is critical that future research examine a wider variety of sleep health domains and RSA using objective and subjective methods in real-world contexts. Using ambulatory devices, we can better understand how sleep indices and real-world physiological regulation predict risk for depression, and intervene in real time. Given that sleep and RSA are transdiagnostic risk factors for mental and physical disorders, it will be important for future research to examine these relationships in other clinical populations, as well as among non-clinical populations to determine whether these patterns predict future risk.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health to Jessica L. Hamilton (F31MH106184, T32HL082610), Jonathan P. Stange (1K23MH112769-01A1), Peter Franzen (R01DA033064), and Lauren B. Alloy (R01MH079369, R01MH101168), and the National Science Foundation Graduate Research Fellowship to Taylor A. Burke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare.
To account for the possibility of respiration impacting RSA during the full stressor portions (speech and math), we also conducted analyses with RSA reactivity during the anticipatory phase (from baseline). Results were comparable to the full stressor, and therefore only these results are presented.
Given research indicating that hypersomnia is also associated with depression (Franzen and Buysse, 2008), we conducted exploratory analyses to test a quadratic effect of sleep duration and depressive symptoms, which would indicate that both shorter and longer sleep predicted depression. These results were not significant and are not included here (but are available upon request).
References
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, Kim RS, Lapkin JB, 2000. The Temple-Wisconsin Cognitive Vulnerability to Depression Project: lifetime history of axis I psychopathology in individuals at high and low cognitive risk for depression. J Abnorm Psychol 109(3), 403–418. [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME, 2008. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord 10(2), 310–322. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM, 2001. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2(4), 297–307. [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW, 2014. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry 55(2), 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, 2015. Respiratory Sinus Arrhythmia: A Transdiagnostic Biomarker of Emotion Dysregulation and Psychopathology. Curr Opin Psychol 3, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Thayer JF, 2015. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol 98(2 Pt 2), 338–350. [DOI] [PubMed] [Google Scholar]
- Bei B, Seeman TE, Carroll JE, Wiley JF, 2017. Sleep and Physiological Dysregulation: A Closer Look at Sleep Intraindividual Variability. Sleep 40(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J, Manber R, 2016. Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev 28, 108–124. [DOI] [PubMed] [Google Scholar]
- Burcusa SL, Iacono WG, 2007. Risk for recurrence in depression. Clin Psychol Rev 27(8), 959–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W, 2008. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep 31(4), 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, Rottenberg J, 2014. Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosom Med 76(1), 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Taylor-Clift A, Rottenberg J, 2011. Emotional reactivity to daily events in major and minor depression. J Abnorm Psychol 120(1), 155–167. [DOI] [PubMed] [Google Scholar]
- Cho S, Philbrook LE, Davis EL, Buss KA, 2017. Sleep duration and RSA suppression as predictors of internalizing and externalizing behaviors. Dev Psychobiol 59(1), 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds KL, Miller CB, Kyle SD, Marshall NS, Gordon CJ, 2017. Heart rate variability in insomnia patients: A critical review of the literature. Sleep Med Rev 33, 88–100. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS, 2007. Children’s sleep and adjustment: the moderating role of vagal regulation. J Sleep Res 16(4), 396–405. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, 1978. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives of general psychiatry 35(7), 837–844. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, 2008. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci 10(4), 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy PA, Hoertel N, Etain B, Bellivier F, Delorme R, Limosin F, Peyre H, 2018. Insomnia and hypersomnia in major depressive episode: Prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J Affect Disord 226, 132–141. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC, 2015. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76(2), 155–162. [DOI] [PubMed] [Google Scholar]
- Hamilton JL, Alloy LB, 2016. Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clin Psychol Rev 50, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JL, Alloy LB, 2017. Physiological markers of interpersonal stress generation in depression. Clin Psychol Sci 5(6), 911–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C, Powell G, Sumner P, 2018. The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behav Res Methods 50(3), 1166–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ, 2015. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 1(1), 40–43. [DOI] [PubMed] [Google Scholar]
- Judd LL, Rapaport MH, Paulus MP, Brown JL, 1994. Subsyndromal symptomatic depression: a new mood disorder? J Clin Psychiatry 55 Suppl, 18–28. [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM, 2010. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 67(11), 1067–1074. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz M, 1997. Prevalence, correlates, and course of minor depression and major depression in the National Comorbidity Survey. J Affect Disord 45(1–2), 19–30. [DOI] [PubMed] [Google Scholar]
- Konjarski M, Murray G, Lee VV, Jackson ML, 2018. Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Med Rev 42, 47–58. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Gevirtz R, 2014. Heart rate variability biofeedback: how and why does it work? Front Psychol 5, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Lam SP, Chan JW, Yu MW, Wing YK, 2012. Residual sleep disturbances in patients remitted from major depressive disorder: a 4-year naturalistic follow-up study. Sleep 35(8), 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall MH, Richard Jennings J, Kamarck TW, 2014. Sleep duration and cardiovascular responses to stress in undergraduate men. Psychophysiology 51(1), 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Zhang M, Schueller SM, 2017. Personal Sensing: Understanding Mental Health Using Ubiquitous Sensors and Machine Learning. Annual Review of Clinical Psychology 13, 23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, 1993. Insomnia: psychological assessment and management Guilford Press, New York. [Google Scholar]
- Muthén LK, Muthén BO, 2013. Mplus 7.11. Statistical Analysis with Latent Variables User’s Guide Muthén & Muthén, Los Angeles, CA. [Google Scholar]
- O’Leary K, Small BJ, Panaite V, Bylsma LM, Rottenberg J, 2017. Sleep quality in healthy and mood-disordered persons predicts daily life emotional reactivity. Cogn Emot 31(3), 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Yu L, Klein DN, Rohde P, Seeley JR, Pilkonis PA, Lewinsohn PM, 2012. Measuring depression using item response theory: an examination of three measures of depressive symptomatology. Int J Methods Psychiatr Res 21(1), 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Yu L, McMakin DL, Forbes EE, Seeley JR, Lewinsohn PM, Pilkonis PA, 2013. Comparisons across depression assessment instruments in adolescence and young adulthood: an item response theory study using two linking methods. J Abnorm Child Psychol 41(8), 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CA, Alfano CA, 2017. Sleep and emotion regulation: An organizing, integrative review. Sleep Med Rev 31, 6–16. [DOI] [PubMed] [Google Scholar]
- Palmer CA, Oosterhoff B, Bower JL, Kaplow JB, Alfano CA, 2018. Associations among adolescent sleep problems, emotion regulation, and affective disorders: Findings from a nationally representative sample. J Psychiatr Res 96, 1–8. [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, Group PC, 2011. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 18(3), 263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, 2007. Cardiac vagal control in depression: a critical analysis. Biol Psychol 74(2), 200–211. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Chambers AS, Allen JJ, Manber R, 2007a. Cardiac vagal control in the severity and course of depression: the importance of symptomatic heterogeneity. J Affect Disord 103(1–3), 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K, 2007b. RSA fluctuation in major depressive disorder. Psychophysiology 44(3), 450–458. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW, 2002. Missing data: our view of the state of the art. Psychol Methods 7(2), 147–177. [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Fresco DM, Alloy LB, 2017a. Flexible parasympathetic responses to sadness facilitate spontaneous affect regulation. Psychophysiology 54(7), 1054–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Olino TM, Fresco DM, Alloy LB, 2017b. Autonomic reactivity and vulnerability to depression: A multi-wave study. Emotion 17(4), 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA, 2014. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed 113(1), 210–220. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH, 2009. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med 37(2), 141–153. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD, 2000. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61(3), 201–216. [DOI] [PubMed] [Google Scholar]
- van Mill JG, Vogelzangs N, van Someren EJ, Hoogendijk WJ, Penninx BW, 2014. Sleep duration, but not insomnia, predicts the 2-year course of depressive and anxiety disorders. J Clin Psychiatry 75(2), 119–126. [DOI] [PubMed] [Google Scholar]
- Visted E, Vollestad J, Nielsen MB, Schanche E, 2018. Emotion Regulation in Current and Remitted Depression: A Systematic Review and Meta-Analysis. Front Psychol 9, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, 2009. The role of sleep in cognition and emotion. Ann N Y Acad Sci 1156, 168–197. [DOI] [PubMed] [Google Scholar]
- Watling J, Pawlik B, Scott K, Booth S, Short MA, 2017. Sleep Loss and Affective Functioning: More Than Just Mood. Behav Sleep Med 15(5), 394–409. [DOI] [PubMed] [Google Scholar]
- Werner GG, Ford BQ, Mauss IB, Schabus M, Blechert J, Wilhelm FH, 2015. High cardiac vagal control is related to better subjective and objective sleep quality. Biol Psychol 106, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2017. Depression and Other Common Mental Disorders Global Health Estimates WHO Press, Geneva. [Google Scholar]
- Yang AC, Tsai SJ, Yang CH, Kuo CH, Chen TJ, Hong CJ, 2011. Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J Affect Disord 131(1–3), 179–185. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Bylsma LM, Rottenberg J, Kovacs M, 2013a. Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biol Psychol 94(2), 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M, 2013b. The utility of combining RSA indices in depression prediction. J Abnorm Psychol 122(2), 314–321. [DOI] [PubMed] [Google Scholar]
- Zhai L, Zhang H, Zhang D, 2015. Sleep Duration and Depression among Adults: A Meta-Analysis of Prospective Studies. Depress Anxiety 32(9), 664–670. [DOI] [PubMed] [Google Scholar]


