Abstract
Background
Environmental exposures are associated with a number of outcomes including adverse pregnancy outcomes. Although inflammation is hypothesized to play a role, the mechanistic pathways between environmental exposures and adverse health outcomes, including associations between exposures and longitudinal measures of systemic and reproductive tract inflammation, need elucidation.
Objectives
This study was conducted to evaluate whether exposure to air pollution is associated with immunologic responses in the systemic circulation and lower reproductive tract, and to evaluate whether systemic and reproductive tract immunologic responses are similar.
Methods
We quantified repeated measures of cytokines from cervico-vaginal exudates and serum obtained concurrently among 104 women with term pregnancies and estimated PM10 and CO exposure using the monitor nearest each participant’s residence. Serum and cervico-vaginal cytokines were compared using Wilcoxon signed-ranks test and Spearman rank correlations for select gestational months. We used intraclass correlation coefficients (ICCs) to quantify reproducibility of cytokine measurements, and Tobit regression to estimate associations between air pollution and cytokines.
Results
Median cervico-vaginal levels of IL-6, Eotaxin, IP-10, MCP-1, MIP-1α, MIP-1β, and TNFα were higher than corresponding serum cytokines, significantly so for IL-6 and IP-10. Cervicovaginal and serum cytokines were not correlated, but cytokines from the same fluid were correlated. ICCs for most serum cytokines were ≤ 0.40, while ICCs were higher in cervicovaginal cytokines (range 0.52–0.83). IP-10 and Eotaxin had the highest ICCs for both cytokine sources. In adjusted models, PM10 was positively associated with serum cytokines IL-6, IP-10, MIP-1β and Eotaxin but inversely associated with cervico-vaginal cytokine TNFα, IP-10, MIP1β, MCP-1 and Eotaxin, controlling for false discovery rate. CO was inversely associated with cervico-vaginal TNFα, IL-6, MIP-1β, MCP-1 and Eotaxin.
Conclusions
Inflammatory processes are compartment-specific. Systemic inflammatory markers may provide information on immunologic processes and response to environmental exposures, but are not proxies for lower reproductive tract inflammation.
Keywords: Environmental pollution, maternal inflammation, longitudinal data, cervico-vaginal cytokines, serum cytokines, PRINCESA cohort
1. Introduction
Pregnant women are exposed to environmental toxicants, including air pollution, but few epidemiologic perinatal studies have evaluated the effect of these agents on systemic and reproductive tract inflammation levels; preterm birth studies using data from cohorts with individual biological measures (versus birth registries) are even fewer(Ferguson et al., 2013). Air pollution is associated with systemic inflammation (van Eeden et al., 2001; Eeden and Hogg, 2002) and oxidative stress (Hansen et al., 2006; Kannan et al., 2006). Additionally, air pollution has been posited to play a role in affecting maternal susceptibility to infection (Hertz-Picciotto et al., 2005; Sagiv et al., 2005; Slama et al., 2008). Air pollution has been associated with premature rupture of membranes (PROM) – rupture of membranes at least an hour before the start of contractions (Pereira et al., 2014), and an increase in preterm premature rupture of membranes (PPROM) – PROM which occurs before 37 weeks of gestation (Dadvand et al., 2014). Reproductive tract infection is an established risk factor for PROM, PPROM and preterm birth (French and McGregor, 1996). Alveolar macrophages are believed to be one of the links between inhaled pollutants and inflammation in the lungs, and the subsequent systemic inflammatory response (Hiraiwa and van Eeden, 2013) that may cross the placental barrier through hematogenous dissemination (Yeo et al., 2005).
Physiologic inflammation plays an important role during normal pregnancy, but dysregulated inflammation, which may result from abnormal response to infection, air pollution and other factors, is posited to be involved in the pathology of preterm birth. The degree to which exposure to air pollution affects inflammatory markers, particularly systemic and reproductive tract inflammatory markers, is poorly understood. A current limitation is identifying the relevant tissue for measurement, as it is uncertain whether systemic inflammatory markers and lower reproductive tract inflammatory markers in pregnancy reflect the same or different pathways. A systematic review and meta-analysis of studies up to 2010 on inflammatory markers and preterm birth in asymptomatic women found that elevated levels of IL-6 obtained from amniotic and cervico-vaginal fluids, and C-reactive protein from amniotic fluid but not plasma, were strongly associated with spontaneous preterm birth among asymptomatic women, potentially suggesting different pathways (Wei et al., 2010). This is important for understanding the role of air pollution in local and systemic inflammation, and if air pollution might affect pregnancy outcomes through one or both inflammatory pathways.
Mexico City, a mega-city where distinctive geographic features and manmade sources contribute to high pollution levels (O’Neill et al., 2002), represents a useful setting to examine the effects of air pollution on inflammatory markers such as cytokines. The aims of this study were i. to evaluate whether exposure to air pollution is associated with systemic and lower reproductive tract inflammation levels, and ii. to evaluate whether systemic and reproductive tract immunologic responses are similar.
2. Material and Methods
2.1. Study participants
Study participants were selected from women participating in the Pregnancy Research on Inflammation, Nutrition, & City Environment: Systematic Analyses (PRINCESA), a longitudinal study based in Mexico City (O’Neill et al., 2013). The parent study included participants with singletons who: lived and or worked in the Mexico City metropolitan area, a requirement that was necessary to obtain air pollution data from the Mexico City Atmospheric Monitoring Network, and did not have an existing diagnosis of a medical or obstetric condition at enrollment. Participants were individuals from the general obstetric population who were included without regard to infection status at enrollment or participation in the remainder of the study. Participants concluded clinic visits when they developed medical or obstetric complications. Demographic and obstetric characteristics of participants in the current study were similar to participants enrolled in the parent study. In order to evaluate the aims of current study, participants in the study were selected based on whether cytokines from serum and cervico-vaginal samples were available for a given visit. Participants were followed at approximately monthly intervals. In addition to other enrollment requirements (O’Neill et al., 2013), informed consent was obtained from each participant. Women in the study agreed to provide clinical, microbiological, and behavioral data at each visit. The University of Michigan Institution Review Board and the Ethics Committees from the Secretaría de Salud del Gobierno de la Ciudad de México (Mexico), and the School of Medicine of the National Autonomous University of Mexico (UNAM) granted approval for the study.
These analyses were limited to participants who delivered at term and for whom same day cervico-vaginal and serum cytokines were available at the time of data analysis (N=104). Gestational months used in the analysis were selected to include one month from each trimester; since cytokine data for all participants were not available, a decision was made to select the gestational months from each trimester that had the highest number of cytokines quantified. We also ensured that none of the selections was consecutive (i.e. we used a skip pattern to have an even spread across pregnancy). Therefore, if the month with the highest number of samples in a trimester was consecutive, we used the month with second highest number of samples. This dataset was used for all analyses. The median number of visits was 3 for this dataset. The median (standard deviation) gestational weeks at sample collection for months 3, 5, and 7 were: 11.5 (1.7), 19.4 (2.5) and 29 (2.6). Samples were collected throughout the day, hour-specific data regarding time of sample collection were not available.
2.2. Assessment of lower reproductive tract infection
Assessment of infection status utilized culture count and clinical sign and symptoms recorded during clinical exams. Points were assigned according to clinical sign and symptoms and patients were classified as infected or non-infected using the following cut-off in the sum of points, a total of 3 points or less was classified as non-infected and 4 or more points was classified as infected. Lactobacillus spp. was classified as follows: absent (3 points), scarce (2 points), intermediate (1 point) and abundant (0 points). Other microorganisms were classified as absent (0 points), scarce (1 point), intermediate (2 points) and abundant (3 points). The opposite classification between Lactobacillus spp. and other microorganisms takes in to account the different effects that each group of microorganisms has on lower reproductive health (Fontaine et al., 1996; Aleshkin et al., 2006). Microorganisms included Bacillus spp., Bifidobacterium spp., Candida albicans, Escherichia coli, Gardnerella vaginalis, Haemophilus spp., Neisseria gonorrhoeae, Proteus spp., Staphylococcus aureus, Staphylococcus spp., Streptococcus spp., and Streptococcus agalactiae.
2.3. Air pollution
We used air pollution data collected by the Mexico City Atmospheric Monitoring System known as SIMAT. (Ministry of the Environment (Federal District)) SIMAT routinely collects air pollution information on carbon monoxide (CO), nitrogen dioxide (NO2), ozone (O3), particulate matter less than ten microns in aerodynamic diameter (PM)-10, PM 2.5, and sulfur dioxide (SO2). Data are collected hourly by 34 automatic monitors located throughout Mexico City and surrounding areas.
We chose to analyze PM10 in micrograms per cubic meter (μg/m3) and CO in parts-per-million (ppm), because each one represents a different type of air pollution (particle versus gaseous) and have been previously evaluated during pregnancy (Reviewed in (Vadillo-Ortega et al., 2014)). In addition, spatial and temporal differences among air pollutants have been reported(Chai et al., 2014). We used daily averages for PM10 and CO estimated from the monitor nearest each participant’s address for the day before each clinic visit. Because all monitors did not collect information on each pollutant, when data from the nearest monitor were not available, data from either the second or third nearest monitor were used (Rivera-González et al., 2015). The selection of an exposure window for air pollution estimates for the day prior was based on previous studies on the effects of air pollution on systemic inflammation that used the same day or the preceding 24-hour period prior to collection of blood (van Eeden et al., 2001; Ruckerl et al., 2007). We used average air pollution estimates for the day prior instead of the day of visit because the exact time of sample collection from study participants was not known.
2.4. Cytokine Quantification
The Millipore MILLIPLEX® MAP human cytokine/chemokine magnetic bead system (Millipore Corporation, Billerica, MA, USA) was used to measure 15 pro-inflammatory, and 5 anti-inflammatory cytokines obtained from monthly serum and cervico-vaginal samples(Millipore). The Millipore MILLIPLEX® MAP system simultaneously quantify various cytokines from a single sample(Millipore). We measured 20 cervico-vaginal cytokines (Eotaxin, Interferon gamma (IFNγ), IL-10, IL-12p40, IL-12p70, IL-17, IL-1rα, soluble IL-2 receptor alpha (sIL-2rα), IL-1α, IL-1β, IL-2, IL-4,Il-6, IL-8, Interferon gamma inducible protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1α), macrophage inflammatory protein-1 beta (MIP-1β), TNFα, and vascular endothelial growth factor (VEGF). If no value was detected for a cytokine, we used in the analysis a value less than the limit of detection (LOD) as determined by the manufacturer. The LODs varied for each cytokine, and undetected observations were substituted with LOD/√2 in all analyses, except where indicated. Observations at the upper limit of detection (> 10,000 pg/mL) were assigned a value of 10,010 for analysis.
2.5. Statistical analysis
The study population was first characterized using standard descriptive statistics. The trimester specific probability of clinician-diagnosed lower reproductive tract infection was estimated by taking an overall average of the number of infections per participant for each trimester divided by the number of visits the participant made during that trimester. The Wilcoxon signed-ranks test, which evaluates whether the median difference between groups is zero, was used to compare cervico-vaginal and serum cytokine concentrations obtained from the same set of participants for gestational months 3, 5 and 7. Because of the very high percent of observations below the LOD, especially among serum cytokines, only pairs of cytokines with 50% or more values observed were evaluated, as was done in a previous study by Vogel et al (Vogel et al., 2007).
To determine the relationships between pairs of cervico-vaginal and serum cytokines across pregnancy, we used Spearman rank correlations for select gestational months. The Spearman correlation coefficients were illustrated in heat maps with a color gradient to indicate the strength and direction of the association between cytokine pairs (Hemedinger, 2013).
Intraclass correlation coefficients (ICCs) were used to provide summary measures across participants of how similar cytokine concentrations were within each participant. Values of the ICC < 0.40; 0.40–0.75; and ≥ 0.75 indicate poor, fair to good, and excellent reproducibility, respectively (Rosner, 2006). The ICCs were calculated for each serum and cervico-vaginal cytokine using log-transformed and inverse fourth root transformation along with the average of the two types of data transformation. These transformations were previously identified using a Box-Cox procedure to accommodate the fact that our cytokine data was not normally distributed, a key assumption for ICCs (Buxton et al.). To calculate the ICCs, we used a maximum likelihood estimation for lognormally distributed data which accounted for values below the limit of detection (Jin et al., 2011).
Finally, we used Tobit regression to estimate associations of the air pollutants PM10 and CO with select cervical and serum cytokine levels (Tobin, 1958). Tobit regression accounts for both undetected values at the lower and upper ends of the data and can be used for repeated measures (Breton et al., 2007). False discovery rate (FDR) was set at 10% and used to adjust the p-values from Tobit regression models to account for multiple testing (Benjamini and Hochberg, 1995). Statistical analyses were performed using SAS Statistical Software version 9.3 (SAS Institute Inc. Cary, NC, USA) and R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Descriptive statistics
The majority of participants were between 20–35 years of age and were either normal weight or overweight before the start of pregnancy (Table 1). Most participants had previously given birth to at least one child. The probability of lower reproductive tract infection increased from the first trimester to the second trimester and then decreased in the third trimester. The median gestational age at enrollment was around 13 weeks of gestation.
Table 1.
Demographic and obstetric characteristics of pregnant women who delivered at term (N=104), PRINCESA cohort, 2009–2014
| Characteristic | n (%) |
|---|---|
| Age | |
| <20 | 18(17.3) |
| 20–35 | 75(72.1) |
| >35 | 11(10.6) |
| Pre-pregnancy BMI | |
| <18.5 kg/m2 | 7(6.7) |
| 18.5–24.9 kg/m2 | 36(34.6) |
| 25–29.9 kg/m2 | 37(35.6) |
| ≥30 kg/m2 | 17(16.4) |
| Missing | 7(6.7) |
| Parity | |
| Nulliparous | 30(28.9) |
| Parous | 58(55.8) |
| Missing | 16(15.4) |
| Probability of reproductive tract infectiona,b | |
| Trimester 1 | 0.3 |
| Trimester 2 | 0.37 |
| Trimester 3 | 0.31 |
Note: BMI, body mass index.
Mean of (number of infection per trimester/number of visits for each participant per trimester)
Microorganisms included Bacillus spp., Bifidobacterium spp., Candida albicans, Escherichia coli, Gardnerella vaginalis, Haemophilus spp., Neisseria gonorrhoeae, Proteus spp., Staphylococcus aureus, Staphylococcus spp., Streptococcus spp., and Streptococcus agalactiae
PM10 averaged 56.0 µg/m3 (SD=21.8) and CO averaged 1.5 ppm (SD=0.5) across the 582 days of observation (table 2).
Table 2.
Summary statistics for air pollutants PM10a (μg/m3) and carbon monoxide (CO) in ppm from the monitor nearest each participant’s home for the day prior to each clinic visit for participants in Mexico City, 2009 −2014.
| Mexico City Standard | Mean | SD | Minimum | Maximum | Number of days | |
|---|---|---|---|---|---|---|
| CO | 11 ppm (8hr avg) | 1.48 | 0.54 | 0.4 | 4.32 | 582 |
| PM10 | 50 μg/m3 (annual mean) | 56.03 | 21.81 | 15.06 | 139.69 | 582 |
Particulate matter less than ten microns in aerodynamic diameter
3.2. Description of cervico-vaginal and serum cytokines
The percent of observations below the limit of detection (LOD) varied across cytokines and the variation was even greater across cytokine source (i.e. serum versus cervico-vaginal samples) (Supplemental table S1). Due to this variability, especially among the serum cytokines where the percent below LOD ranged from 0–97.8% (data not shown), further analyses were restricted to cytokines with at least 50% of observations above the LOD in both cervico-vaginal and serum samples. The seven cytokines that met this criterion were: IL-6, Eotaxin, IP-10, MCP-1, MIP-1α, MIP-1β, and TNFα. All cervico-vaginal cytokine levels were substantially higher than the corresponding serum cytokines, but significant median differences between cervico-vaginal and serum cytokines were found only for IL-6 and IP-10 across all months evaluated (gestational months 3, 5, and 7) (Table 3).
Table 3.
Comparison between concurrently measured cervico-vaginal and serum cytokine concentrations (in pg/mL) at select points in pregnancy, PRINCESA cohort 2009–2014 (n=104).
| Cytokine | LODa | Range |
Median, month 3 |
|
Median, month 5 |
|
Median, month 7 |
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CVEb | Serum | CVE | Serum | pc | CVE | Serum | pc | CVE | Serum | pc | ||
| IL-6 | 0.9 | <LOD − 10,010 | <LOD − 259.8 | 11.0 | 0.6 | 0.001 | 8.5 | 3.0 | 0.01 | 12.3 | 1.9 | <0.0001 |
| Eotaxin | 4 | <LOD − 10,010 | <LOD − 286.8 | 19.7 | 29.6 | 0.60 | 23.1 | 23.8 | 0.17 | 24.8 | 24.4 | 0.05 |
| IP-10 | 8.6 | <LOD − 10,010 | 23.5 − 1,334 | 323.4 | 257.1 | 0.03 | 347.7 | 228.3 | 0.02 | 352.8 | 256.7 | 0.003 |
| MCP-1 | 1.9 | <LOD − 10,010 | 3.2 − 2,277 | 174.3 | 347.0 | 0.49 | 222.8 | 322.1 | 0.48 | 226.5 | 276.5 | 0.16 |
| MIP-1α | 2.9 | <LOD − 10,010 | <LOD − 1,166 | 13.1 | 29.1 | 0.40 | 16.5 | 76.3 | 0.44 | 7.6 | 53.2 | 0.50 |
| MIP-1β | 3 | <LOD − 10,010 | <LOD − 952.7 | 39.4 | 60.0 | 0.62 | 45.4 | 73.8 | 0.06 | 31.4 | 67.9 | 0.66 |
| TNFα | 0.7 | <LOD − 6,405 | <LOD − 278.9 | 9.6 | 11.2 | 0.36 | 9.2 | 16.1 | 0.04 | 6.6 | 15.3 | 0.43 |
Median difference was tested only for cytokines (n=7) in which both CVE and serum had 50% or more of observations above the LOD. LOD/√2 was substituted for values below the limit of detection for each cytokine and values greater than 10,000 (in pg/mL) were assigned a value of 10,010.
Lower limit of detection
CVE, cervico-vaginal exudate
p from Wilcoxon signed rank tests
3.3. Reproducibility of cervico-vaginal and serum cytokines
Serum cytokines showed poor to good reproducibility over the course of pregnancy. (Table 4) Average ICCs for most serum cytokines were less than 0.40, indicating poor reproducibility, except IP-10 and Eotaxin which exhibited fair and good reproducibility, respectively (Rosner, 2006). Reproducibility was better for the corresponding cervico-vaginal cytokines; ICC values were higher and reproducibility ranged from fair to excellent (average ICCs 0.52–0.83) Similar to serum ICCs, IP-10 and Eotaxin had the highest ICC values. (Table 4)
Table 4:
Intraclass correlation coefficients for serum and cervico-vaginal cytokines among term births, PRINCESA cohort, 2009–2014 (n=104).
| Cytokine | N | Log | Inverse Fourth Root |
|---|---|---|---|
| Serum | |||
| sIL6a | 402 | 0.28 | 0.13 |
| sTNFα | 402 | 0.20 | 0.23 |
| sIP-10 | 402 | 0.57 | 0.59 |
| sMCP-1 | 402 | 0.38 | 0.33 |
| sMIP-1α | 402 | 0.32 | 0.25 |
| sMIP-1β | 402 | 0.24 | 0.24 |
| sEotaxin | 402 | 0.71 | 0.69 |
| Cervico-vaginal | |||
| cIL6b | 405 | 0.58 | 0.55 |
| cTNFα | 405 | 0.61 | 0.54 |
| cIP-10 | 405 | 0.64 | 0.69 |
| cMCP-1 | 405 | 0.53 | 0.50 |
| cMIP-1α | 405 | 0.57 | 0.54 |
| cMIP-1β | 405 | 0.64 | 0.56 |
| cEotaxin | 405 | 0.84 | 0.81 |
s prefix indicates serum cytokine
c prefix indicates cervico-vaginal cytokine
3.4. Correlation between cervico-vaginal and serum cytokines
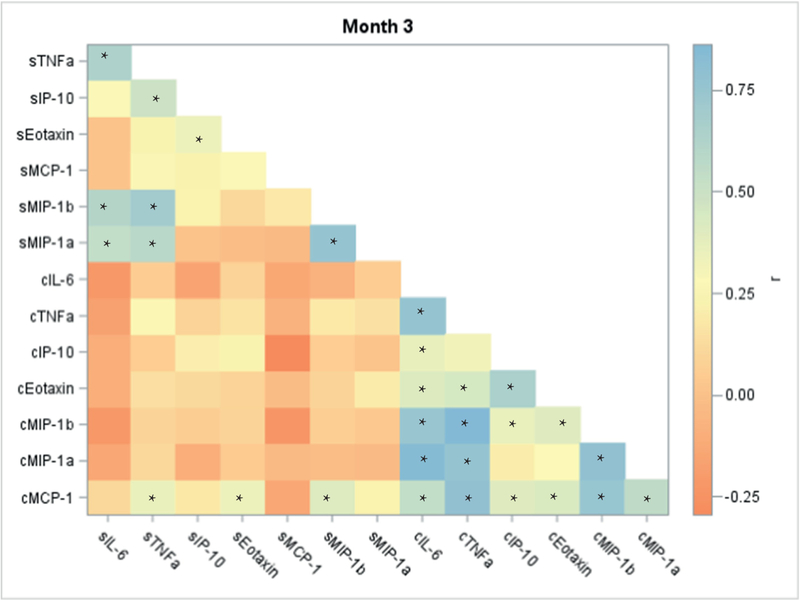
Overall and individually, and at every time point, cervico-vaginal and serum cytokines were not correlated, even between matching pairs (for example, serum IL-6 vs. cervico-vaginal IL-6). Heat maps of these correlations are presented in figures 1, S1, and S2 (asterisks indicate significant Spearman correlation coefficients p ≤ 0.05). Even for the few serum/ cervico-vaginal pairs with a statistically significant association, correlations were very weak. By contrast, cytokines from the same biological compartment tended to correlate. The strongest correlations were among cervico-vaginal cytokines TNFα and MIP-1β for months 3 (rho=0.86), 5 (rho=0.89), and 7(rho=0.92). MIP-1α and MIP-1β had the strongest correlations among serum cytokines for all time points evaluated (rho = 0.76, 0.84, 0.77 for month 3, 5, and 7, respectively).
Figure 1.
Correlation map based on Spearman coefficients for cervico-vaginal and serum cytokines for women who delivered at term using data from gestation month 3, PRINCESA cohort 2009–2014.
*p ≤ 0.05
c prefix indicates cervico-vaginal cytokines
s prefix indicates serum cytokines
3.5. PM10 and CO association with cervico-vaginal and serum cytokines
Overall standard deviations for cervico-vaginal cytokines were 2.15, 2.38, 1.78, 2.48, 1.96, 2.22 and 2.02 for TNFα, IL-6, IP-10, MIP-1α, MIP-1β, MCP-1 and Eotaxin, respectively. Standard deviations for the corresponding serum cytokines were lower. In Tobit models adjusted for age, lower reproductive tract infection status, pre-pregnancy body mass index, gestational age at sample collection, education, maximum temperature for the day prior to clinic visit and season, air pollutants were positively associated with serum cytokine concentrations (table 5). PM10 was marginally or significantly associated with serum cytokines IL-6 Tobit regression estimate (standard error) 0.19 (0.09), IP-10 0.09 (0.03), MIP-1β 0.07 (0.03), and Eotaxin 0.09 (0.05) after adjusting for FDR. Associations were mostly negative for CO and serum cytokines, but MIP-1β −0.16 (0.09) was the only cytokine that was significant (p≤ 0.1) . On the other hand, PM10 and CO were negatively associated with cervico-vaginal cytokines. This negative association was marginally significant or significant for PM10 and the following cytokines: TNFα −0.22 (0.12), IP-10 −0.25 (0.10) MIP-1β −0.21 (0.11), MCP-1 −0.26 (0.12) and Eotaxin −0.29 (0.13) while significant negative associations were seen for CO and cervico-vaginal cytokines TNFα −0.54 (0.31), IL-6 −0.79 (0.42), MIP-1β −0.60 (0.28), MCP-1 −1.01 (0.31) and Eotaxin −0.62 (0.30).
Table 5.
Associations of PM10a and COb with serum and cervico-vaginal inflammatory markers in 104 pregnant women, Mexico City, PRINCESA Cohort, 2009–2014.
| TNFα | IL-6 | IP-10 | MIP-1α | MIP-1β | MCP-1 | Eotaxin | |||
|---|---|---|---|---|---|---|---|---|---|
| CVE (Betas (SEe)) | |||||||||
| Overall SDf | 2.15 | 2.38 | 1.78 | 2.48 | 1.96 | 2.22 | 2.02 | ||
| PM10 c | −0.22 (0.12)c | −0.22 (0.15) | −0.25 (0.10)c | −0.18 (0.13) | −0.21 (0.11)c | −0.26 (0.12)c | −0.29 (0.13)c | ||
| CO | −0.54 (0.31)c | −0.79 (0.42)c | −0.2 0 (0.29) | −0.50 (0.40) | −0.60 (0.28)c | −1.01 (0.31)d | −0.62 (0.30)c | ||
| Serum (Beta and (SE)) | |||||||||
| Overall SD | 1.12 | 1.7 | 0.71 | 1.72 | 0.92 | 0.59 | 1.02 | ||
| PM10 | 0.05 (0.05) | 0.19 (0.09)c | 0.09 (0.03)d | 0.07 (0.06) | 0.07 (0.03)c | 0.02 (0.02) | 0.09 (0.05)c | ||
| CO | −0.01 (0.12) | 0.01 (0.27) | 0.13 (0.10) | −0.14 (0.18) | −0.16 (0.09)c | −0.02 (0.07) | 0.08 (0.16) | ||
Note: Units for pollutants: PM10 (per 10 μg/m3); CO (per 10 ppm). Results are from Tobit regression models, which used air pollution data (1 day prior to clinic visit) from the monitor nearest each participant’s residence and adjusted for pre-pregnancy body mass index, gestational age at the time of sampling (weeks), education, maximum temperature for the day prior to visit and season.
PM10, particulate matter with an aerodynamic diameter 10 μm or less
CO, carbon monoxide
p ≤ 0.1 after adjusting for False Discovery Rate (FDR)
p ≤ 0.05 after adjusting for FDR
SE, standard error
SD, standard deviation
4. Discussion
We compared cervico-vaginal and serum cytokines obtained over the course of term pregnancy and evaluated their associations with outdoor particulate matter and carbon monoxide levels measured on the day prior to specimen collection. The relationship between inflammatory markers from these two compartments is of fundamental interest in perinatal epidemiology because of the important role inflammation plays throughout healthy pregnancy, and because dysregulated inflammation, in response to infection, oxidative stress, and other factors, is suspected to be involved in the pathology of preterm birth.
Our first key finding was that serum and cervico-vaginal cytokines were not correlated, and cervico-vaginal cytokines concentrations were substantially higher than the corresponding serum cytokines. These findings indicate that inflammatory processes are compartment specific, with minimal crossover between compartments, at least when evaluated at the same point in time. Vogel et al. also found no correlation between cervico-vaginal and serum cytokines in a study of 57 pregnant women in Birmingham, Alabama with a previous preterm birth (Vogel et al., 2007). Similarly, cervico-vaginal and serum cytokines were not correlated among 26 post-menopausal women with and without vaginal symptoms (Stute et al., 2014). This suggests that compartmentspecific immunologic response is not an anomaly of pregnancy but rather a normal physiologic process across conditions and/or stages of life.
Our second key finding was cervico-vaginal and serum cytokines had different associations with PM10, and carbon monoxide, suggesting that the biological mechanisms associated with systemic versus localized immunologic response to air pollution and other environmental exposures leading to oxidative stress/inflammation may differ.
Our finding reinforces the importance of a recommendation by Ferguson and colleagues. In a repeated measures study evaluating the association between exposure to six phthalates (estimated from urinary metabolites) and five inflammatory markers obtained from plasma during pregnancy (in 30 individual models), they found only one significant and three marginally significant associations. Ferguson et al. concluded that inflammatory markers from more specific sources might be important to evaluate the association between phthalate exposure and the subsequent inflammatory response that can lead to preterm birth (Ferguson et al., 2015). These results have implications for studies using biomarkers to evaluate the effects of inflammation on pregnancy outcomes: namely, that inflammation measured from a source contiguous to the site of the developing fetus might be more representative of the environment to which the fetus is exposed, compared to inflammation measured in the systemic circulation. Georgiou and colleagues, in a recent review of advances made in the use of biomarkers to predict preterm birth, also concluded that because of its compositional characteristic of being a mixture of secretions from gestational and reproductive tissues, cervico-vaginal fluid is ideal for consideration of biomarkers related to labor (Georgiou et al., 2015). By contrast, biomarkers obtained from blood components (plasma and serum) have been reported to be non-specific due to peripheral blood availability across body tissues and the potential that these biomarkers are either diluted or lack information about the exact source from which inflammation originated. The idea that cytokines obtained from serum/plasma are non-specific is supported by the ICCs obtained for serum cytokines in this study. Serum cytokines exhibited more variability compared to cervico-vaginal samples and this may be an indication of the myriad factors capable of influencing cytokine levels in the peripheral circulation. Interestingly, ICCs for the corresponding cervico-vaginal cytokines were higher than serum cytokines among the same participants for samples collected during the same visit, again suggesting different processes occurring during these same time points and that these processes are compartment specific. Increases in serum cytokines linked with air pollution were expected but the negative association of estimated air pollution exposure with cervico-vaginal cytokines was surprising. The mechanisms associated with the opposite expression of cytokines from systemic and lower reproductive tract and air pollution are unclear and need to be further evaluated. However, one possible explanation is that immunologic response may vary by pollutant type and part of the body being evaluated, and may be categorized in two ways: direct or indirect. For example, response to physical coarse particles in the lungs is direct and is associated with the release of pro-inflammatory cytokines (van Eeden et al., 2001) while remote locations such as the reproductive tract might operate through other reported “sequelae pathways” such as the suppression of certain cell types (CDC, 2010). Although inconsistent across studies and not well defined, the immunosuppressive effects of smoking have been associated with a decrease in response by lymphocytes to mitogen, which is needed for the start of cell division, (mitogen., (n.d)) and also with impaired chemotactic activities of polymorphonuclear leukocytes. (Reviewed in (CDC, 2010)). Leukocytes are producers of pro-inflammatory chemokines such as MIP-1a, MCP-1, and IP-10; these cytokines are involved in recruitment of monocytes and macrophages, which are producers of other pro-inflammatory cytokines (Ryckman et al., 2009). Another example of an indirect pathway is the postulated link between air pollution and intestinal inflammation, where movement of large PM particles from one part of the body (lungs) to another (intestines) occurs through mucociliary clearance. (reviewed in (Beamish et al., 2011)) Similar pathways might be at play in more distal compartments of the body such as the reproductive tract, but may be associated with a lagged response. Future studies should evaluate whether a lagged response to “secondary exposure” in parts of the body that are further away from the lungs exists. Consideration should also be given to whether reduced cytokine levels seen in this study reflect a competition for cytokine-producing cells during active inflammation in the lungs.
Finally, the use of inflammatory markers collected longitudinally on the same participants on the same dates to evaluate median differences, reproducibility, and correlation between local and systemic sources is a major strength of this study. However, some limitations should be considered. The data used in the study contained values below and above the LOD. Except for the Tobit regression models which properly accounted for all censored observations and ICC calculations, which only accounted for observations below the LOD, censored values were replaced with common substitution methods. Simple replacement methods are commonly used for censored data but are known to have limitations especially when the percent below the LOD reaches 25 percent (Croghan and Egeghy, 2003). Another limitation is that other sources of inflammation and anti-inflammatory medication use were not accounted for.
5. Conclusion
In summary, inflammatory markers obtained from peripheral blood are not specific and may represent different as well as remote processes. Therefore, they should not be used as proxies of lower reproductive tract inflammation. These findings also imply that environmental pollutants may impact health and human physiology through different pathways and inflammatory mechanisms, depending on which compartment or organ system is being evaluated.
Supplementary Material
Highlights.
Median cervico-vaginal cytokines were higher than corresponding serum cytokines.
Cervico-vaginal and serum cytokines were not correlated.
Cytokines from the same source (cervico-vaginal or serum) were correlated.
Cervico-vaginal cytokines exhibited better reproducibility than serum cytokines.
Air pollution and cytokine associations suggest different inflammatory mechanisms.
Acknowledgements
The authors thank Ricardo de Majo, the O’Neill Research Group at the University of Michigan School of Public Health, the PRINCESA cohort Mexico City Research Group for collecting and processing the data.
This project received support from the following sources: National Institute for Environmental Health Sciences grants R01 ES016932, R01 ES017022, T32 ES007062, and P30 ES017885; National Institute for Occupational Safety and Health grant T42 OH008455–09; the University of Michigan Rackham Graduate School; and the University of Michigan Center for Research on Ethnicity, Culture and Health (National Institute of General Medical Sciences grant 5R25GM058641–18).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
There are no competing financial interests to declare. None of the funding agencies played a role in the design and conduct of this study, data analysis and interpretation or preparation of the manuscript.
References
- Aleshkin VA, Voropaeva EA and Shenderov BA (2006). “Vaginal microbiota in healthy women and patients with bacterial vaginosis and nonspecific vaginitis.” Microbial Ecology in Health and Disease 18(2): 71–74. [Google Scholar]
- Beamish LA, Osornio-Vargas AR and Wine E (2011). “Air pollution: An environmental factor contributing to intestinal disease.” Journal of Crohn’s and Colitis 5(4): 279–286. [DOI] [PubMed] [Google Scholar]
- Benjamini Y and Hochberg Y (1995). “Controlling the false discovery rate: a practical and powerful approach to multiple testing.” Journal of the Royal Statistical Society. Series B (Methodological): 289–300. [Google Scholar]
- Breton CV, Kile ML, Catalano PJ, Hoffman E, Quamruzzaman Q, Rahman M, Mahiuddin G and Christiani DC (2007). “GSTM1 and APE1 genotypes affect arsenic-induced oxidative stress: a repeated measures study.” Environ Health 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton MA, Meraz-Cruz N, Sánchez BN, Foxman B, Gronlund CJ, Beltran-Montoya J, CastilloCastrejon M, O’Neill MS and Vadillo-Ortega F “Repeated Measures of Cervicovaginal Cytokines during Healthy Pregnancy: Understanding “Normal” Inflammation to Inform Future Screening.” Amer J Perinatol(EFirst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2010). How tobacco smoke causes disease: The biology and behavioral basis for smokingattributable disease: A report of the surgeon general, Centers for Disease Control and Prevention (US). [PubMed] [Google Scholar]
- Chai F, Gao J, Chen Z, Wang S, Zhang Y, Zhang J, Zhang H, Yun Y and Ren C (2014). “Spatial and temporal variation of particulate matter and gaseous pollutants in 26 cities in China.” Journal of Environmental Sciences 26(1): 75–82. [DOI] [PubMed] [Google Scholar]
- Croghan C and Egeghy PP (2003). Methods of Dealing with Values Below the Limit of Detection using SAS Presented at the Southeastern SAS User Group; St. Petersburg, FL. [Google Scholar]
- Dadvand P, Basagana X, Figueras F, Martinez D, Beelen R, Cirach M, de Nazelle A, Hoek G, Ostro B and Nieuwenhuijsen MJ (2014). “Air pollution and preterm premature rupture of membranes: a spatiotemporal analysis.” Am J Epidemiol 179(2): 200–207. [DOI] [PubMed] [Google Scholar]
- Eeden S. F. v. and Hogg JC (2002). “SYSTEMIC INFLAMMATORY RESPONSE INDUCED BY PARTICULATE MATTER AIR POLLUTION: THE IMPORTANCE OF BONE-MARROW STIMULATION.” Journal of Toxicology and Environmental Health, Part A 65(20): 1597–1613. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Mukherjee B, Loch-Caruso R and Meeker JD (2015). “Associations between maternal biomarkers of phthalate exposure and inflammation using repeated measurements across pregnancy.” PloS one 10(8): e0135601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, O’Neill MS and Meeker JD (2013). “Environmental contaminant exposures and preterm birth: a comprehensive review.” J Toxicol Environ Health B Crit Rev 16(2): 69–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine EA, Claydon E and Taylor-Robinson D (1996). “Lactobacilli from Women With or Without Bacterial Vaginosis and Observations on the Significance of Hydrogen Peroxide.” Microbial Ecology in Health and Disease 9(4): 135–141. [Google Scholar]
- French JI and McGregor JA (1996). “The pathobiology of premature rupture of membranes.” Semin Perinatol 20(5): 344–368. [DOI] [PubMed] [Google Scholar]
- Georgiou HM, Di Quinzio MK, Permezel M and Brennecke SP (2015). “Predicting Preterm Labour: Current Status and Future Prospects.” Dis Markers 2015: 435014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Neller A, Williams G and Simpson R (2006). “Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia.” BJOG 113(8): 935–941. [DOI] [PubMed] [Google Scholar]
- Hemedinger C (2013). “How to build a correlations matrix heat map with SAS.” The SAS Dummy [Google Scholar]
- A SAS® blog for the rest of us Retrieved July 5 2018, from http://blogs.sas.com/content/sasdummy/2013/06/12/correlations-matrix-heatmap-with-sas/.
- Hertz-Picciotto I, Herr CE, Yap PS, Dostal M, Shumway RH, Ashwood P, Lipsett M, Joad JP, Pinkerton KE and Sram RJ (2005). “Air pollution and lymphocyte phenotype proportions in cord blood.” Environ Health Perspect 113(10): 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa K and van Eeden SF (2013). “Contribution of Lung Macrophages to the Inflammatory Responses Induced by Exposure to Air Pollutants.” Mediators of Inflammation 2013: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Hein MJ, Deddens JA and Hines CJ (2011). “Analysis of lognormally distributed exposure data with repeated measures and values below the limit of detection using SAS.” Ann Occup Hyg 55(1): 97–112. [DOI] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT and Krishnakumar A (2006). “Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition.” Environ Health Perspect 114(11): 16361642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millipore Luminex® xMAP® Technology Boston, MA, USA, Millipore Corporation. [Google Scholar]
- Millipore MILLIPLEX MAG HUMAN CYTOKINE / CHEMOKINE MAGNETIC BEAD PANEL KIT Boston, MA, USA, Millipore Corporation. [Google Scholar]
- Ministry of the Environment (Federal District, M. “Mexico - Mexico City Automatic Air Quality Monitoring Network Database Mexico City, Mexico: Ministry of the Environment (Federal District, Mexico).”. [Google Scholar]
- mitogen. ((n.d)). Medical Dictionary for the Health Professions and Nursing (2012). Retrieved June 10 2018. [Google Scholar]
- O’Neill MS, Loomis D, Torres Meza V, Retama A and Gold D (2002). “Estimating particle exposure in the Mexico City metropolitan area.” J Expo Anal Environ Epidemiol 12(2): 145–156. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Castillo-Castrejon M, Mordhukovich IB, Brown DG and Vadillo-Ortega F (2013). “Air pollution, inflammation and preterm birth in Mexico City: study design and methods.” Sci Total Environ 448: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Bell ML, Belanger K and de Klerk N (2014). “Fine particulate matter and risk of preterm birth and pre-labor rupture of membranes in Perth, Western Australia 1997–2007: a longitudinal study.” Environ Int 73: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-González LO, Zhang Z, Sánchez BN, Zhang K, Brown DG, Rojas-Bracho L, OsornioVargas A, Vadillo-Ortega F and O’Neill MS (2015). “An Assessment of Air Pollutant Exposure Methods in Mexico City, Mexico.” Journal of the Air & Waste Management Association (1995) 65(5): 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B (2006). Fundamentals of biostatistics Belmont, CA, Thomson-Brooks/Cole. [Google Scholar]
- Ruckerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Kuchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J and Peters A (2007). “Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors.” Environ Health Perspect 115(7): 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman KK, Simhan HN, Krohn MA and Williams SM (2009). “Cervical cytokine network patterns during pregnancy: the role of bacterial vaginosis and geographic ancestry.” J Reprod Immunol 79(2): 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Mendola P, Loomis D, Herring AH, Neas LM, Savitz DA and Poole C (2005). “A timeseries analysis of air pollution and preterm birth in Pennsylvania, 1997–2001.” Environ Health Perspect 113(5): 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, Glinianaia S, Hoggatt KJ, Kannan S, Hurley F, Kalinka J, Sram R, Brauer M, Wilhelm M, Heinrich J and Ritz B (2008). “Meeting report: atmospheric pollution and human reproduction.” Environ Health Perspect 116(6): 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stute P, Kollmann Z, Bersinger N, von Wolff M, Thurman AR and Archer DF (2014). “Vaginal cytokines do not differ between postmenopausal women with and without symptoms of vulvovaginal irritation.” Menopause 21(8): 840–845. [DOI] [PubMed] [Google Scholar]
- Tobin J (1958). “Estimation of Relationships for Limited Dependent Variables.” Econometrica 26(1): 2436. [Google Scholar]
- Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Viveros-Alcaraz M, Castillo-Castrejon M, Beltran-Montoya J, Brown DG and O’Neill MS (2014). “Air pollution, inflammation and preterm birth: a potential mechanistic link.” Med Hypotheses 82(2): 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R and Hogg JC (2001). “Cytokines Involved in the Systemic Inflammatory Response Induced by Exposure to Particulate Matter Air Pollutants (PM10).” American Journal of Respiratory and Critical Care Medicine 164(5): 826–830. [DOI] [PubMed] [Google Scholar]
- Vogel I, Goepfert AR, Thorsen P, Skogstrand K, Hougaard DM, Curry AH, Cliver S and Andrews WW (2007). “Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth.” Journal of Reproductive Immunology 75(2): 133–140. [DOI] [PubMed] [Google Scholar]
- Wei SQ, Fraser W and Luo ZC (2010). “Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review.” Obstet Gynecol 116(2 Pt 1): 393–401. [DOI] [PubMed] [Google Scholar]
- Yeo BK, Lim LP, Paquette DW and Williams RC (2005). “Periodontal disease -- the emergence of a risk for systemic conditions: pre-term low birth weight.” Ann Acad Med Singapore 34(1): 111–116. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.