Highlights
-
•
Youth were more prosocial to in-group than out-group peers (i.e., intergroup bias).
-
•
Prosocial behavior increases with higher reward inequity favoring others’ outcomes.
-
•
VS-pSTS connectivity increased with more inequitable rewards favoring the out-group.
-
•
VS-pSTS connectivity to more inequitable out-group rewards attenuated biased giving.
Keywords: Intergroup bias, Prosocial behavior, Youth, fMRI, Corticostriatal connectivity
Abstract
Although prosocial behavior is preferentially directed toward the in-group across many species, prioritizing the outcomes of both the in- and out-group earlier in development can reduce intergroup biases. The current study examined the role of corticostriatal recruitment and connectivity in buffering the effect of intergroup bias on costly giving behavior during childhood and adolescence, a period when other-oriented preferences and associated brain functions undergo significant change. During functional magnetic resonance imaging, youth (n = 51; 8–16 years) made decisions that could potentially benefit in-group and out-group peers at a cost to themselves. Youth were more prosocial toward in-group relative to out-group peers, but were relatively more prosocial to out-group peers when there was a greater discrepancy between potential rewards for others over oneself (i.e., higher reward inequity). Although they showed no differences in corticostriatal activation, youth evinced greater connectivity between the ventral striatum (VS) and posterior superior temporal sulcus (pSTS) when considering more inequitable prosocial decisions that favored the outcomes of out-group peers, which attenuated intergroup biases in prosocial behavior. We found no age-related differences at the behavioral or neural level, suggesting that in-group preferences already bias prosocial behavior and its neurocognitive processes by late childhood and do not change across adolescence.
1. Introduction
In-group preferences permeate human prosocial behavior as early as childhood (Dunham et al., 2011), resulting in differential treatment that favors the in-group and discriminates against the out-group (Dunham et al., 2011), a phenonomen known as intergroup bias. Even arbitrarily-defined groups elicit preferential prosocial behavior toward in-group over out-group peers in children (Dunham et al., 2011). This research suggests that individuals’ fundamental need to belong not only biases their decisions to help others from an early age, but also emerges within minutes of being assigned to a novel social group. As modern society becomes increasingly diverse, it is critical to mitigate the effect of intergroup biases on prosocial behaviors before stronger biases take root. Most studies to date have either examined the early/evolutionary antecedents of intergroup bias during infancy and early childhood (e.g., Engelmann et al., 2018, 2013; Jin and Baillargeon, 2017; Jordan et al., 2014; Yu et al., 2016) or explored potential neurocognitive mechanisms by which established intergroup biases can be reduced in adulthood (Baumgartner et al., 2015, 2013; Hein et al., 2016; Molenberghs, 2013; Telzer et al., 2015). However, the role of intergroup biases on prosocial behavior is poorly understood across the period of late childhood to adolescence, a time of significant change in other-oriented preferences (Fehr et al., 2013; Güroğlu et al., 2014a; Meuwese et al., 2015; van den Bos et al., 2010) and the importance of group affiliation (Abrams et al., 2003; Brewer, 1979; Pfeifer et al., 2009). To address this gap, the current study used functional magnetic resonance imaging (fMRI) to examine how intergroup biases are encoded in the developing brain and impact prosocial decision making–specifically in the form of costly giving–from childhood to adolescence.
Prosocial behaviors, particularly those that come at a cost to the individual, are guided by the subjective value that an individual associates with the outcomes of others relative to the self, which has been assessed using both behavioral and neural measures (Hackel et al., 2017; Meuwese et al., 2015; Zaki and Mitchell, 2011). According to economic models of decision making, prosocial, other-oriented choices often emerge under conditions in which valued resources favor another person more than oneself, referred to as disadvantageous inequity (Fliessbach et al., 2012; Güroğlu et al., 2014b; Williams and Moore, 2014) or costly prosociality (Telzer et al., 2013, 2015; Telzer et al., 2011). Because social information (e.g., consequences for others) is increasingly incorporated into decision making with age (Crone and Dahl, 2012; Güroğlu, van den Bos, et al., 2014; Güroğlu, Will, et al., 2014; Powers et al., 2018), even youth have been shown to forgo personal gains to allocate a greater amount of resources to another individual (Telzer et al., 2014, 2010). Thus, in some contexts, youths’ prosocial motivation to increase the outcomes of others may outweigh their selfish inclinations to keep rewards for themselves. However, little is known about whether youths’ preferences for prosocial, other-focused choices vary as a function of the magnitude of reward inequity between two individuals, and how this reward inequity may guide prosocial decision making in an intergroup context.
Prior work in adults has identified the role of corticostriatal circuitry in moderating the effect of reward inequity and intergroup bias on costly giving. The ventral striatum (VS), a region known to implement computations of reward value, is not only recruited more during costly giving to in-group relative to out-group peers (Hackel et al., 2017; Telzer et al., 2015), but also shows parametric increases in activation as resources allocated to the self versus other become increasingly inequitable (Hackel et al., 2017; Zaki and Mitchell, 2011). Moreover, greater recruitment of cortical regions involved in social perception and mentalizing (e.g., posterior superior temporal sulcus (pSTS), temporoparietal junction (TPJ), and dorsomedial prefrontal cortex (dmFPC)) to out-group relative to in-group peers has been linked to the reduction of intergroup biases on costly giving (Telzer et al., 2015). Thus, taking the perspectives of out-group peers may be important for overcoming strong intergroup biases. Taken together, these studies suggest that brain regions involved in value processing and mentalizing are sensitive to differences in reward inequity and group membership during prosocial decision making.
Increasingly, researchers are implementing functional connectivity techniques to understand how dynamic interactions between corticostriatal regions—beyond distinct brain patterns—integrate reward and sociocognitive information that, in turn, motivate intergroup giving behavior. The VS, which codes the motivational value of a given decision, receives input from interconnected, cortical regions that support higher-order cognitive functions (e.g., mentalizing) via looped, anatomical pathways (Haber et al., 2000; Haber and Knutson, 2010; Pennartz et al., 2009). Stronger connections between corticostriatal regions are thought to reflect an upregulation of higher order cognitive processes that, in some contexts, prompts other-regarding behaviors that are necessary to obtain a desired outcome (e.g., social connection) (Gesiarz and Crockett, 2015; Telzer et al., 2011). For example, individuals who value helping others to a greater extent show greater functional connectivity between the VS and mentalizing-related regions during costly giving (Telzer et al., 2011). Thus, stronger functional connectivity between brain regions involved in reward and social cognitive processing may promote prosocial, other-oriented considerations more than selfish needs and underlie individual differences in costly giving to in-group and out-group members.
1.1. Current study
Despite converging evidence on the role of corticostriatal recruitment and connectivity in costly giving in adults, it is unknown whether such neural responses similarly distinguish between in-group and out-group peers during childhood and adolescence. Given corticostriatal regions undergo significant functional reorganization during this period (Casey, 2015;Insel et al., 2017;Somerville and Casey, 2010), an intriguing question is whether still-maturing brain systems that support intergroup processing confer an opportunity to promote more impartial prosocial behaviors earlier in development. The current study elicited intergroup biases by assigning youth (n = 51; 8–16 years) to novel social groups (Tajfel et al., 1971). Youth completed an intergroup giving task during fMRI, where they made decisions to keep points for themselves or forgo those points to give a greater amount of points to an in-group or out-group peer. Prosocial behavior was operationalized as decisions to give to in-group or out-group members at a cost of gaining a personal reward. The relative rewards for self versus peer were always unequal, such that the peer could earn 1–5 more points than the participant on each decision. A greater point difference between self versus peer reflected decisions with higher reward inequity that maximized the outcomes of others more. This experimental design allowed us to model the point difference for each decision trial as a parametric regressor in fMRI analyses, thereby capturing how brain activity during intergroup giving decisions selectively tracks (i.e., is modulated by) increasingly inequitable outcomes that favor greater rewards for others over oneself.
We first aimed to examine the behavioral and neural correlates of intergroup giving among youth. We tested the hypothesis that youth would more frequently forgo personal gains in order to give to in-group peers than out-group peers, particularly when there was a smaller discrepancy between the potential rewards for the self versus an in-group or out-group peer (i.e., lower reward inequity). At the neural level, we examined whether functional activation and connectivity in corticostriatal circuitry (1) change as a function of the level of inequity between potential rewards for the self versus an in-group or out-group peer and (2) predict individual differences in costly giving to the in-group versus out-group. Based on research demonstrating the importance of reward- and mentalizing-related regions in intergroup giving, we hypothesized that activation in reward-related regions would increase with a greater potential reward for the in-group, whereas activation in mentalizing-related regions would increase with a greater potential reward for the out-group. Given functional connectivity with mentalizing-related brain regions is associated with more impartial intergroup behavior (Baumgartner et al., 2015, 2013; Telzer et al., 2015), we hypothesized stronger connectivity between the VS and mentalizing-related regions during decisions with greater potential rewards for the out-group would be associated with less biased giving.
Finally, we examined developmental differences in intergroup giving behavior and its underlying neurocognitive processes. Given prior work showing that young children already exhibit biased prosocial behaviors (Dunham et al., 2011), one hypothesis is that in-group preferences are reinforced at the neural level and influence prosocial behavior regardless of age, suggesting that intergroup biases may already be engrained in the developing brain before childhood. However, an alternative hypothesis is that the ongoing maturation of other-oriented preferences and corticostriatal circuitry exacerbate intergroup biases and further decentivize prosocial behavior toward out-group peers across development. Characterizing the age-related correlates of intergroup prosocial behavior during childhood and adolescence will provide important insight into possible sensitive periods for attenuating in-group preferences before they escalate into adulthood.
2. Materials and methods
2.1. Sample characteristics
Fifty-six healthy youth between the age of 8 and 16 years were recruited from the local community, but five participants were excluded from analyses for excessive motion (>2 mm) in fMRI data (n = 1), technical errors (n = 3), and incomplete task data (n = 1). The final sample size comprised 51 youth (Mage = 12.94 years, SDage = 2.70; 28 female). Participants whose parent reported they had MRI contraindications (e.g., braces) and/or diagnosis of neurological, developmental, or psychiatric disorders were excluded during telephone screening. The ethnicity/race of the sample was 76.50% European American, 3.90% African American, 3.90% Asian American, 3.90% Hispanic/Latino, and 11.80% multi-ethnic. Annual family income was binned into the following five categories: 10.7% <$14,999-$29,999, 27.7% $30,000-$59,999, 10.7% $60,000-$89,999, 31.9% $90,000-$119,999, 19.2% $120,000-$150,000 + . There was a wide range reported for parents’ highest level of education (maternal | paternal): less than high school diploma (3.9% | 6.4%), high school diploma (6.1% | 12.8%), some college (20.4% | 6.4%), associate’s degree (14.3% | 12.8%), bachelor’s degree (32.7% | 27.7%), some graduate school (0% | 2.1%), master’s degree (e.g., M.A., M.S.) (18.4% | 27.7%), and professional degree (e.g., M.D., Ph.D.) (4.1% | 4.3%). Informed assent/consent was obtained from youth and their parents in accordance with the University of Illinois Institutional Review Board.
2.2. Pre-scan procedure
Following a novel group paradigm, participants were assigned to represent the University of Illinois as part of a team competition against participants from The Ohio State University. Participants received t-shirts with their team’s colors (blue and orange), which experimenters also wore throughout the study to increase the salience of group membership. Participants viewed a photo of participants at The Ohio State University receiving t-shirts in their team’s colors (red and grey). After team assignment, participants had their pictures taken by an experimenter to include in subsequent tasks.
Before the scan, participants completed a learning task, where they viewed pictures of all the peers (72 total), half of whom were in their in-group (University of Illinois) and half in their out-group (The Ohio State University). All photos were forward facing and smiling, and were presented one at a time in a randomized order. To ensure they were familiar with the team membership of their peers, participants were instructed to categorize each peer as belonging to “my team” or the “other team” by pressing one of two buttons on a keyboard. The teams were said to be comprised of participants who previously completed the study at each university, but in reality, were pictures of youth taken from several developmentally-appropriate databases (e.g., National Institute of Mental Health Child Emotional Faces Picture Set (Egger et al., 2011)), with equal representation by age (7–17 years old), gender, and race (White, Black, Asian). Peer stimuli were matched for attractiveness based on ratings collected during pilot testing (M = 4.1 ranging from 1 (not at all) to 7 (very much)). In order to provide a visual cue for team membership, pictures were cropped and superimposed onto the colored backgrounds that corresponded with their team colors, such that in-group peers appeared on blue backgrounds and out-group peers appeared on red backgrounds. To control for potential differences in the composition of each team (e.g., based on attractiveness), peers’ assignment to the in-group or out-group teams was counterbalanced so that participants were equally likely to see each peer face as an in-group or out-group member. Participants also saw their own picture, which was superimposed on the blue-colored background that corresponded to the in-group, and categorized their own picture twice to enhance their identification with the in-group team.
2.3. Intergroup prosocial task
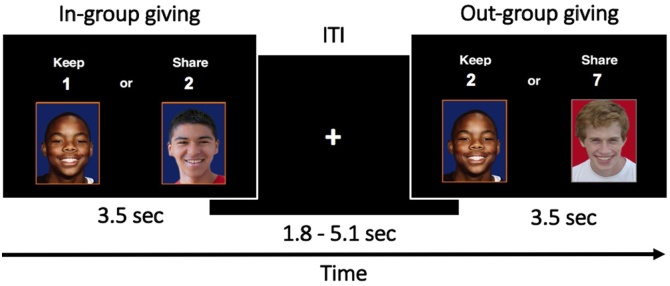
During an fMRI scan, participants completed an intergroup prosocial task (Telzer et al., 2015), during which they could earn points for themselves and their in-group and out-group peers (Fig. 1). Using the same peer stimuli from the learning task, participants viewed pictures of themselves and an in-group or out-group peer. Above each picture was a variable amount of points that participants could either choose to “keep” for themselves or “share” (i.e., give) with an in-group or out-group peer. Notably, participants were instructed that their choice to “share” actually corresponded to giving all points to a peer. Prosocial behavior was operationalized by decisions to give to in-group or out-group peers at a cost of gaining a personal reward. The point values associated with the “keep” versus “share” response options ranged from 1-10. The relative rewards for self versus peer were always unequal, such that the peer could earn 1–5 more points than the participant on each decision (e.g., Keep 1 point or Share 6 points with an in-group peer, reflecting a 5 point difference). A greater point difference between self versus peer reflected decisions with higher inequity that prioritized rewards for others over oneself (Fig. 1A), which was calculated by subtracting the number of points that could be kept for the self from the number of points that could be given to the peer. There was no running total of the participants’ or peers’ earnings presented during the task. Participants were informed that points kept for oneself in the task could be exchanged for non-monetary prizes (e.g., candy) after the visit, and points given to others would help earn them non-monetary prizes. No information was provided about how others would receive points or prizes, nor whether or how the decisions of previous participants would affect their own payoffs. This ensured that youths’ decisions to give could not be motivated by potential reciprocity benefits.
Fig. 1.
Schematic representation of intergroup prosocial task. During each trial of the intergroup prosocial task, participants viewed pictures of themselves and either an in-group (blue background) or out-group (red background) peer. Participants made decisions to keep points for themselves or give points to an in-group or out-group peer (prosocial decision), each of which maximized the rewards of others over oneself to varying degrees. ITI = inter-trial interval.
Five trial types were included in the task, each of which differentially impacted participants’ and their in-group and out-group peers’ earnings. During in-group giving, participants could forgo earning points to give a greater amount of points to an in-group peer. During out-group giving, participants could forgo earning points to give a greater amount of points to an out-group peer. During forced intergroup giving, participants were forced to give an equal number of points to either an in-group peer or out-group peer, with no option to keep points for themselves. During in-group pure rewards, participants could earn points at no cost to an in-group peer. During out-group pure rewards, participants could earn points at no cost to an out-group peer. Following previous work on intergroup giving (Telzer et al., 2015), the current study focused on the contrast between in-group giving and out-group giving trials, which provides a unique comparison of how peers’ group membership and the level of reward inequity influenced the neural correlates of costly giving. The other three trial types (i.e., forced intergroup giving, in-group pure rewards, out-group pure rewards) were not the main focus of the current paper, but were included to vary the difficulty and range of responses and keep the participants engaged. This is consistent with previous research examining costly giving (Harbaugh et al., 2007; Moll et al., 2006; Telzer et al., 2013, 2014, 2015, 2011).
Participants completed 120 trials across two functional runs, which consisted of 30 in-group giving, 30 out-group giving, 30 forced intergroup giving, 15 in-group pure rewards, and 15 out-group pure rewards trials. Fewer pure reward trials were administered because participants typically provide highly consistent responses on pure reward trials (i.e., keeping ˜100% of the time) relative to giving trials (i.e., keeping roughly ˜50% of the time) (Telzer et al., 2015, 2011). Trial types were presented in a randomized order. Each decision was presented for 3.5 s followed by a fixation cross that was jittered for a range of 1.8–5.1 seconds (M = 2.5 s). To control for motor-related activation and decrease heuristic responding, the location of the participant’s picture (right or left) and corresponding “Keep” and “Share” response options were counterbalanced across trials.
2.4. Manipulation check
Participants completed a brief post-scan questionnaire as a manipulation check of the novel group paradigm. To examine how much they identified with the in-group and out-group teams, participants used a 4-point Likert scale (1=“Not at all” to 4=“Definitely”) to rate how much they: (1) valued being a member of the University of Illinois Team (or Ohio State University Team); (2) were proud to be a member of the University of Illinois Team (or Ohio State University Team); and (3) felt that being a member of the University of Illinois Team (or Ohio State University Team) was an important part of their identity (Bavel et al., 2012). Responses were averaged across the three items to calculate overall in-group collective identification (Cronbach α = .76) and out-group collective identification (Cronbach α = .95) scores. To examine their intentions to give to in-group versus out-group peers, participants used the same Likert scale to report the extent to which they tried to give more points to the (1) University of Illinois participants (in-group peers) and (2) Ohio State University participants (out-group peers) during the intergroup prosocial task. We calculated a difference between in-group and out-group scores of collective identification and intentions to give as an index of intergroup bias. Higher, positive difference scores reflect stronger biases in youths’ identification with the in-group and their intentions to give to in-group peers, respectively.
2.5. Functional MRI data acquisition and analysis
Images were collected on a 3 T Siemans Trio MRI scanner. Blood oxygen-level dependent echo-planar images (EPI) were acquired with a T2*-weighted gradient echo sequence [slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25 msec; matrix = 92 × 92; FOV = 230 mm; voxel size 2.5 × 2.5 × 3 mm3]. A T1*-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) scan [TR: 2.3 s; TE: 2.1 msec; FOV: 256; matrix: 192 × 192; sagittal plane; slice thickness: 1 mm; 160 slices] and a T2*-weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4 s; TE = 63 msec; FOV = 230; matrix = 256 × 256; sagittal plane; slice thickness = 1 mm; 187 slices) were acquired for registration purposes. EPI and MBW scans were acquired at an oblique axial orientation in order to maximize brain coverage and reduce signal drop out.
Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, United Kingdom). Individual-level functional data were realigned to correct for slice-to-slice head motion (no participant exceeded 2-mm movement in any direction). The functional data were then co-registered to the high-resolution MPRAGE, which was segmented into grey matter, white matter, and cerebrospinal fluid. Transformation matrices used in the MPRAGE segmentation were applied to the MBW and EPI images to warp them into standard stereotaxic space as defined by the Montreal Neurological Institute (MNI) and International Consortium for Brain Mapping. The warped functional data had a resampled resolution of 3-mm cubic voxel and were spatially smoothed using a 6-mm full-width-at-half-maximum Gaussian kernel to increase the signal-to-noise ratio.
Individual-level, fixed-effects analyses were conducted using the general linear model in SPM8. The functional time-series were modeled as a series of stick functions convolved with a canonical hemodynamic response function. Non-linear high-pass temporal filtering (128 s cutoff) was applied to remove low-frequency drift in the time-series, and a restricted maximum-likelihood algorithm with a first-order autoregressor error structure was used to estimate serial autocorrelations in the time-series. For each participant’s fixed-effects analysis, the decision onsets (i.e., onset of decision to keep versus share) of each trial type were modeled as separate events of interest, which included in-group giving, out-group giving, forced intergroup giving, in-group pure rewards, and out-group pure rewards. Our conditions of interest did not separately model the decision (i.e., keep or share) but instead focused on the entire decision phase of each trial, regardless of participants’ actual decision. Using a brain-as-predictor approach (Berkman and Falk, 2013), we used neural responses during the modeled decision phase to predict individual differences in costly giving to the in-group versus out-group on the experimental task. The current study focused on the contrast between in-group giving and out-group giving to examine unique differences between considering decisions with the potential to benefit in-group versus out-group peers more than oneself. In order to capture how brain activity during intergroup prosocial decision making selectively tracks increasingly inequitable outcomes that favor others, we included a parametric modulator (PM) at the trial level for in-group giving and out-group giving trials. The PM was the difference between points that could be kept versus given (range: 1–5 point difference), with a greater point difference reflecting greater discrepancy between the payoff for self versus other that favors the other person. By including these parametric regressors at the individual level, we sought to investigate trial-by-trial differences in how brain regions tracked changes in the magnitude of reward inequity when considering whether or not to give to in-group versus out-group peers, regardless of their actual decisions. All PM values were mean-centered within each participant. The jittered inter-trial intervals were not explicitly modeled and served as an implicit baseline. Six motion regressors were modeled as covariates of non-interest. Finally, contrasts were generated at the individual level for our primary contrasts of interest: in-group giving > baseline, out-group giving > baseline, in-group giving > out-group giving, and out-group giving > in-group giving.
In order to examine corticostriatal connectivity, we conducted generalized form of context-dependent psychophysiological interaction (gPPI) analyses (Friston et al., 1997; McLaren et al., 2012). We used an anatomically-defined bilateral VS as the seed region because of its role in the experience of rewards associated with prosocial decisions, both among youth (Telzer et al., 2013, 2014) and within intergroup contexts (Hein et al., 2010; Telzer et al., 2015). The bilateral VS seed region was defined using the Wake Forest University Pickatlas Tool (Maldjian et al., 2003; Tzourio-Mazoyer et al., 2002), which was restricted from MNI z=-13 to z = 3 in the axial plane. For each participant, we used the automated gPPI toolbox in SPM (McLaren et al., 2012) to (1) extract the deconvolved time-series from the VS seed region (physiological regressor); (2) convolve the parametric modulator of each trial type (e.g., in-group giving) with the canonical HRF (psychological regressor); and (3) multiply the time-series of the physiological regressor by the psychological regressor (PPI term), which identified brain regions that co-varied with the VS as a function of the task. At the individual-level, a gPPI model was created using one regressor to represent the deconvolved VS BOLD signal, as well as the psychological (i.e., PM) and PPI regressors for each condition type. Contrasts were generated at the individual level for our primary contrasts of interest: in-group giving > baseline, out-group giving > baseline, in-group giving > out-group giving, and out-group giving > in-group giving.
A random-effects, group level model was computed for all individual subject contrasts using GLMFlex. GLMFlex corrects for variance-covariance inequality, partitions error terms, removes outliers and sudden changes in brain activation, and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). All analyses were conducted at the whole-brain level. First, we statistically controlled for mean-centered age to examine neural activity and functional connectivity in brain regions that tracked increasing reward inequity, independent of age. Second, we investigated whether brain activity and connectivity that tracked increasing reward inequity predicted the frequency of costly giving to in-group versus out-group peers. For each participant, we calculated a difference score between their frequency (percentage) of giving to in-group peers and out-group peers as an index of intergroup bias in prosocial behavior (i.e., biased giving favoring the in-group). We entered biased giving scores as a regressor in a whole-brain regression analysis and controlled for mean-centered age. Our final analyses included mean-centered age as a regressor in a whole-brain regression analysis to explicitly test for age-related patterns in brain activity and connectivity that tracked increasing reward inequity.
Corrections for multiple comparison were run at the cluster level using the updated version (November 2016) of the 3dFWHMx and 3DClustSim programs from AFNI (Ward, 2000). This method considers the actual group-level data structure and smoothness of the data to correct for multiple independent statistical tests, thus controlling for Type I errors. We used the group-level brain mask for each analysis to compute a corresponding whole-brain corrected threshold. The Monte Carlo simulations and a voxel-wise threshold of p < .005 resulted in cluster extent minimums ranging from 49 to 65 voxels for the whole brain analyses, corresponding to p < .05 family-wise error corrected. Unthresholded maps from the neuroimaging analyses are available on Neurovault (Gorgolewski et al., 2015): https://neurovault.org/collections/XTHKADYR/.
3. Results
3.1. Behavioral results
As a manipulation check of the novel group paradigm, we first examined the effect of group membership on youths’ self-reported feelings of collective identification, controlling for age. A repeated measures analysis of covariance (ANCOVA) with group membership (in-group or out-group) as a within-subject factor and mean-centered age as a covariate revealed a main effect of group membership on collective identification [F(145) = 471.33, p < .000, ηp2 = .91]. Specifically, participants identified more with the in-group team (M = 3.32, SD = .68) compared to the out-group team (M = 1.07, SD = .29), suggesting that the novel group paradigm was effective at eliciting strong in-group identification, even within minutes of being introduced to the novel teams. There was a marginally significant effect of age on collective identification F(1,45) = 3.93, p = .05, ηp2 = .08], but a significant interaction between group membership and age [F(1,45) = 4.54, p = .04, ηp2 = .09]. Post-hoc tests probing the significant interaction suggest that biases in youths’ identification with the in-group over out-group tend to decrease across age [b=-.08, SE = .04, β=-.30, p = .04].
We also examined the effect of group membership on youths’ self-reported intentions to give, controlling for age. A repeated measures ANCOVA with group membership (in-group or out-group) as a within-subject factor and mean-centered age as a covariate revealed a main effect of group membership [F(1, 49) = 122.19, p < .000, ηp2 = .71], such that participants reported stronger intentions to give to in-group peers (M = 3.04, SD = .96) than out-group peers (M = 1.37, SD = .49) on the intergroup giving task. These results suggest that youth knowingly endorsed in-group preferences when making decisions to give. There were no age differences or interaction between group membership and age, suggesting no developmental differences in intentions to give.
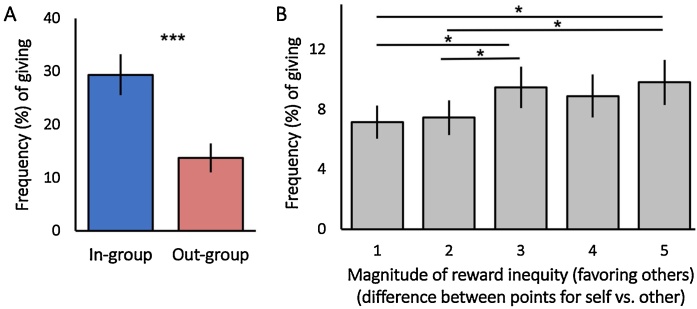
Next, we tested for effects on the intergroup giving task. In order to examine how decisions to give were differentially affected by group membership and the magnitude of reward inequity, we conducted a 2 (group membership: in-group or out-group) x 5 (point difference: 1–5 points) repeated measures ANCOVA on the frequency of costly giving, with mean-centered age included as a covariate. Note that the frequency of selfish choices was not analyzed because it is the complement of prosocial choices. There was a significant main effect of group membership [F(1,49) = 26.61, p < .000, ηp2 = .35], such that youth gave to in-group peers (M = 29.40%, SD = 27.72%) more frequently than out-group peers (M = 13.74%, SD = 19.36%) (Fig. 2A). There was a significant main effect of reward inequity [F(2.80,137.12) = 3.33, p = .02, ηp2 = .06]1 . Post hoc paired-samples t-tests suggest that youth gave more frequently at higher levels of reward inequity, regardless of group membership (Fig. 2B). There were neither two-way interactions between group membership and age or group membership and inequity, nor a three-way interaction between age, group membership, and inequity.
Fig. 2.
Behavioral results from the intergroup prosocial task. A) Youth were significantly more prosocial toward in-group compared to out-group peers, independent of age. B) The frequency of prosocial decisions increased as reward inequity favoring others’ outcomes increased, regardless of group membership and independent of age. The y-axis depicts the mean frequency of prosocial decisions, whereas the x-axis represents the magnitude of reward inequity, or the discrepancy in the payoff for self versus other (collapsed across group membership for visualization purposes). The magnitude of reward inequity was determined by calculating a difference score between the number of points that could be given to another peer and the number of points that could be kept for oneself on each trial (e.g., Share 7 points - Keep 2 points = 5 point inequity; range: 1–5 point difference), such that higher reward inequity reflected a greater preference for maximizing others’ rewards over one’s own rewards. ***p < .001, *p < .05. Error bars represent the standard error of the mean.
3.2. fMRI results
3.2.1. Neural correlates of intergroup giving
First, we examined whether there were brain regions that differentially tracked increasing reward inequity when youth considered making decisions to give to in-group relative to out-group peers. At the whole-brain level, no brain regions showed significant parametric changes as a function of reward inequity, controlling for age.
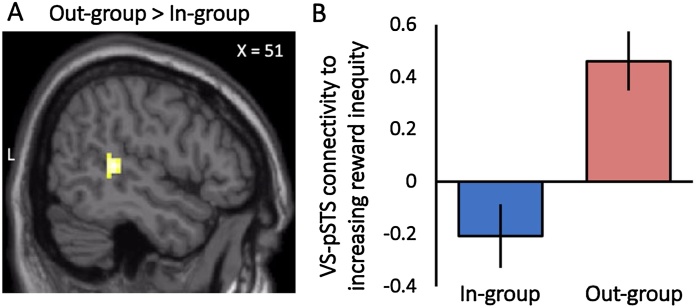
Next, we examined how the magnitude of reward inequity was related to functional connectivity with the VS as youth considered giving to in-group versus out-group peers. With increasing reward inequity, youth exhibited greater functional connectivity between the VS and the right pSTS (xyz = 51, -37, 7; t = 4.05; k = 71 contiguous voxels; p < .005 corrected) when they considered giving to out-group versus in-group peers (Fig. 3A). For descriptive purposes, we extracted parameter estimates of VS-pSTS connectivity separately for out-group and in-group giving trials. As shown in Fig. 3B, considering decisions that could increasingly benefit out-group peers evoked increases in functional connectivity between the VS and pSTS (i.e., parametric increases in connectivity that tracked reward inequity). In contrast, considering decisions that increasingly benefited in-group peers evoked decreases in VS-pSTS functional connectivity (i.e., parametric decreases in connectivity that tracked reward inequity). No other regions showed parametric changes in functional connectivity with the VS as reward inequity increased.
Fig. 3.
Youth show greater corticostriatal connectivity when considering prosocial decisions that favor greater rewards for the out-group relative to the in-group. A) With increasing reward inequity, youth exhibited greater functional connectivity between the ventral striatum and right posterior superior temporal sulcus (pSTS) when making decisions to give to out-group compared to in-group peers. B) For descriptive purposes, parameter estimates were extracted from the VS-pSTS connectivity cluster and plotted separately for the in-group and out-group. Note that the y-axis represents the linear relationship between VS-pSTS connectivity and level of reward inequity. Youth showed parametric decreases in VS-pSTS connectivity during decisions with a greater potential reward for the in-group, but parametric increases in the VS-pSTS connectivity during decisions with greater potential reward for the out-group. fMRI results are reported at p<.005, with a corrected cluster extent of 65 contiguous voxels. Error bars represent the standard error of the mean.
3.2.2. Linking the neural correlates of intergroup giving to the frequency of giving
Using a whole-brain regression analysis, we tested whether brain activity when considering increasingly inequitable giving predicted the frequency of prosocial behavior to in-group and out-group peers, controlling for mean-centered age. No brain regions showed a correlation with the frequency of prosocial behavior.
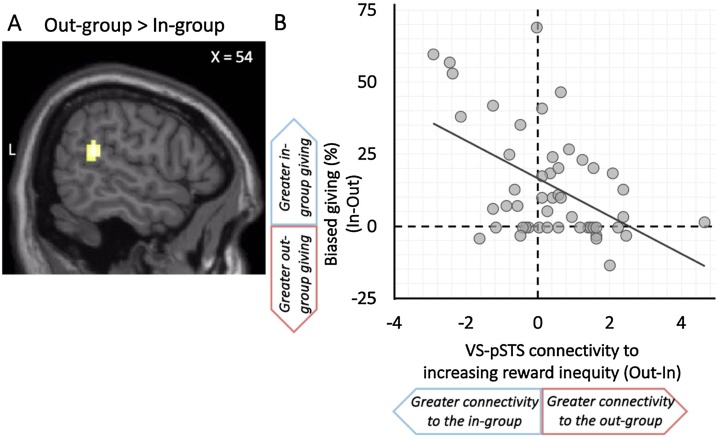
Next, we investigated whether inequity-dependent increases in functional connectivity with the VS was associated with the frequency of prosocial behavior to in-group relative to out-group peers. After controlling for mean-centered age and excluding an outlier (who exhibited brain connectivity >3 SD from the mean), we found that inequity-dependent functional connectivity between the VS and right pSTS to out-group versus in-group giving (xyz = 57, -43, 19; t = 3.88; k = 54 contiguous voxels; p < .005 corrected; Fig. 4A) was negatively correlated with biased giving behavior. For descriptive purposes, we extracted parameter estimates of VS-pSTS connectivity from out-group versus in-group giving trials and plotted against the biased giving scores, with the y-axis illustrating how functional connectivity tracks increases in the magnitude of reward inequity. As illustrated in Fig. 4B, youth who exhibited parametric increases in VS-pSTS connectivity with higher reward inequity on behalf of the out-group showed the lowest intergroup bias—choosing to give equally to in-group and out-group peers (i.e., biased giving scores around 0). In contrast, youth who exhibited parametric increases in VS-pSTS connectivity with higher reward inequity on behalf of the in-group were more likely to give preferentially to in-group peers (i.e., positive values of biased giving scores). Thus, functional connectivity between brain regions implicated in reward processing and mentalizing may be critical for processing potential rewards for out-group peers, which can attenuate the effect of intergroup bias on prosocial behavior among youth. The frequency of prosocial behavior was not associated with other regions that showed inequity-dependent connectivity with the VS.
Fig. 4.
Corticostriatal connectivity is associated with a reduction of intergroup bias and more impartial giving. A) Youth who showed parametric increases in VS-pSTS connectivity to increasingly inequitable out-group giving B) showed lower intergroup bias (i.e., more impartiality) in costly giving to in-group versus out-group peers, as illustrated by biased giving scores around 0 on the y-axis. Note: The x-axis shows the linear relationship between the strength of VS-pSTS connectivity and the level of reward inequity when considering whether to give to the out-group relative to in-group. A positive value reflects greater functional connectivity between the VS and pSTS as youth considered giving more rewards to out-group peers over themselves, whereas a negative value reflects greater VS-pSTS connectivity as youth considered giving more rewards to in-group peers over themselves. The y-axis shows the level of biased giving, such that a positive value indicates a greater preference for giving to in-group peers over out-group peers (i.e., intergroup bias), whereas a value closer to 0 indicates no differential giving between in-group and out-group peers (i.e., impartial or equitable giving). fMRI results are reported at p < .005, with a corrected cluster extent of 49 contiguous voxels. L=Left.
3.2.3. Age-related differences in the neural correlates of intergroup giving
Finally, we investigated the effect of age on the brain regions that track parametric increases in reward inequity as youth considered giving to in-group versus out-group peers. Results from a parametric, whole-brain analysis with mean-centered age as a regressor revealed no age differences in brain regions that track the magnitude of reward inequity. There were also no regions that showed age-related differences in functional connectivity with the VS to increasingly inequitable giving to in-group relative to out-group peers.
4. Discussion
Intergroup biases result in preferential treatment to in-group peers, often at a cost to out-group peers, which can reinforce group boundaries in society. In a youth sample, we tested how intergroup bias and the level of reward inequity (i.e., the extent to which a decision maximizes the outcomes of others over oneself) influence the neural correlates of costly giving decisions to in-group and out-group peers. We not only find strong in-group preferences in behavior, but also show that functional connectivity during decisions that tracked increases in reward inequity (favoring either in-group or out-group peers over oneself) predicted individual differences in biased giving, effects that did not change developmentally. Overall, these findings highlight the need to promote more impartial intergroup interactions in childhood and adolescence, a time when other-oriented preferences and the brain systems that support intergroup giving are still undergoing maturation.
Behaviorally, we found that youth gave more frequently to in-group relative to out-group peers, an effect that was already present in the youngest participants and did not change developmentally across our sample. This replicates an extensive body of research showing youth (Dunham et al., 2011), adults (Hackel et al., 2017; Telzer et al., 2015), and even non-human primates (Burkart et al., 2009) demonstrate greater prosocial behaviors toward peers from their in-group compared to their out-group, thus highlighting the ubiquity of intergroup biases on prosocial behavior. Using novel social groups to study the influence of group biases on behavior is particularly advantageous as it ensures the absence of a history of biases or the ability to develop social connection with in-group peers. Yet, despite having no prior knowledge about the other peers in this study, youth still strongly identified with the in-group and reported explicit intentions to give more frequently to in-group relative to out-group peers within minutes of being assigned to these novel teams. A greater preference to give to in-group over out-group peers may reflect youths’ motivation to engage in behaviors that enhance the positive welfare of their own in-group more so than the out-group, perhaps because it feels personally rewarding to vicariously experience the rewards of a similar, in-group peer (Tajfel, 1974). Notably, youth gave more frequently when others stood to benefit more (i.e., higher inequity favoring others’ outcomes), even to the out-group. Taken together, these results suggest that contexts where another individual stands to benefit significantly more than oneself may implicitly emphasize the value of acting on behalf of others more so than oneself, regardless of their group affiliation.
At the neural level, youth exhibited greater functional connectivity between the VS and pSTS when considering increasingly inequitable decisions to give to out-group peers compared to in-group peers. The VS is implicated in prosocial allocation of rewards to others (Braams et al., 2014; Telzer et al., 2013, 2014, 2011), scaling with the magnitude of self versus other reward discrepancies (Fliessbach et al., 2007; Nihonsugi et al., 2015; Tricomi et al., 2010). In contrast to the VS’s role in prosocial behaviors, the pSTS, a region involved in social cognition, is thought to be more engaged in prosocial decision making that involves considerations of intention rather than reward or value (Behrens et al., 2009). Although corticostriatal regions have been recruited during costly giving decisions that involve either differences in reward inequity (Güroğlu, Will, et al., 2014; Hackel et al., 2017; Zaki and Mitchell, 2011) or group membership (Hackel et al., 2017; Telzer et al., 2015), we did not find evidence that youth exhibited differences in activation during in-group relative to out-group decisions. Our findings instead highlight the importance of functional connectivity between corticostriatal regions to support increasingly costly decisions to give in an intergroup context. Given the pSTS is associated with better mental state reasoning toward in-group relative to out-group peers (Adams et al., 2009), increases in VS-pSTS connectivity during decisions that have the potential to benefit the out-group more may reflect a mechanism by which intergroup biases can be mitigated. Specifically, a greater orientation toward the perspectives and outcomes of out-group peers—particularly when they involve unequal payoffs for the self versus other—may elicit a richer encoding of out-group peers’ mental states at the neural level (Baumgartner et al., 2013; Cheon et al., 2011). Indeed, several studies show that individuals mentalize less spontaneously and accurately for out-group peers (Adams et al., 2009; McLouglin and Over, 2017), a social cognitive skill found to be particularly important for initiating prosocial behaviors toward out-group peers by 9–10 years old (Adams et al., 2009; McLouglin and Over, 2017; Yu et al., 2016). Taken together, deciding whether to give to out-group peers in the face of increasing reward inequity involves increased connectivity between, but not activity within, corticostriatal circuitry.
Corticostriatal connectivity that tracked parametric increases in potential rewards for the out-group was associated with reductions in intergroup bias, resulting in more impartial giving toward in-group and out-group peers. Results are consistent with prior work in adults showing that stronger connectivity between regions within the mentalizing network leads to reductions in the effect of intergroup bias on mentalizing processes and social interactions (Baumgartner et al., 2015). Moreover, individuals who value helping others more (via self-report) exhibit greater functional connectivity between the VS and mentalizing-related regions during costly giving behavior (Telzer et al., 2011). On one hand, greater VS-pSTS connectivity may reflect the fact that decisions favoring greater reward outcomes for the out-group involve greater activity in the VS, which then upregulates activation in the pSTS toward the outcomes of out-group members. Thus, increased activation in value-related regions to greater potential rewards for out-group peers may promote a better understanding of the perspectives of out-group peers, encouraging more impartial giving behavior. Alternatively, perhaps greater perspective-taking on behalf of out-group peers can increase the value associated with giving to out-group peers, increasing the likelihood of impartial giving to in-group and out-group peers. Despite the ubiquity of intergroup bias from as early as childhood (Dunham et al., 2011), functional connectivity between reward- and mentalizing-related regions may encourage mentalizing processes that reduce the salience of group differences, thereby moderating the effect of intergroup bias on costly giving behavior in youth.
Despite prior research showing developmental changes in giving (Güroğlu, van den Bos, et al., 2014; Meuwese et al., 2015) and corticostriatal circuitry (Casey, 2015; Insel et al., 2017; Somerville and Casey, 2010), we did not find age-related differences in behavior or brain during decisions to give to novel in-group or out-group peers. Unlike the novel group paradigm, an individual’s affiliation with real-life social groups is often more subjective and confounded by his/her relationship history. Because the definitions of an “in-group” and “out-group” vary significantly across individuals, ages, and social contexts, reputational incentives to act on behalf of familiar others can facilitate age-related differences in costly giving. Indeed, prior research has found that even young children care more about their reputation with in-group than out-group members (Engelmann et al., 2013), which is associated with higher levels of prosociality toward the in-group (Engelmann et al., 2018). However, in the absence of a relationship history, our study highlights that intergroup biases form within minutes, impacting neural processes and giving behavior to novel in-group relative to out-group peers. Moreover, these findings converge with evidence suggesting that in-group preferences emerge and permeate prosocial norms and decision making by infancy and early childhood (Baillargeon et al., 2015, 2014; Hamlin, 2014; Jin and Baillargeon, 2017). To our knowledge, this is the first study to show that the effect of intergroup biases on costly giving decisions is also evident at the neural level by childhood, remaining relatively stable throughout adolescence.
Because intergroup biases often emerge implicitly and can be difficult to link to behavior using self-report measures, a major strength of the current study is its use of neuroimaging methods to probe the underlying processes that support intergroup giving decisions, particularly during a time of significant change in other-oriented preferences (Fehr et al., 2013; Güroğlu, van den Bos, et al., 2014; Meuwese et al., 2015; van den Bos et al., 2010) and corticostriatal development (Casey, 2015; Insel et al., 2017; Somerville and Casey, 2010). However, one limitation is that our fMRI analyses focus on the decision making process that underlies intergroup prosocial behavior, regardless of youths’ actual choices to give to in-group and out-group members. We were unable to compare neural processes when giving to in-group versus out-group members directly due to low rates of prosocial behavior toward out-group members, which limited our statistical power to detect group differences at the neural level. Thus, the current findings delineate the neurobiological processes that underlie youths’ decisions as they considered giving to in-group versus out-group members, particularly as the potential rewards for others increases. Future studies should consider employing experimental designs that elicit enough instances of out-group prosocial behavior to more accurately measure differences in neural processing when youth act prosocially toward in-group versus out-group members. For example, using continuous (e.g., how much do you want to give?) rather than binary (e.g., do you want to give or keep?) indices of prosocial behavior may increase the likelihood of capturing prosocial decisions that benefit out-group members.
In conclusion, the present study offers novel evidence that group membership and reward inequity (i.e., the extent to which a decision maximizes the outcomes of others over oneself) moderate the neural responses that underlie intergroup prosocial decisions in youth. While youth already demonstrate strong biases in their prosocial behavior in favor of the in-group over the out-group, they differentiate less by group membership when the potential to maximize the outcomes of others over oneself increases (i.e., higher magnitude of reward inequity). This suggests that, in some social contexts, the motivation to increase another individual’s outcomes above one’s own outcomes can trump youths’ instinct for in-group loyalty during prosocial decision making. Furthermore, the extent to which youth exhibited greater corticostriatal connectivity (between the VS and pSTS/TPJ) as they considered making prosocial decisions that were increasingly unequal in self-other payoffs—though more beneficial for out-group peers—related to more impartial prosocial behavior.
The implications of the current findings are significant for real life behaviors, where group biases—based on race or political affiliations, for instance—have already developed for many years, thus underscoring the need to intervene earlier in development to promote more positive intergroup interactions. Recent work suggests that experiencing unexpectedly positive out-group interactions (e.g., receiving help from out-group peers) leads adults to develop more positive attitudes toward out-group members (Hein et al., 2016). At a broader scale, societies in which positive contact with out-groups is more common ultimately endorse social norms that support and facilitate positive intergroup interactions (Christ et al., 2014). Thus, interventions should focus on increasing the diversity of youths’ social interactions and environments, which may not only help change individual attitudes toward the out-group, but also reduce society-level prejudices.
Author contributions
E.H.T. designed and performed research; K.T.D. analyzed data and wrote the paper with input from E.H.T.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgements
We would like to thank members of the Developmental Social Neuroscience Lab for helpful comments. We are also thankful to the research participants and for the staff of the Biomedical Imaging Center at the University of Illinois for assistance in data collection. This research was supported by the National Institutes of Health (R01DA039923 to E.H.T.), Department of Psychology at the University of Illinois (to E.H.T.), and the National Science Foundation Graduate Research Fellowship (to K.T.D.).
Footnotes
Mauchly’s test of sphericity indicated the assumption of sphericity was only violated for the reward inequity variable (χ2(9)=41.78, p<.000), thus the Greenhouse-Geisser correction was applied. Assumptions for repeated-measures ANCOVA were otherwise met for the remaining variables and analyses.
References
- Abrams D., Rutland A., Cameron L. The development of subjective group dynamics: children’s judgments of normative and deviant in-group and out-group individuals. Child Dev. 2003;74(6):1840–1856. doi: 10.1046/j.1467-8624.2003.00641.x. [DOI] [PubMed] [Google Scholar]
- Adams R.B., Rule N.O., Franklin R.G., Wang E., Stevenson M.T., Yoshikawa S. Cross-cultural reading the mind in the eyes: an fMRI investigation. J. Cogn. Neurosci. 2009;22(1):97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Baillargeon R., Setoh P., Sloane S., Jin K. Infant social cognition: psychological and sociomoral reasoning. In: Gazzaniga M.S., Mangun G.R., editors. The Cognitive Neurosciences. 5th ed. MIT Press; Cambridge, MA: 2014. pp. 7–14. [Google Scholar]
- Baillargeon R., Scott R.M., He Z., Sloane S., Setoh P., Jin K. Psychological and sociomoral reasoning in infancy. In: Mikulincer M., Shaver P.R., Borgida E., Bargh J.A., editors. vol. 1. American Psychological Association; Washington, D.C: 2015. pp. 79–150. (APA Handbook of Personality and Social Psychology). Attitudes and Social Cognition. [Google Scholar]
- Baumgartner T., Schiller B., Hill C., Knoch D. Impartiality in humans is predicted by brain structure of dorsomedial prefrontal cortex. NeuroImage. 2013;81:317–324. doi: 10.1016/j.neuroimage.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Nash K., Hill C., Knoch D. Neuroanatomy of intergroup bias: a white matter microstructure study of individual differences. NeuroImage. 2015;122:345–354. doi: 10.1016/j.neuroimage.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Bavel J.J., Van, Cunningham W.A. A social identity approach to person memory: group membership, collective identification, and social role shape attention and memory. Pers. Soc. Psychol. Bull. 2012;38(12):1566–1578. doi: 10.1177/0146167212455829. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Hunt L.T., Rushworth M.F.S. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B. Beyond brain mapping: using neural measures to predict real-world outcomes. Curr. Dir. Psychol. Sci. 2013;22(1):45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Peters S., Peper J.S., Güroglu B., Crone E.A. Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. NeuroImage. 2014;100:281–289. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Brewer M.B. In-group bias in the minimal intergroup situation: a cognitive-motivational analysis. Psychol. Bull. 1979;86(2):307–324. [Google Scholar]
- Burkart J.M., Hrdy S.B., Van Schaik C.P. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 2009;18(5):175–186. [Google Scholar]
- Casey B.J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 2015;66(1):295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Cheon B.K., Im D., Harada T., Kim J.-S., Mathur V.A., Scimeca J.M., Chiao J.Y. Cultural influences on neural basis of intergroup empathy. NeuroImage. 2011;57(2):642–650. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Christ O., Schmid K., Lolliot S., Swart H., Stolle D., Tausch N. Contextual effect of positive intergroup contact on outgroup prejudice. Proc. Natl. Acad. Sci. 2014;111(11):3996–4000. doi: 10.1073/pnas.1320901111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dunham Y., Baron A.S., Carey S. Consequences of “minimal” group affiliations in children. Child Dev. 2011;82(3):793–811. doi: 10.1111/j.1467-8624.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., Leibenluft E., Ernst M., Towbin K.E., Angold A. The NIMH child emotional faces picture set (NIMH-ChEFS): a new set of children’s facial emotion stimuli. Int. J. Methods Psychiatr. Res. 2011;20(3):145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.M., Over H., Herrmann E., Tomasello M. Young children care more about their reputation with ingroup members and potential reciprocators. Dev. Sci. 2013;16(6):952–958. doi: 10.1111/desc.12086. [DOI] [PubMed] [Google Scholar]
- Engelmann J.M., Herrmann E., Tomasello M. Concern for group reputation increases prosociality in young children. Psychol. Sci. 2018;29(2):181–190. doi: 10.1177/0956797617733830. [DOI] [PubMed] [Google Scholar]
- Fehr E., Glätzle-Rützler D., Sutter M. The development of egalitarianism, altruism, spite and parochialism in childhood and adolescence. Eur. Econ. Rev. 2013;64:369–383. [Google Scholar]
- Fliessbach K., Weber B., Trautner P., Dohmen T., Sunde U., Elger C.E., Falk A. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318:1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Fliessbach K., Phillipps C.B., Trautner P., Schnabel M., Elger C.E., Falk A. Neural responses to advantageous and disadvantageous inequity. Front. Hum. Neurosci. 2012;6(165):1–9. doi: 10.3389/fnhum.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gesiarz F., Crockett M.J. Goal-directed, habitual and Pavlovian prosocial behavior. Front. Behav. Neurosci. 2015;9:135. doi: 10.3389/fnbeh.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S.S., Maumet C. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güroğlu B., van den Bos W., Crone E.A. Sharing and giving across adolescence: an experimental study examining the development of prosocial behavior. Front. Psychol. 2014;5(291):1–13. doi: 10.3389/fpsyg.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güroğlu B., Will G.-J., Crone E.A. Neural correlates of advantageous and disadvantageous inequity in sharing decisions. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. Rev. 2010;35129(10):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Fudge J.L., McFarland N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. http://doi.org/http://www.jneurosci.org/content/20/6/2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel L., Zaki J., Bavel J., Van Social identity shapes social valuation: evidence from prosocial behavior and vicarious reward. Soc. Cogn. Affect. Neurosci. 2017;12(8):1219–1228. doi: 10.1093/scan/nsx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J.K. The origins of human morality: complex socio-moral evaluations by preverbal infants. In: Decety J., Christen Y., editors. Vol. 21. Springer International Publishing; Cham, Switzerland: 2014. pp. 165–188. (Research and Perspectives in Neurosciences). New Frontiers in Social Neuroscience. [Google Scholar]
- Harbaugh W.T., Mayr U., Burghart D.R. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C.D., Singer T. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron. 2010;68:149–160. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hein G., Engelmann J.B., Vollberg M.C., Tobler P.N. How learning shapes the empathic brain. Proc. Natl. Acad. Sci. U. S. A. 2016;113(1):80–85. doi: 10.1073/pnas.1514539112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel C., Kastman E.K., Glenn C.R., Somerville L.H. Development of corticostriatal connectivity constrains goal-directed behavior during adolescence. Nat. Commun. 2017;8(1605):1–10. doi: 10.1038/s41467-017-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K.-S., Baillargeon R. Infants possess an abstract expectation of ingroup support. Proc. Natl. Acad. Sci. 2017;114(31):8199–8204. doi: 10.1073/pnas.1706286114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J.J., McAuliffe K., Warneken F. Development of in-group favoritism in children’s third-party punishment of selfishness. Proc. Natl. Acad. Sci. U. S. A. 2014;111(35):12710–12715. doi: 10.1073/pnas.1402280111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLouglin N., Over H. Young children are more likely to spontaneously attribute mental states to members of their own group. Psychol. Sci. 2017;28(10):1503–1509. doi: 10.1177/0956797617710724. [DOI] [PubMed] [Google Scholar]
- Meuwese R., Crone E.A., de Rooij M., Güroğlu B. Development of equity preferences in boys and girls across adolescence. Child Dev. 2015;86(1):145–158. doi: 10.1111/cdev.12290. [DOI] [PubMed] [Google Scholar]
- Molenberghs P. The neuroscience of in-group bias. Neurosci. Biobehav. Rev. 2013;37(8):1530–1536. doi: 10.1016/j.neubiorev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Moll J., Krueger F., Zahn R., Pardini M., de Oliveira-Souza R., Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. U. S. A. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihonsugi T., Ihara A., Haruno M. Selective increase of intention-based economic decisions by noninvasive brain stimulation to the dorsolateral prefrontal cortex. J. Neurosci. 2015;35(8):3412–3419. doi: 10.1523/JNEUROSCI.3885-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz C.Ma, Berke J.D., Graybiel A.M., Ito R., Lansink C.S., van der Meer M., Voorn P. Corticostriatal interactions during learning, memory processing, and decision making. J. Neurosci. 2009;29(41):12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80(4):1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers K.E., Yaffe G., Hartley C.A., Davidow J.Y., Kober H., Somerville L.H. Consequences for peers differentially bias computations about risk across development. J. Exp. Psychol. Gen. 2018;0(999):1–12. doi: 10.1037/xge0000389. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Casey B. Developmental neurobiology of cognitive control and motivational systems. Curr. Opin. Neurobiol. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajfel H. Social identity and intergroup behaviour. Soc. Sci. Inf. 1974;13(2):65–93. [Google Scholar]
- Tajfel H., Billig M.G., Bundy R.P., Flament C. Social categorization and intergroup behaviour. Eur. J. Soc. Psychol. 1971 [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. Gaining while giving: an fMRI study of the rewards of family assistance among white and Latino youth. Soc. Neurosci. 2010;5(5–6):508–518. doi: 10.1080/17470911003687913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Masten C.L., Berkman E.T., Lieberman M.D., Fuligni A.J. Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage. 2011;58(1):242–249. doi: 10.1016/j.neuroimage.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Dev. Cogn. Neurosci. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proc. Natl. Acad. Sci. U. S. A. 2014;111(18):6600–6605. doi: 10.1073/pnas.1323014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N., Qu Y. The ties that bind: group membership shapes the neural correlates of in-group favoritism. NeuroImage. 2015;115:42–51. doi: 10.1016/j.neuroimage.2015.04.035. [DOI] [PubMed] [Google Scholar]
- Tricomi E., Rangel A., Camerer C.F., O’ doherty J.P. Neural evidence for inequality-averse social preferences. Nature. 2010;463(25):1089–1092. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Bos W., Westenberg M., van Dijk E., Crone E.A. Development of trust and reciprocity in adolescence. Cogn. Dev. 2010;25(1):90–102. [Google Scholar]
- Ward B.D. 2000. Simultaneous Inference for fMRI Data. Biophysics Research Institute. Medical College of Wisconsin. [Google Scholar]
- Williams A., Moore C. Exploring disadvantageous inequality aversion in children: how cost and discrepancy influence decision-making. Front. Psychol. 2014;5(1088):1–6. doi: 10.3389/fpsyg.2014.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhu L., Leslie A.M. Children’s sharing behavior in mini-dictator games: the role of in-group favoritism and theory of mind. Child Dev. 2016;87(6):1747–1757. doi: 10.1111/cdev.12635. [DOI] [PubMed] [Google Scholar]
- Zaki J., Mitchell J.P. Equitable decision making is associated with neural markers of intrinsic value. Proc. Natl. Acad. Sci. U. S. A. 2011;108(49):19761–19766. doi: 10.1073/pnas.1112324108. [DOI] [PMC free article] [PubMed] [Google Scholar]