Abstract
Background:
Within the growing geriatric population, there is an increasing need for emergency operations. Optimizing outcomes may require a structured system of surgical care based on key quality indicators. To investigate this, the present study sought to answer two questions. First, to what degree does hospital emergency operative volume impact mortality for geriatric patients undergoing emergency general surgery (EGS) operations? Second, at what procedure-specific hospital volume will geriatric patients undergoing an emergency operation achieve at or better than average mortality risk?
Study Design:
Retrospective cohort study of geriatric patients (≥65years) who underwent one of ten EGS operations identified from the California State Inpatient Database (2010-2011). Beta-logistic generalized linear regression was employed, with the hospital as the unit of analysis, to investigate the relationship between hospital operative volume and in-hospital risk-adjusted mortality. Hospital operative volume-thresholds to optimize probability of survival were defined.
Results:
41,860 surgeries were evaluated at 299 hospitals. For each operation, mortality decreased as hospital emergency operative volume increased (p<0.001 for each operation); for every standardized increase in volume (meaning +1 natural logarithm of volume), the reduction in mortality ranged from 14% for colectomy to 61% for appendectomy. Hospital volume-thresholds, which optimize to 95% probability of survival, varied by operation, with a mean of 14 operations over 2 years. More than 50% of hospitals did not meet the threshold benchmarks, representing 22% of patients.
Conclusions:
Survival rates for geriatric patients were significantly improved when emergency operations were performed at hospitals with higher operative volumes. Consistent with all active Quality Programs of the American College of Surgeons, hospital operative volume appears to be an important metric of surgical quality for older patients undergoing emergency operations.
Keywords: Emergency general surgery, volume-to-outcomes, geriatric patients
PRÉCIS:
Survival rates for geriatric patients were significantly higher when emergency general surgery (EGS) operations were performed at hospitals with higher emergency geriatric operative volumes. Operative volume seems to be a key quality indicator and determinant of survival for older EGS patients, and a principle driver of variation in EGS-hospital performance.
INTRODUCTION:
As the United States (US) geriatric population increases(1), there is significant interest in ensuring safe and high-quality surgical care for older persons. The American College of Surgeons (ACS), in concert with the John A. Hartford Foundation, has formed the Coalition for Quality in Geriatric Surgery (CQGS) with over 50 stakeholder organizations working to create verifiable hospital-based standards to improve outcomes for older surgical patients.(2) The ACS National Surgical Quality Improvement Program (NSQIP) and the American Geriatric Society have jointly published best practice guidelines on the “Optimal Perioperative Management of the Geriatric Patient.”(3) The National Quality Forum, one of the nation’s leading patient safety organizations, has endorsed specific quality measures for geriatric surgical patients.(4) And the ACS NSQIP Geriatric Surgery Pilot has developed and tested a quality improvement dataset with metrics targeted to an older population, including cognition, decision-making, mobility, and function.(5, 6)
At present, much of the focus is on optimizing outcomes for older patients undergoing elective operations.(7, 8) However, as the US population ages, the number of older patients who will require emergency surgical intervention will increase.(9-11) Current data show that, depending on the general surgery operation, anywhere from 10% to 44% of operations in older persons are emergent.(12, 13) This presents additional challenges since emergency surgery in older patients is associated with higher morbidity and mortality(14-16) as well as increased costs(17, 18) relative to elective operations. Accordingly, there is a significant opportunity, with broad clinical implications, to improve the care of older persons with acute surgical diseases.(19-21) The ACS Quality Programs(22) provide a valuable, tested framework on which to base improvements in emergency surgery for geriatric patients. For many surgical disciplines, quality improvement is synonymous with an accreditation process which verifies surgical centers using specific criteria to ensure standards of care and optimize outcomes. Across multiple surgical subspecialties, including bariatrics(23), trauma(24), and pediatric surgery(25), discipline-specific hospital volume is a fundamental criterion of accreditation, and serves as a quality indicator that institutions must meet to become a verified center.
To test the concept that hospital emergency operative volume is a key quality indicator and determinant of mortality in older patients undergoing surgical emergencies, the present study sought to answer two questions. First, to what degree does hospital emergency operative volume influence mortality for geriatric patients undergoing common emergency general surgery (EGS) operations? Second, at what procedure-specific hospital volume will geriatric patients undergoing an emergency operation achieve at or better than average mortality risk? The hypothesis was that higher hospital volume would be associated with lower post-operative in-hospital mortality. An additional hypothesis was that hospital emergency operative volume reaches a threshold above which nearly all hospitals performing the emergency operation in older patients would realize the average mortality risk or lower.
METHODS:
Datasets and Variables:
This is a population-based, retrospective cohort study of all geriatric patients (≥65 years) who underwent one of ten EGS operations in the state of California over a 24-month period, from January 1, 2010 to December 31, 2011. The ten operations analyzed were: appendectomy; cholecystectomy; colectomy; inguinal & femoral hernia repair (analyzed together); lysis of adhesions (LOA; no bowel resections were performed in the LOA group, by definition); excision of necrotizing soft tissue infection (NSTI); repair of perforated peptic ulcer disease (either gastric or duodenal ulcers); small bowel resection; umbilical hernia repair; and ventral hernia repair. Both laparoscopic and open operations were included; trauma operations were excluded. Two datasets were used. The first was the State Inpatient Database (SID) for California (data from 2010 and 2011). The state of California was chosen as it is the most populous state in the US (population of 37 million in 2011), with a diverse population and varied geography, with both urban and rural areas. The SID is part of a family of datasets developed by the Healthcare Cost and Utilization Project, and sponsored by the Agency for Healthcare Research and Quality.(26) Data abstracted included patient demographics, chronic health conditions, hospital-based metrics, and in-hospital mortality. The second dataset was the American Hospital Association (AHA) Annual Survey of Hospitals Database for 2010 and 2011.(27) The same California acute care hospitals in the SID and the AHA were paired, thus enabling risk-adjustment at the hospital level.
For the current analyses, only patients undergoing urgent/emergency operations with specific EGS diagnoses were included. Patients were identified using International Classification of Disease, 9th Edition (ICD-9), procedural codes (Appendix A); only patients who were listed in the SID dataset as having undergone one of the ten operations as a primary core operation were included (as opposed to a secondary operation/procedure). ICD-9 diagnosis codes (Appendix B) identified patients with a specific diagnosis of an EGS condition. Given the ability to longitudinally track patients within SID, patients were not included more than once. The chosen acute surgical conditions are among most prevalent emergent surgical diseases requiring operative intervention in the US, and have a non-trivial risk of postoperative morbidity and mortality.(28-30) An operation was defined as being performed urgently/emergently if it was associated with an admission not scheduled, as defined by the SID unscheduled admission variable.
The transfer status of patients was accounted for in the inclusion/exclusion criteria; “transfer status” refers to the actual movement of a patient to or from one acute care hospital to another in California. If a patient urgently/emergently underwent an operation at one acute care hospital and was later transferred out to a second hospital, mortality was attributed to the transferring hospital that initially provided care; this is in keeping with the public reporting of mortality rates.(31) Hospital volume was defined as the total number of patients having urgent/emergent operations at each acute care hospital over the two-year period. Pediatric hospitals and rehabilitation hospitals were excluded from the analysis, as were governmental hospitals such as Veteran’s Affairs (VA) hospitals. Any hospital that performed fewer than three of the specific operation of interest over the two years was excluded from the analyses. Hospitals doing an average of one emergent operation per year were not representative of the hospital types of interest, nor did they contribute reliable information regarding a mortality rate. Operative mortality was defined as a death which occurred during the patient’s index hospitalization.
Statistical Analyses and Outcome Measures:
The first research question evaluated the degree to which hospital operative volume influences hospital mortality rate, for each of the ten specified procedures. An ecological analysis was performed based on the hospital characteristics as predictors of the hospital’s mortality rates, not the individual patient’s characteristics as predictors of the patient’s mortality; in other words, the ‘hospital’ was the unit of analysis. An ecological analysis is a reasonable and valid approach for a study of the relationship between a hospital-level contextual risk factor (in this case volume) and a hospital-level contextual outcome (incidence rate of mortality).(32)
Beta-logistic generalized linear regression(33) was used to examine the relationship between hospital emergency operative volume and postoperative inpatient mortality, with adjustment for both a hospital’s patient case-mix as well as other relevant hospital-type characteristics. The primary outcome measure was the proportion (a value from 0 to 1) of patients with in-hospital mortality over the 2-year period (defined as the number of patients who died during that admission after undergoing an emergency operation divided by all patients undergoing that same type of emergency operation at that hospital over the two years). Mortality proportion data typically exhibit an S-shaped, or sigmoidal, curve with asymptotes at the limits of zero and one when plotted against a predictor. In contrast to the beta-logistic generalized linear regression with logit link function used in the current study, ordinary regression does not capture this relationship. The beta distribution supports a range from zero to one, and the logit link ensures that the predicted mean stays within bounds (0, 1). The model regressed the mortality proportion at each hospital on the natural logarithm of the hospital volume plus hospital-level characteristics as covariates to adjust for variation in case-mix across hospitals. The general equations for the beta regression models, by operation, are available upon request to the corresponding author. The natural logarithm transformation of volume was used in the model for the operative volume predictor. The results are based on a +1 standardized increase in the natural logarithm transformation of volume. To interpret this from an operative volume standpoint, one must transform the natural log integer into a hospital operative volume – see footnotes in Table 2 and Appendix C for full explanations of this. There are two major reasons why this natural logarithm transformation was chosen for the models. The first is because the logit is the canonical link function for the beta regression, and therefore both the predictor of interest (hospital urgent/emergent operative volume) and the outcome of interest (proportion of mortality) would both be on the natural log scale. The second is that, from a practical interpretation standpoint, hospital volume is believed to have a multiplicative effect rather than an additive effect. For example, an additive effect would suggest that a difference of 20 operations between hospitals with volumes of 5 operations vs 25 operations would be equivalent to the difference of 20 operations between hospitals with volumes of 100 operations vs 120 operations; the multiplicative effect would interpret this differently, saying the difference between hospitals with volumes of 5 operations vs 25 operations would be 5 times as large, and the difference between hospitals with volumes of 100 operations vs 120 operations would be 1.2 times as large. Patient case-mix characteristics were included as hospital-level means or percentages to serve as covariates in the models that adjust for case-mix differences between hospitals. These included mean age, mean Elixhauser-van Walraven comorbidity index, and percentages of gender, race, and payer status. The Elixhauser-van Walraven is a widely-used, validated, weighted measure of a patient’s chronic disease burden.(34) Coexisting conditions were identified in the SID dataset using ICD-9 diagnosis codes, which were then used to calculate an Elixhuaser-van Walraven comorbidity index. Unadjusted as well as multivariable risk-adjusted models were tested to predict in-hospital mortality; odds ratios represented the effects of hospital volume on survival proportion.
Table 2.
Beta Regression Estimates for the Impact of Hospital Operative Volume on Hospital Mortality Rate, by Operation
| Operation | Multivariable risk-adjusted model | ||
|---|---|---|---|
| Estimate a | 95% CI | p Value | |
| Appendectomy | 0.39 | 0.36-0.42 | <0.001 |
| Cholecystectomy | 0.51 | 0.48-0.56 | <0.001 |
| Colectomy | 0.86 | 0.78-0.96 | <0.001 |
| Inguinal and femoral hernia repair | 0.45 | 0.40-0.50 | <0.001 |
| Lysis of adhesions | 0.61 | 0.54-0.68 | <0.001 |
| Necrotizing soft tissue infection excision | 0.53 | 0.40-0.69 | <0.001 |
| Repair of perforated peptic ulcer disease | 0.64 | 0.50-0.82 | <0.001 |
| Small bowel resection | 0.74 | 0.65-0.84 | <0.001 |
| Umbilical hernia repair | 0.40 | 0.32-0.50 | <0.001 |
| Ventral hernia repair | 0.46 | 0.41-0.53 | <0.001 |
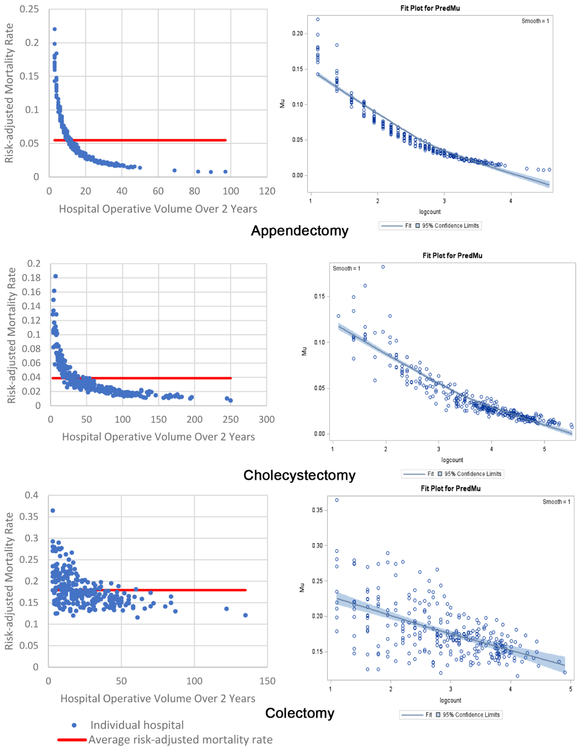
These are beta regression coefficient estimates for hospital volume (aka procedure count) They quantitatively demonstrate the decrease in hospital mortality proportion for each operation type when the natural log of hospital volume is increased by +1. For example, a +1 unit change in natural log volume (meaning an integer increase from 1-->2 or 2-->3 or 3-->4) for colectomy will decrease morality by 14% (as defined in the above table). This 14% predicted decrease in mortality proportion occurs at each increase in integer interval, meaning that if a hospital increases colectomy natural log volume by 2-->3 they can expect a 14% decrease in mortality, and if they increase natural log operative volume by 3-->4 they can expect another 14% decrease in mortality proportion. Note that by comparison, a +1 unit change in natural log volume from 1-->2 or 2-->3 or 3-->4 for small bowel resection will decrease morality by 26% over each interval. However, natural log volumes are difficult to conceptualize, so it is helpful to transform these natural log volume integers (such as 1, 2, 3, 4, etc) back into actual hospital operative volumes. The natural log integer can be back-converted to an actual operative volume like this: for the natural log volume integer 2: 2 = ln(x) --> x = e^2 = 7.4 operations; for the volume integer 3: 3 = ln(x) --> x = e^3 = 20.1 operations; for the natural log volume integer 4: 4 = ln(x) --> x = e^4= 54.6 operations; etc. Therefore, in terms of actual operative volume, a +1 unit change in natural log volume integer from 2-->3 (7.4 operations vs 20.1 operations = +12.7 operations) is not equivalent to the change from 3-->4 (20.1 operations vs 54.6 operations = +34.5 operations) – this highlights the exponential function of the natural log. Please see Figure I to appreciate this visually, as there are graphs for mortality proportion (on the y axis) plotted against both actual operative volume as well as natural log volume integer (on the x axis).
A pseudo-R2 statistic(35, 36) was calculated for each beta regression model as a generalized linear model analog to the linear regression R2 statistic, denoting the proportion of the variance of the dependent variable explained by the model predictor of interest with covariates. Importantly, the values for the beta regression model pseudo-R2 statistic are very different than the values for the linear regression R2 statistic: a wider range of pseudo-R2 values represent good model fit compared to the standard R2.
Patient-level characteristics were compared between those who died and those who survived. Chi-squared (χ2) tests were used to compare differences in proportions for categorical variables; these data were summarized by frequencies with percentages. Group means were compared using two-sample t-tests for normally distributed continuous variables; data were summarized by mean values with standard deviations (SD). Hospital-level characteristics (medical school affiliation; trauma center status; high technology capability) were evaluated and presented as frequencies with percentages. A hospital had a “medical school affiliation” if it was defined in the AHA dataset as a teaching hospital as reported to the American Medical Association, which accredits medical schools.(37) A hospital was considered a “trauma center” if it was either a Level 1 or Level 2 trauma center as defined and verified by the American College of Surgeons Committee on Trauma(24) (note the AHA dataset designation of trauma center, which was missing many variables and is based on state-level standards, was not used). A hospital was defined as having “high technology capability” if it performed adult open heart surgery and/or major liver or heart organ transplantation; this variable is therefore a proxy for a hospital’s ability to manage and treat intensely sick patients in the perioperative period, as these operations often demand intensive care unit admission and blood banking abilities, among others.(27, 38) The second research question was at what hospital operative volume would patients undergoing an emergency operation realize the average or lower mortality risk for that operation. The volume-threshold was analyzed using the results of the beta regression models. The threshold (also referred to as the “threshold benchmark” or “benchmark”) was defined as the hospital operative volume above which ≥95% of the hospitals were performing at or better than the average risk-adjusted mortality rate. For example, if a patient were to have a non-elective cholecystectomy at a hospital with a cholecystectomy operative volume greater than the volume-threshold, there would be a 95% chance that that patient’s mortality risk (as defined by hospital mortality proportion) would be lower than the average risk-adjusted mortality for all hospitals performing cholecystectomies. Average mortality risk was defined and calculated by operation as the mean in-hospital risk-adjusted mortality at all acute care hospitals included in the analysis performing that specific EGS operation.
A p-value <0.05 was defined as statistically significant. All statistical analyses were conducting using SAS 9.4 (SAS Institute Inc., Cary, NC). This study was approved by the Human Investigation Committee (HIC) of the Yale University Human Research Protection Program (HRPP) for biomedical research.
RESULTS:
Of the 425 acute care hospitals in California, the number meeting the inclusion criterion performing emergent operations ranged from 62 performing umbilical hernia repairs to 298 performing cholecystectomies; 299 different acute care hospitals were included. Further breakdown of hospital level characteristics by operation are found in Appendix D. The three highest volume operations were cholecystectomy (n=17427), colectomy (6727), and appendectomy (4857). The three lowest volume operations were umbilical hernia repair (268), excision of NSTI (666), and repair of perforated peptic ulcer disease (871).
At 299 different acute care hospitals in California, 41,860 patients underwent EGS operations (Table 1). Overall unadjusted mortality rate was 5.5%, though it varied significantly by operation (Appendix E): 0.6% for appendectomy; 1.5% for cholecystectomy; 14.2% for colectomy; 2.3% for inguinal and femoral hernia repair; 6.2% for LOA; 11.4% for NSTI excision; 17.7% for repair of perforated peptic ulcer disease; 12.6% for small bowel resection; 1.1% for umbilical hernia repair; and 2.4% for ventral hernia repair. Relative to the non-decedents, the decedents were more likely to be white, have a payor source of Medicare, older and had higher van Walraven co-morbidity scores (Table 1). Open versus laparoscopic operations did not significantly impact mortality outcome. Further breakdown of patient level characteristics, by operation, is found in Appendix E.
Table 1.
Characteristics of 41,860 Geriatric Patients Undergoing Emergency General Surgery Operations in California in 2011 and 2012, Recorded in the State Inpatient Database
| Variable | Survived | Died | p Value* | |
|---|---|---|---|---|
| n | 39,550 | 2,310 | -- | |
| Female sex, n (%) | 21,780 (55.1) | 1,279 (55.4) | 0.91 | |
| Race and ethnicity, n (%) | <0.001 | |||
| White | 24,401 (64.0) | 1,521 (67.8) | ||
| Black | 1,697 (4.4) | 135 (6.0) | ||
| Non-black, non-white | 4,296 (11.3) | 204 (9.1) | ||
| Hispanic | 7,753 (20.3) | 385 (17.1) | ||
| Age, y, mean (SD) | 76.3 (7.5) | 79.9 (7.7) | <0.001 | |
| Comorbidities, van Walraven score, mean (SD) | 5.5 (7.1) | 13.9 (8.1) | <0.001 | |
| Payor source, n (%) | <0.001 | |||
| Medicare | 33754 (85.3) | 2083 (90.2) | ||
| Medicaid | 1977 (5.0) | 112 (4.8) | ||
| Private insurance | 3385 (8.6) | 104 (4.5) | ||
| Self pay or other | 433 (1.1) | 11 (0.5) | ||
Shows overall patient characteristics; for patient characteristics by operation type, see Appendix E Table E1-E10. Additionally, because of missing individual data for “Race and ethnicity” and “Payor source” the total number of patients for those variables are lower than the patient totals for the survived and died columns.
p Values are from two-sided t-test for continuous variables and χ2 for categorical variables.
Multivariable beta regression models found that risk-adjusted mortality significantly decreased as volume increased for all ten EGS operations, although the relative magnitude of this inverse relationship varied substantially by procedure (Table 2). Relative to other covariates in the beta regression models, hospital operative volume was the most important characteristic with significant impact on mortality for every operation studied (Appendix C). Even with operations that have a lower risk of mortality (<2%), there remained a survival benefit to having the operation done at a higher volume hospital.
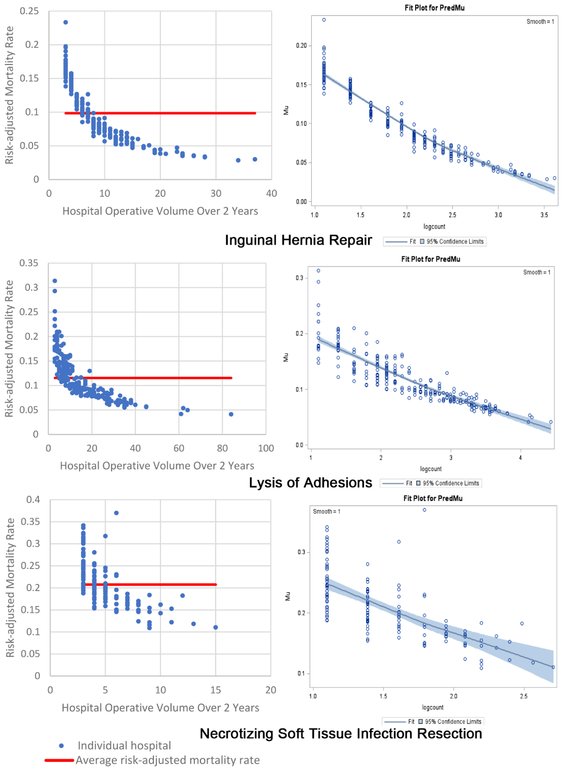
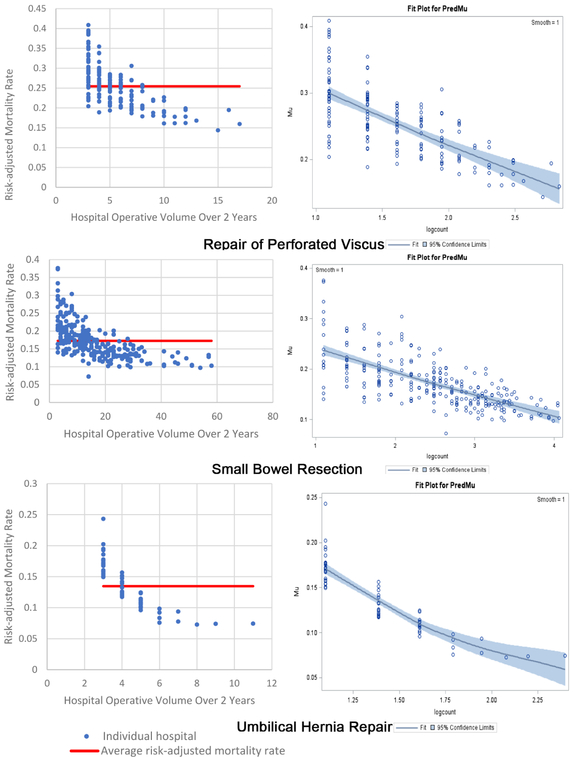
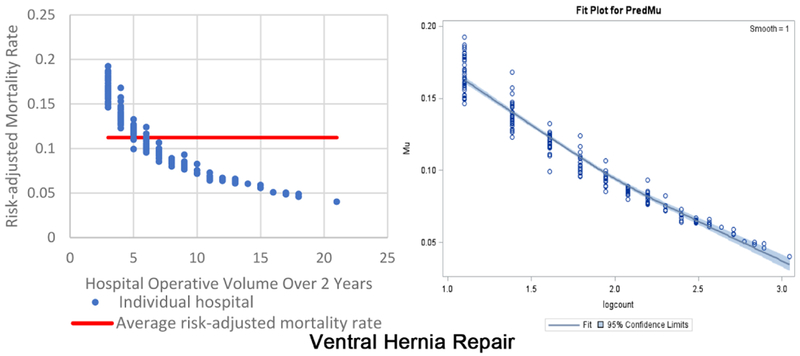
The inverse volume to mortality relationship is shown graphically in the beta fit plots for all ten operations (Figure 1). For each operation, the high-volume hospitals tend to cluster together, indicating less variability and higher precision, while at the lower volume hospitals there is wide variation in mortality. In terms of model fit, each operation’s beta regression pseudo R2 indicated that each model fit the data well; pseudo R2 values ranged from 0.16 for repair of perforated viscus to 0.74 for appendectomy. Full results of beta regression by operation, including hospital-level and patient-level covariates that were significantly associated with death, are found in Appendix C.
Figure 1.
Two beta fit plots for each of the 10 emergency general surgery operations. The left plot shows the inverse volume to mortality relationship: the x-axis is hospital operative volume over 2 years; the y-axis is risk-adjusted hospital mortality rate; the thick flat line represents the average risk-adjusted mortality; every blue dot represents an individual hospital. The right plot is the relationship modeled in the beta regression analyses (plots on right are titled “Fit Plot for PredMu” meaning predicted mortality): the x-axis is natural log-transformed hospital operative volume; the y-axis is risk-adjusted hospital mortality rate (in the figure labeled “Mu”); every dot represents an individual hospital.
The hospital operative volume-thresholds at which there was a 95% chance that that institution performed at or better than the average risk-adjusted mortality rate varied by operation (Table 3). from 38 cases over two years for colectomy to 5 cases over two years for umbilical hernia repair. On average, more than 50% of the hospitals did not meet the volume-thresholds, representing 22% of patients. The number of hospitals failing to meet these threshold mortality standards varied by operation type, from 30% of all hospitals for cholecystectomy to 81% of institutions for repair of perforated peptic ulcer disease. The number of operations performed at these below-threshold institutions also varied, from 7% of all cases for cholecystectomy to 64% of the operations for repair of perforated peptic ulcer disease.
Table 3.
Hospital Operative Volume Threshold Analysis
| Operation | Average risk- adjusted mortality, % |
Mortality rate, lowest volume hospitals, % |
Mortality rate, highest volume hospitals |
Hospital volume threshold with 95% chance of being better than average mortality* |
Total no. of hospitals |
Hospitals not achieving volume threshold, % |
Total operative volume |
No. of operations performed at hospitals below volume threshold |
Operations at hospitals below volume threshold, % |
|---|---|---|---|---|---|---|---|---|---|
| Appendectomy | 5.5 | 22.0 | 0.7 | 11 | 267 | 35 | 4,857 | 609 | 13 |
| Cholecystectomy | 3.9 | 18.2 | 0.7 | 28 | 298 | 30 | 17,427 | 1,187 | 7 |
| Colectomy | 17.9 | 36.4 | 11.5 | 38 | 274 | 76 | 6,727 | 3264 | 49 |
| Inguinal and femoral hernia repair | 9.8 | 23.3 | 2.9 | 8 | 222 | 48 | 1,978 | 490 | 25 |
| Lysis of Adhesions | 11.5 | 31.4 | 4.1 | 12 | 252 | 49 | 3,910 | 800 | 20 |
| Necrotizing soft tissue infection excision | 20.7 | 37.0 | 10.9 | 7 | 131 | 77 | 666 | 403 | 61 |
| Repair of perforated peptic ulcer disease | 25.5 | 40.8 | 14.4 | 8 | 156 | 81 | 871 | 560 | 64 |
| Small bowel resection | 17.3 | 37.6 | 7.2 | 19 | 256 | 68 | 4,008 | 1,574 | 39 |
| Umbilical hernia repair | 13.5 | 24.3 | 7.3 | 5 | 62 | 65 | 268 | 137 | 51 |
| Ventral hernia repair | 11.2 | 19.2 | 4.0 | 6 | 170 | 49 | 1,148 | 320 | 28 |
Hospital operative volume that optimizes probability of survival for a given operation, defined as the 2-year volume above which 95% of hospitals have better than average risk-adjusted mortality.
DISCUSSION:
Consistent with the study’s primary hypothesis, higher hospital emergency operative volume was independently and significantly associated with higher probability of survival for patients ≥65 years undergoing each of ten urgent/emergency EGS operations. For each procedure, hospital emergency operative volume reaches a specific threshold above which nearly all hospitals achieve at or better than average risk-adjusted survival. This volume-threshold provides an operationally defined, empirically-based, objective indicator of operative performance. These results suggest that hospital operative volume is an important metric of surgical quality for older patients undergoing emergency operations.
The ACS CQGS is working to create verifiable hospital-based standards to improve outcomes for older surgical patients. In a recent publication(39), a modified RAND-UCLA Appropriateness Method was used to establish a list of valid standards to improve the surgical care of older adults. Hospital operative volume was not on this list. This is understandable, as using hospital operative volume as a benchmark-criteria for surgical quality is not perfect, and has its critics.(40) However, based on the current study, the CQGS should consider establishing hospital operative volume as one of the fundamental benchmarks of high-quality emergency surgical care and including volume in the accreditation process for geriatric surgical centers. This criterion has been successfully implemented for other surgical subspecialties such as bariatrics(23), trauma(24), and pediatric surgery(25), which use hospital volume as one of the standards for the verification process.
Variability is inherent to any outcome in medicine and surgery.(41) One of the goals of quality improvement initiatives is to minimize this variability so that outcomes are more predictable, thus saving lives and decreasing morbidity. Part of the survival variability in the current study is explained by hospital operative volume, which turns out to be the most significant predictor of survival for all EGS operations in this geriatric cohort. For some EGS operations, the survival variability is also explained by patient-level characteristics (see Appendix C), though their relative importance was less consistent. Patient characteristics such as comorbidity status (for cholecystectomy; colectomy; lysis of adhesions; small bowel resection) and age (for appendectomy; repair of perforated peptic ulcers; umbilical hernia repair) were significantly associated with mortality.
The differences observed between procedure volume and patient characteristics on in-hospital mortality after EGS operations demonstrates that predicting outcomes in an older population is operation dependent. Given the lack of consistency in associations for patient characteristics in the regression models, the results may also mean that traditional patient-level metrics of risk-adjustment (age, gender, race, comorbidities, etc) lack specificity in an older population. Future outcomes research on older populations should look beyond these traditional metrics and assess more geriatric-centered variables (frailty, function, cognition, etc), which may better characterize an aged population. In the SID dataset that was employed for the present study, such geriatric-specific variables were not available.
The survival variability seen among hospitals in this study is also likely due to processes of care and institutional cultures (such as high reliable organizations) that are characteristic of certain medical centers. While such indices are best measured with qualitative data, they are inherently captured – though not quantified – in the beta regression models which use the hospital as the unit of analysis. This is a distinct strength of the current study – though additional qualitative investigation is needed to specifically define these processes, structures, and cultures. The use of an ecological analysis, with outcome as a hospital’s mortality incidence rate rather than a patient’s probability of death, is a strength of this study. It allows a comparison of hospitals as a function of procedure volume, rather than comparing patient-types within or between hospitals. The risk of mortality for individual patients within hospitals via multilevel models with random hospital effects was not modelled as this would assume conditional independence of patient outcomes within hospitals. Such a modeling paradigm ignores contextual effects on mortality risk; for example, if you were the only high-risk patient at a hospital, or among exclusively high-risk patients at a hospital with the same volume, you would have the same risk of procedure failure. In addition to multilevel models with random hospital effects, another alternative to beta regression would be generalized estimating equations with ‘sandwich’ variance estimation. Such a model, however, assumes all patients are from the same population, but adjusts variance estimates to acknowledge that there are correlated, not independent, observations. Relative to these alternative analytic strategies, ecological analysis is a more reasonable approach for modeling the true relationship between hospital operative volume and mortality proportion.
Several issues regarding emergency operative intervention in an older population warrant further discussion. The first is the transfer of patients between institutions. Based on the volume-threshold definitions, the results suggest that 22% of geriatric EGS patients, approximately 9344 people over the 2 year study period in California, would potentially have benefited from transfer to a higher-volume institution for their operation. This could be problematic as it has the potential to overwhelm higher volume centers. Furthermore, emergency surgical conditions are inherently time-sensitive diagnoses requiring time-sensitive operations. As such, delaying an urgent/emergent operation by a transfer can also be potentially problematic especially given the evidence that transferred patients have poorer outcomes for time-sensitive surgical emergencies.(42, 43) However, there are few reliable data to support these claims in the geriatric EGS population. Given the underappreciated complexity of emergency operations in older patients, some have advocated that urgent/emergent EGS operations should be daytime-only procedures.(44) Taken together, based on the present knowledge of EGS disease processes, there may exist a safe window of time during which a majority of older patients with EGS conditions could be temporized with standard medical treatment and transferred to higher-volume centers for operative intervention, perhaps within 6-12 hours of presentation and diagnosis. Further investigation is necessary and warranted.
The second consideration – which builds on the first and is based on the fact that most rural hospitals are lower operative volume institutions – is the practical limitation of emergency surgical care in more remote settings. A structured system of EGS care that mandates transfer of older patients away from rural areas may not prove beneficial as the attendant delays to therapy may negate the benefits of a higher volume center. Therefore, examining the reasons that some hospitals performing less complex cases such as appendectomy and cholecystectomy have higher mortality in geriatric patients is important; these data seem to imply that “low risk” emergency operations are not uniformly low risk at all hospitals, and can become “high risk” operations in certain institutions. The ACS CQGS should prioritize such research. The overall goal should be to investigate best-practices and establish standards to allow lower volume hospitals to achieve acceptable outcomes for less complex operations in older patients.
The third consideration is the influence of individual surgeon volume to outcomes in geriatric EGS patients. In the elective surgical literature, much has been written about the value of both hospital volume(45-51) and surgeon volume(51-53); these relationships continue to be explored, clarified, and researched today. Unlike elective general surgery, where patients can choose their surgeon ahead of an operation, this is rare in unplanned non-elective surgical situations. Therefore, akin to the exceedingly safe field of anesthesia, the field of general surgery should strive to make EGS operations in older patients safe regardless of individual surgeon experience or volume. It is justified not to investigate the individual surgeon level in a setting where a patient cannot choose their individual surgeon; to do so would be incongruent with the real-world setting of emergency surgical care. Additionally, given the limitations of the SID HCUP datasets, information on individual surgeons was not available.
The current study has several limitations. First, it employed the use of a retrospective administrative dataset, so the results should be interpreted in that context. For example, information was not available on cause of death or type of anesthesia used during the operation. Second, the ability to risk-adjust the data did not include physiologic parameters (such as heart rate or blood pressure) or geriatric-specific characteristics (such as frailty or functional independence) due to limitation of the SID HCUP data; the present study was additionally unable to assess involvement of geriatricians in post-operative care as well as do not resuscitate (DNR) status in the perioperative period. Third, an “emergency” patient is a construct of the Healthcare Cost and Utilization Project and the current study, and generalizing to all older patients requiring an urgent/emergency operation may not be valid. Fourth, due to the nature of the California SID database, the current study could only evaluate in-hospital mortality. Because variations in timing of patient discharge across hospitals can influence rates of in-hospital mortality, 30-day and 90-day mortality outcomes are considered more accurate metrics. Fifth, the data are from the state of California beginning eight years ago, and generalizations to a national level and current practice patterns may not be valid. And lastly, the hospital volume-thresholds defined herein are a construct of the study itself. While the study defined the benchmark calculation a priori, there may be other potential methodologies to define a volume-threshold.
CONCLUSIONS:
Across the spectrum of EGS, survival rates for geriatric patients were associated with significant improvement when emergency operations were performed at hospitals with higher emergency geriatric operative volumes. Operative volume for older EGS patients therefore seems to be a key quality indicator and determinant of survival, as well as a principle driver of variation in EGS hospital performance. To lessen the negative impact of the wide variation in survival rates at lower volume hospitals, geriatric patients may benefit from a formal system of emergency surgical care that consolidates operative emergencies to higher volume accredited surgery centers, as defined by the volume-threshold benchmark. Further investigation needs to be conducted to define and validate other important determinants of morbidity and mortality in geriatric patients undergoing surgical emergencies.
Supplementary Material
Acknowledgments
Support: Dr Becher was supported in part by the Yale Center for Clinical Investigation CTSA (grant number KL2 TR001862) from the National Center for Advancing Translational Science (NCATS), a component of the NIH; and the American Association for the Surgery of Trauma (AAST) Emergency General Surgery Research Scholarship Award. Dr. Gill was supported in part by the Academic Leadership Award (grant number K07AG043587) and Claude D Pepper Older Americans Independence Center (grant number P30AG021342) from the National Institute on Aging (NIA).
ABBREVIATIONS:
- ACS
American College of Surgeons
- AHA
American Hospital Association
- CQGS
Coalition for Quality in Geriatric Surgery
- EGS
Emergency general surgery
- ICD-9
International Classification of Disease, 9th Edition
- LOA
Lysis of adhesions
- NSQIP
National Surgical Quality Improvement Program
- NSTI
Necrotizing soft tissue infection
- SID
State Inpatient Database
- US
United States
Footnotes
Disclosure Information: Nothing to disclose.
Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the NCATS, NIH, AAST, or NIA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.United States Census Bureau. Older People Projected to Outnumber Children. Available at: https://www.census.gov/newsroom/press-releases/2018/cb18-41-population-projections.html. Accessed January 5, 2019.
- 2.American College of Surgeons. The Coalition for Quality in Geriatric Surgery. Available at: https://www.facs.org/quality-programs/geriatric-coalition. Accessed January 5, 2019.
- 3.American College of Surgeons. Optimal Perioperative Care of the Geriatric Patient. Available at: https://www.facs.org/quality-programs/acs-nsqip/geriatric-periop-guideline. Accessed January 5, 2019.
- 4.National Quality Forum. NQF Measure #0697 “Risk Adjusted Case Mix Adjusted Elderly Surgery Outcome Measure." Available at: http://www.qualityforum.org/QPS. Accessed January 2019.
- 5.Robinson TN, Rosenthal RA. The ACS NSQIP geriatric surgery pilot project: improving care for older surgical patients. Bull Am Coll Surg 2014;99:21–23. [PubMed] [Google Scholar]
- 6.Berian JR, Zhou L, Hornor MA, et al. Optimizing surgical quality datasets to care for older adults: lessons from the American College of Surgeons NSQIP geriatric surgery pilot. J Am Coll Surg 2017;225:702–712.e1. [DOI] [PubMed] [Google Scholar]
- 7.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012;215:453–466. [DOI] [PubMed] [Google Scholar]
- 8.Bergenfelz A, Søreide K. Improving outcomes in emergency surgery. Br J Surg 2014;101:e1–2. [DOI] [PubMed] [Google Scholar]
- 9.Deiner S, Westlake B, Dutton RP. Patterns of surgical care and complications in elderly adults. J Am Geriatr Soc 2014;62:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda N, Wada J, Niki M, et al. Factors predicting mortality in emergency abdominal surgery in the elderly. World J Emerg Surg 2012;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015;385:549–562. [DOI] [PubMed] [Google Scholar]
- 12.National Confidential Enquiry into Patient Outcome and Death (NCEPOD). An Age Old Problem: A review of the care received by elderly patients undergoing surgery 2013. Available at: http://patientsafety.health.org.uk/resources/age-old-problem-review-of-care-received-elderly-patients-undergoing-surgery. Accessed January 5, 2019.
- 13.Stewart B, Khanduri P, McCord C, et al. Global disease burden of conditions requiring emergency surgery. Br J Surg 2014;101:e9–22. [DOI] [PubMed] [Google Scholar]
- 14.Becher RD, Hoth JJ, Miller PR, et al. A critical assessment of outcomes in emergency versus nonemergency general surgery using the American College of Surgeons National Surgical Quality Improvement Program database. Am Surg 2011;77:951–959. [PubMed] [Google Scholar]
- 15.Shah AA, Haider AH, Zogg CK, et al. National estimates of predictors of outcomes for emergency general surgery. J Trauma Acute Care Surg 2015;78:482–490; discussion 490-491. [DOI] [PubMed] [Google Scholar]
- 16.Ingraham AM, Cohen ME, Raval MV, et al. Variation in quality of care after emergency general surgery procedures in the elderly. J Am Coll Surg 2011;212:1039–1048. [DOI] [PubMed] [Google Scholar]
- 17.Rich PB, Adams SD. Health care: economic impact of caring for geriatric patients. Surg Clin North Am 2015;95:11–21. [DOI] [PubMed] [Google Scholar]
- 18.Haider AH, Obirieze A, Velopulos CG, et al. Incremental cost of emergency versus elective surgery. Ann Surg 2015;262:260–266. [DOI] [PubMed] [Google Scholar]
- 19.Desserud KF, Veen T, Søreide K. Emergency general surgery in the geriatric patient. Br J Surg 2016;103:e52–61. [DOI] [PubMed] [Google Scholar]
- 20.Torrance ADW, Powell SL, Griffiths EA. Emergency surgery in the elderly: challenges and solutions. Open Access Emerg Med 2015;7:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Søreide K, Desserud KF. Emergency surgery in the elderly: the balance between function, frailty, fatality and futility. Scand J Trauma Resusc Emerg Med 2015;23:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Surgeons. ACS Quality Programs homepage. Available at: https://www.facs.org/quality-programs. Accessed January 5, 2019.
- 23.Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. MBSAQIP homepage. Available at: https://www.facs.org/quality-programs/mbsaqip. Accessed January 5, 2019.
- 24.American College of Surgeons Committee on Trauma. ACS-COT Homepage. Available at: https://www.facs.org/quality-programs/trauma. Accessed January 5, 2019.
- 25.American College of Surgeons Task Force for Children’s Surgical Care. ACS Children’s Surgery Verification Quality Improvement Program homepage. Available at: https://www.facs.org/quality-programs/childrens-surgery/childrens-surgery-verification. Accessed January 5, 2019.
- 26.Healthcare Cost and Utilization Project (HCUP). State Inpatient Databases (SID) homepage. Available at: https://www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed January 5, 2019.
- 27.American Hospital Association (AHA). AHA Annual Survey of Hospitals Database homepage. Available at: https://www.ahadataviewer.com/additional-data-products/AHA-Survey/. Accessed January 5, 2019.
- 28.Becher RD, Hoth JJ, Miller PR, et al. A critical assessment of outcomes in emergency versus nonemergency general surgery using the American College of Surgeons National Surgical Quality Improvement Program database. Am Surg 2011;77:951–959. [PubMed] [Google Scholar]
- 29.Shiloach M, Frencher SK, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2010;210:6–16. [DOI] [PubMed] [Google Scholar]
- 30.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg 2008;207:698–704. [DOI] [PubMed] [Google Scholar]
- 31.Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA 2013;309:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin KA. Study design VI - ecological studies. Evid Based Dent 2006;7:108. [DOI] [PubMed] [Google Scholar]
- 33.SAS. Usage Note 57480: Modeling continuous proportions: Normal and Beta Regression Models. Available at: http://support.sas.com/kb/57/480.html. Accessed January 5, 2019.
- 34.van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47:626–633. [DOI] [PubMed] [Google Scholar]
- 35.Long JS. Regression Models for Categorical and Limited Dependent Variables. . In: Thousand Oaks: SAGE Publications, Inc, 1997. [Google Scholar]
- 36.Shafrin Jason. Healthcare Economist: What is a Pseudo R-squared? Available at: http://healthcare-economist.com/2016/12/28/what-is-a-pseudo-r-squared/. Accessed January 5, 2019.
- 37.Association of American Medical Colleges (AAMC). AAMC Organization Directory homeage. Available at: https://members.aamc.org/eweb/DynamicPage.aspx?webcode=AAMCOrgSearchResult&orgtype=Medical%20School. Accessed January 5, 2019.
- 38.McHugh MD, Kelly LA, Smith HL, et al. Lower mortality in magnet hospitals. Med Care 2013;51:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berian JR, Rosenthal RA, Baker TL, et al. Hospital standards to promote optimal surgical care of the older adult: a report from the Coalition for Quality in Geriatric Surgery. Ann Surg 2018;267:280–290. [DOI] [PubMed] [Google Scholar]
- 40.LaPar DJ, Kron IL, Jones DR, et al. Hospital procedure volume should not be used as a measure of surgical quality. Ann Surg 2012;256:606–615. [DOI] [PubMed] [Google Scholar]
- 41.Birkmeyer JD, Dimick JB. Understanding and reducing variation in surgical mortality. Annu Rev Med 2009;60:405–415. [DOI] [PubMed] [Google Scholar]
- 42.DeWane MP, Davis KA, Schuster KM, et al. Transfer status: A significant risk factor for mortality in emergency general surgery patients requiring colon resection. J Trauma Acute Care Surg 2018;85:348–353. [DOI] [PubMed] [Google Scholar]
- 43.Holena DN, Mills AM, Carr BG, et al. Transfer status: a risk factor for mortality in patients with necrotizing fasciitis. Surgery 2011;150:363–370. [DOI] [PubMed] [Google Scholar]
- 44.Peitzman AB, Watson GA, Marsh JW. Acute cholecystitis: When to operate and how to do it safely. J Trauma Acute Care Surg 2015;78:1–12. [DOI] [PubMed] [Google Scholar]
- 45.Flood AB, Scott WR, Ewy W. Does practice make perfect? Part I: The relation between hospital volume and outcomes for selected diagnostic categories. Med Care 1984;22:98–114. [PubMed] [Google Scholar]
- 46.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 47.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 48.Flood AB, Scott WR, Ewy W. Does practice make perfect? Part II: The relation between volume and outcomes and other hospital characteristics. Med Care 1984;22:115–125. [PubMed] [Google Scholar]
- 49.Hannan EL, O’Donnell JF, Kilburn H, et al. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA 1989;262:503–510. [PubMed] [Google Scholar]
- 50.Dudley RA, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA 2000;283: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 51.Schrag D, Panageas KS, Riedel E, et al. Surgeon volume compared to hospital volume as a predictor of outcome following primary colon cancer resection. J Surg Oncol 2003;83:68–78; discussion 78–79. [DOI] [PubMed] [Google Scholar]
- 52.Chowdhury MM, Dagash H, Pierro A. A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg 2007;94:145–161. [DOI] [PubMed] [Google Scholar]
- 53.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.