Atrial fibrillation (AF) is increasing in prevalence and remains a leading cause of morbidity in the elderly. As such, orthogonal strategies to estimate and mitigate AF risk are increasingly necessary. Largely based on epidemiological studies, arterial stiffness is proposed to increase risk for AF through resultant left ventricular hypertrophy and left atrial alteration, pulsatile load, and compensatory changes from endothelial dysfunction1. However, it remains unclear whether arterial stiffness is causally related to AF. Arterial stiffness index (ASI) measurement using finger infrared photoplethysmography (PPG) is a scalable, non-invasive approach to assess arterial stiffness and is correlated with aortic (carotid-femoral) pulse wave velocity, the gold standard approach for arterial stiffness measurement2. Understanding whether arterial stiffness causally mediates AF may help identify relevant biology, motivate novel preventive and therapeutic approaches, and establish a new titratable biomarker for AF risk.
Mendelian randomization uses human genetics for causal inference by leveraging the random assortment of genetic variants associated with an exposure during meiosis at conception, minimizing influences from confounding. We recently comprehensively described the genetic basis of PPG-derived ASI among 131,686 participants of the UK Biobank through a genome-wide association study (GWAS) 3. Here, we leverage those observations and use Mendelian randomization to determine whether a genetic predisposition to increased ASI is independently associated with increased risk for AF.
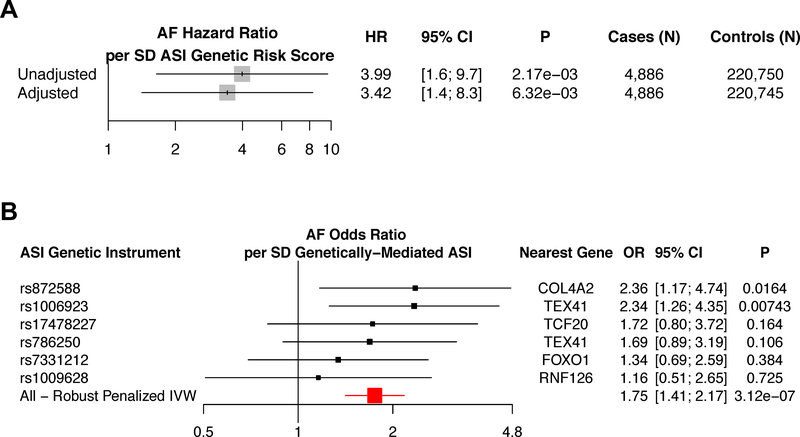
We derived an additive ASI genetic risk score (GRS) comprised of the top 6 variants independently associated with ASI (P<5×10−7, LD r2<0.25) from 131,686 participants3. We applied this ASI GRS to two studies including 225,636 UK Biobank participants and 588,190 individuals from the largest published multi-ethnic AF GWAS to date 4. In the UK Biobank, we associated the ASI GRS with risk of incident AF (4,886 cases) over median 7 (range: 0–10.6) years follow-up. The ASI GRS was significantly associated with incident AF in the UK Biobank in both an unadjusted univariate model (HR 4.0 per SD ASI phenotype [95% CI, 1.6–9.7]; P=2.2×10−3), and in a multivariate model adjusted for age, sex, smoking status, prevalent heart failure, prevalent hypertension, prevalent coronary artery disease, prevalent hypercholesterolemia, prevalent diabetes, heart rate, alcohol intake, and exercise frequency (HR 3.4 per SD ASI phenotype [95% CI, 1.4–8.2]; P=6.5×10−3) (Figure 1A).
Figure 1:
Mendelian randomization analyses between an arterial stiffness index genetic risk score and atrial fibrillation. A) Association of the ASI genetic risk score with incident AF was derived using individual-level data from the UK Biobank (225,636 total individuals, and 4,886 incident AF cases). Analyses are provided both unadjusted, and separately, adjusted by cardiometabolic risk factors (age, sex, smoking status, prevalent heart failure, prevalent coronary artery disease, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, heart rate, alcohol intake, and exercise frequency). B) Association of ASI genetic instruments, individually and combined, with the largest published multi-ethnic AF genome-wide association study (4) (588,190 total individuals, and 65,446 cases) using robust, penalized inverse-variance weighted 2-sample Mendelian randomization. Variant-level hazard ratios (A) and odds ratios (B) are reported per standard deviation of ASI instrumented by the genetic instrument.
AF = atrial fibrillation or flutter, ASI = Arterial stiffness index, HR = hazard ratio, IVW = inverse variance weighted
To replicate this association, we pursued 2-sample Mendelian randomization between ASI and AF using variant-level summary statistics from 588,190 individuals including 65,446 AF cases 4. Robust, penalized inverse-variance weighted (IVW) 2-sample Mendelian randomization demonstrated a significant association between genetically-elevated ASI and AF (OR 1.8 per SD ASI phenotype [95% CI, 1.4–2.2], P=3.1×10−7), with each ASI-raising allele consistently increasing AF risk (Figure 1B).
Sensitivity analyses were performed to evaluate the robustness of the 2-sample Mendelian randomization association. Firstly, since the multi-ethnic AF study used included the UK Biobank within its sample set, we performed additional analyses using summary statistics without the UK Biobank and found consistent results in 2-sample MR (OR =1.76; 95% CI [1.30–2.38], P=2.7×10−4, N=237,173). Furthermore, a consistent and significant association between the ASI GRS and AF is observed among multiple 2-sample Mendelian randomization methods, with no evidence of significant horizontal pleiotropy (MR-Egger Intercept P=0.61), or heterogeneity (IVW heterogeneity test P=0.68). Additionally, a leave-one-out analysis was performed, re-computing the IVW association statistics after leaving one variant at a time out of the genetic instrument, demonstrating consistently significant results. Lastly, multi-variable IVW Mendelian randomization was performed with 5 available ASI GRS variants or proxies present in the multi-ethnic AF GWAS, the UK Biobank ASI GWAS, and the International Consortium for Blood Pressure GWAS 5, finding that the association of AF with ASI remains at least nominally significant after adjusting for the effect of the genetic variants on systolic blood pressure (OR 1.6 per SD ASI phenotype [95% CI, 1.01–2.8], P=0.044). These findings are consistent with concordant relationships between ASI-raising alleles and AF risk without substantial influence of pleiotropy.
Finally, some prior work has suggested that the presence of AF itself precedes and thereby influences ASI (as opposed to the reverse). Thus, we additionally performed 2-sample Mendelian randomization to evaluate the association of an AF genetic instrument with ASI. 106 independent, genome-wide significant loci associated with atrial fibrillation among Europeans 4 were used as a genetic instrument and associated with their respective summary statistics from the ASI GWAS study3. No significant association was observed using IVW 2-sample Mendelian randomization (Beta=0.001 [95% CI, −0.007–0.009], P=0.89), although we cannot exclude the possibility of reduced power to explain the lack of reverse association.
In conclusion, a genetic predisposition to higher ASI was unidirectionally associated with increased risk of incident and prevalent AF. These results are consistent with a causal association between ASI and AF. Randomized controlled studies are required to confirm whether lowering ASI will lower risk of incident AF.
Anonymized individual-level data are available by application from the UK Biobank (https://www.ukbiobank.ac.uk). All supporting data are available within the present article.
Acknowledgments:
S.M.Z. is supported by the National Institutes of Health’s Medical Scientist Training Program at the Yale School of Medicine and the Paul and Daisy Soros Fellowship. P.N. is supported by a Hassenfeld Scholar Award from the Massachusetts General Hospital, and K08 HL140203 from the National Heart, Lung, and Blood Institute. P.T.E. was supported by the National Institutes of Health (1R01HL092577, R01HL128914, K24HL105780), by an Established Investigator Award from the American Heart Association (13EIA14220013) and by the Fondation Leducq (14CVD01). S.A.L. is supported by NIH grant 1R01HL139731 and American Heart Association 18SFRN34250007. The authors would like to acknowledge the participants, study staff, and investigators of the UK Biobank and the AFGen Consortium.
Disclosures: P.N. reports grant support from Amgen and Boston Scientific, and consulting income from Apple. P.T.E. is supported by a grant from Bayer AG to the Broad Institute focused on the genetics and therapeutics of cardiovascular disease. S.A.L. receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer HealthCare, and Boehringer Ingelheim, and has consulted for Abbott, Quest Diagnostics, Bristol Myers Squibb / Pfizer.
References:
- 1.Shaikh AY, et al. Relations of Arterial Stiffness and Brachial Flow-Mediated Dilation With New-Onset Atrial Fibrillation: The Framingham Heart Study. Hypertension. 2016;68:590–6. [DOI] [PubMed] [Google Scholar]
- 2.Woodman RJ, et al. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18:249–60. [DOI] [PubMed] [Google Scholar]
- 3.Zekavat SM, et al. Genetic Association of Finger Photoplethysmography-Derived Arterial Stiffness Index With Blood Pressure and Coronary Artery Disease. Arterioscler Thromb Vasc Biol. XXX;39:00–00. DOI: 10.1161/ATVBAHA.119.312626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roselli C, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Consortium for Blood Pressure Genome-Wide Association, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]