Key Points
Question
What is the effect of immediate vs delayed pushing during the second stage of labor on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia?
Findings
In this randomized clinical trial that involved 2414 women, immediate vs delayed pushing did not result in a significant difference in the rates of spontaneous vaginal delivery (85.9% vs 86.5%, respectively).
Meaning
Among nulliparous women receiving neuraxial analgesia, the timing of pushing during the second stage of labor did not affect the rate of spontaneous vaginal delivery.
Abstract
Importance
It is unclear whether the timing of second stage pushing efforts affects spontaneous vaginal delivery rates and reduces morbidities.
Objective
To evaluate whether immediate or delayed pushing results in higher rates of spontaneous vaginal delivery and lower rates of maternal and neonatal morbidities.
Design, Setting, and Participants
Pragmatic randomized clinical trial of nulliparous women at or beyond 37 weeks’ gestation admitted for spontaneous or induced labor with neuraxial analgesia between May 2014 and December 2017 at 6 US medical centers. The interim analysis suggested futility for the primary outcome and recruitment was terminated with 2414 of 3184 planned participants. Follow-up ended January 4, 2018.
Interventions
Randomization occurred when participants reached complete cervical dilation. Immediate group participants (n = 1200) began pushing immediately. Delayed group participants (n = 1204) were instructed to wait 60 minutes.
Main Outcomes and Measures
The primary outcome was spontaneous vaginal delivery. Secondary outcomes included total duration of the second stage, duration of active pushing, operative vaginal delivery, cesarean delivery, postpartum hemorrhage, chorioamnionitis, endometritis, perineal lacerations (≥second degree), and a composite outcome of neonatal morbidity that included neonatal death and 9 other adverse outcomes.
Results
Among 2414 women randomized (mean age, 26.5 years), 2404 (99.6%) completed the trial. The rate of spontaneous vaginal delivery was 85.9% in the immediate group vs 86.5% in the delayed group, and was not significantly different (absolute difference, −0.6% [95% CI, −3.4% to 2.1%]; relative risk, 0.99 [95% CI, 0.96 to 1.03]). There was no significant difference in 5 of the 9 prespecified secondary outcomes reported, including the composite outcome of neonatal morbidity (7.3% for the immediate group vs 8.9% for the delayed group; between-group difference, −1.6% [95% CI, −3.8% to 0.5%]) and perineal lacerations (45.9% vs 46.4%, respectively; between-group difference, −0.4% [95% CI, −4.4% to 3.6%]). The immediate group had significantly shorter mean duration of the second stage compared with the delayed group (102.4 vs 134.2 minutes, respectively; mean difference, −31.8 minutes [95% CI, −36.7 to −26.9], P < .001), despite a significantly longer mean duration of active pushing (83.7 vs 74.5 minutes; mean difference, 9.2 minutes [95% CI, 5.8 to 12.6], P < .001), lower rates of chorioamnionitis (6.7% vs 9.1%; between-group difference, −2.5% [95% CI, −4.6% to −0.3%], P = .005), and fewer postpartum hemorrhages (2.3% vs 4.0%; between-group difference, −1.7% [95% CI, −3.1% to −0.4%], P = .03).
Conclusions and Relevance
Among nulliparous women receiving neuraxial anesthesia, the timing of second stage pushing efforts did not affect the rate of spontaneous vaginal delivery. These findings may help inform decisions about the preferred timing of second stage pushing efforts, when considered with other maternal and neonatal outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02137200
This randomized clinical trial compares the effects of immediate vs delayed pushing on spontaneous vaginal delivery and maternal and neonatal morbidities among nulliparous women at or beyond 37 weeks’ gestation admitted for spontaneous or induced labor.
Introduction
More than 3 million women give birth in the United States every year.1 Despite the frequency of childbirth, many aspects of labor management lack an evidence-based approach. The second stage of labor, defined as the interval from complete cervical dilation through delivery of the fetus, is the most physiologically demanding period of labor for both the mother and the fetus.2,3 Despite the effect labor management can have on mode of delivery, and the fact that operative deliveries are associated with higher risks of adverse neonatal and maternal outcomes (such as acidosis, febrile morbidities, and hemorrhage) than spontaneous vaginal deliveries,4 the optimal technique for managing maternal pushing during the second stage of labor is unknown.
The 2 most common approaches to the second stage of labor management are to either initiate pushing with uterine contractions once complete cervical dilation occurs (immediate pushing) or to allow for spontaneous descent (delayed pushing). Both approaches are commonly used, and neither is considered the gold standard. Prior investigations regarding the optimal management strategy were conflicting regarding the effect on spontaneous vaginal delivery and maternal and neonatal morbidities.5,6,7,8,9 The largest trial to date found that delayed pushing reduced the rate of deliveries using mid-pelvic forceps with an equivocal effect on neonatal outcomes. Deliveries using mid-pelvic forceps were prevalent 20 years ago when that study was conducted10; however, these types of deliveries are obsolete in modern obstetric practice in the United States. A subsequent systematic review and meta-analysis including that trial10 and other trials found no differences between delayed and immediate pushing in spontaneous vaginal delivery rates when only high-quality studies were summarized.11 Thus, the optimal second stage labor management strategy regarding the timing of pushing remains controversial.
A pragmatic, multicenter randomized clinical trial was conducted to determine the effect of immediate pushing compared with delayed pushing during the second stage of labor on rates of spontaneous vaginal delivery and maternal and neonatal morbidities among nulliparous women receiving neuraxial analgesia. It was hypothesized that the rate of spontaneous vaginal delivery would increase among nulliparous women with immediate pushing compared with delayed pushing.
Methods
Trial Design
The trial was approved by the institutional review boards at all 6 study sites prior to enrollment. All participants provided written informed consent to be included in this research study. Participants were randomly assigned to immediate pushing or delayed pushing once they reached complete cervical dilation in this open-label, pragmatic, multicenter randomized clinical trial.12 Other than the timing of initiation of pushing, participants were managed according to usual obstetric care. The trial protocol appears in Supplement 1.
Patient Selection
Nulliparous pregnant women at or beyond 37 weeks’ gestation admitted for spontaneous or induced labor with neuraxial analgesia were eligible for this trial (Figure 1). Women were recruited between May 19, 2014, and December 16, 2017, at 6 geographically representative academic and community medical centers across the United States (Washington University in St Louis Medical Center, Missouri Baptist Medical Center, Hospital of the University of Pennsylvania, Pennsylvania Hospital, University of Alabama, Birmingham, and Oregon Health & Science University). Multiparous patients, scheduled cesarean deliveries, multiple gestations, major fetal anomalies, and those with nonreassuring fetal status were excluded. The rate of immediate pushing across the participating centers was approximately 50% before the trial was initiated.
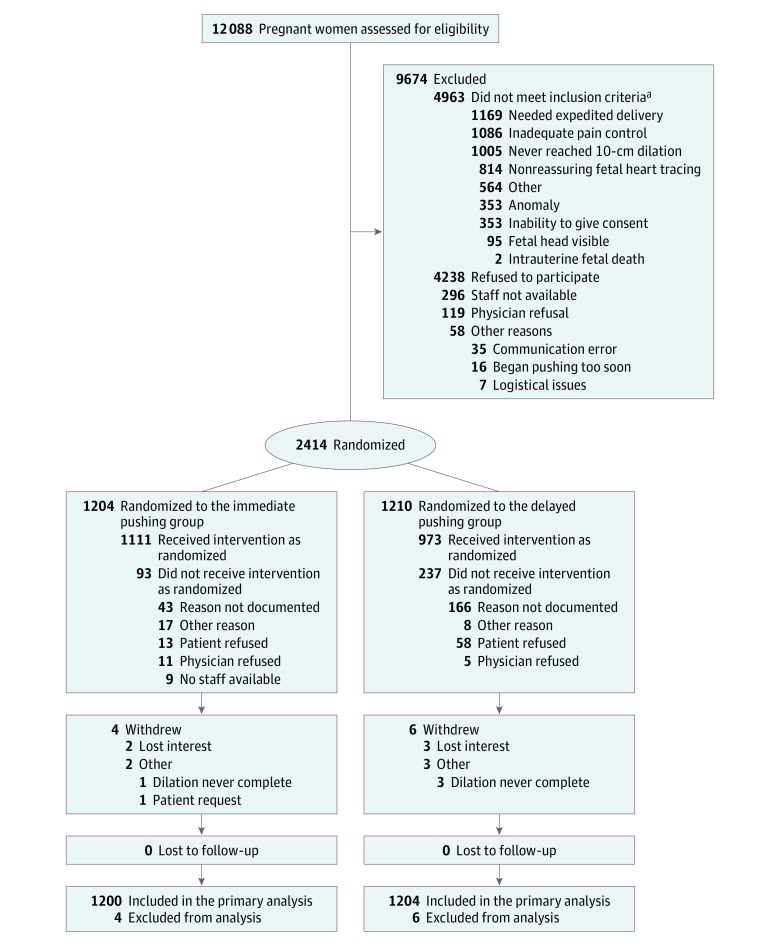
Figure 1. Recruitment, Randomization, and Flow of Participants Through the Trial.
aSome individuals had more than 1 reason for not meeting the inclusion criteria.
Treatment Allocation
At complete cervical dilation (10 cm), patients were randomized centrally in a 1:1 ratio to immediate pushing or delayed pushing. A computer-generated randomization sequence was prepared using variable block sizes that were stratified according to study site by the study statistician.13 Each woman’s group assignment was obtained from a secure website only after a study number and parity were entered and locked and after complete (10 cm) cervical dilation was reached.
Trial Interventions
Women in the immediate pushing group were instructed to initiate pushing at randomization. Women randomized to delayed pushing were instructed to wait 60 minutes prior to initiation of pushing unless instructed to do otherwise by their clinician or unless they had an irresistible urge to push. Maternal position, technique (closed or opened glottis), and duration and frequency of maternal pushing efforts were at the discretion of each participant’s nurse or clinician. Mothers and their infants were followed up through 6 weeks’ postpartum.
Trial End Points
The primary end point was spontaneous vaginal delivery defined as delivery that occurred without the use of forceps, vacuum, or cesarean delivery. Prespecified secondary outcomes included total duration of the second stage of labor, duration of active pushing, operative vaginal delivery (forceps or vacuum), cesarean delivery, and several individual maternal morbidity assessments including postpartum hemorrhage (estimated blood loss >500 mL for vaginal delivery and >1000 mL for cesarean delivery), chorioamnionitis during the second stage of labor, endometritis, and perineal lacerations (≥second degree). A composite outcome of neonatal morbidity also was assessed and defined as occurrence of 1 or more of the following: neonatal death, birth injury, neonatal acidemia (umbilical cord arterial pH <7.1), respiratory distress, transient tachypnea, meconium aspiration with pulmonary hypertension, hypoxic-ischemic encephalopathy, hypoglycemia (venous blood glucose level <40 mg/dL), hypothermia treatment, or suspected neonatal sepsis.
Other prespecified secondary outcomes were occult levator ani muscle injury on 3D transperineal ultrasound, frequency and extent of signs of pelvic organ prolapse using the validated Pelvic Organ Prolapse Quantification system, and rates of patient-reported symptoms of urinary incontinence, fecal incontinence, and pelvic organ prolapse on validated quality-of-life questionnaires at 1 to 5 days, 4 to 8 weeks, and 5 to 7 months’ postpartum. Data were collected for these outcomes but the findings are not reported herein.
Prespecified exploratory outcomes included the individual components of the neonatal morbidity composite outcome and the specific degree of perineal lacerations (ie, second, third, or fourth degree). Patient satisfaction with their birthing experience was assessed using a modified Mackey Childbirth Satisfaction Rating Scale (range, 0 [worst] to 5 [best]).14 Post hoc outcomes included estimated blood loss, blood transfusion, shoulder dystocia, severe perineal laceration (third or fourth degree), and admission to the neonatal intensive care unit.
Patient demographics, antepartum and labor course, and outcomes were extracted from the electronic medical records. Clinical outcomes were extracted by obstetric research nurses who were blinded to group assignment. Race/ethnicity was included in this study for representativeness and generalizability, and was self-reported by the participants in response to an open-ended question. All definitions of outcome measures used in this trial appear in Supplement 2.
Trial Oversight
The trial was overseen by an independent data and safety monitoring board and a steering committee. Two planned interim analyses were conducted after 50% and 75% of the participants were randomized. The clinical investigators were not informed of the results of the prespecified interim analyses during the conduct of the trial. The Haybittle-Peto rule was designated as the guide for stopping the trial early for efficacy.15,16 Under this rule, the prespecified interim analyses of the primary outcome had to demonstrate an extreme difference between groups (P < .001) to justify stopping the trial. This rule has the advantage that the overall type I error rate is preserved at 0.05.
Adverse Events
Study-defined adverse events were prespecified by the data and safety monitoring board and included neonatal acidemia (umbilical cord arterial pH <7.1), chorioamnionitis during the second stage of labor, severe postpartum hemorrhage (estimated blood loss >1000 mL for vaginal delivery and >2000 mL for cesarean delivery), and admission to the neonatal intensive care unit for longer than 12 hours. Serious adverse events were maternal death, life-threatening maternal event, maternal admission to the ICU, unplanned hysterectomy, life-threatening neonatal event, and serious neonatal birth injury including skull fracture, brachial plexus injury, and cephalohematoma.
Sample Size
The sample size was calculated based on a spontaneous vaginal delivery rate of 72% in the delayed pushing group.9 Based on a review and meta-analysis,11 it was estimated that a sample size of 3184 (1592 in each group) was sufficient to detect a 5% absolute difference (from a rate of 72% to ≥77%) in the spontaneous vaginal delivery rate with 90% power using a 2-tailed test and a type I error rate of 0.05.
Data Analysis
The primary data analysis followed the intention-to-treat principle in that all patients were analyzed in the group that they were randomly assigned, regardless of what intervention they received.17 Baseline and labor characteristics were compared between the groups using the χ2 test or Fisher exact test for categorical variables and the t test or Mann-Whitney test for continuous variables.
Rates of spontaneous vaginal delivery and other dichotomous secondary outcomes were compared using the χ2 test or Fisher exact test as appropriate. Relative risk (RR) or risk differences and 95% CIs were calculated. Generalized estimating equations were used to account for study site and the nonindependence of participants at a given site. Continuous outcomes were tested for normality using the Kolmogorov-Smirnov test and compared using the t test or the Mann-Whitney test as appropriate. Prespecified subgroup analyses were performed for the primary outcome using the Breslow-Day test to measure interaction and assess whether the relative effectiveness of immediate or delayed pushing differed across subgroups. Per-protocol and as-treated analyses also were performed.
There were no missing data for the primary outcome. For the other variables, there were missing data for less than 5% of the variables; thus, no imputation was used. Because there was no adjustment for multiplicity, the secondary outcomes should be interpreted as exploratory. All tests were 2-sided and the significance level was set at .05. The statistical package SAS version 9.2 (SAS Institute Inc) was used for all statistical analyses. Additional details appear in the statistical analysis plan in Supplement 3.
Results
After the second interim analysis at 75% recruitment, the data and safety monitoring board recommended recruitment be stopped due to futility and concern for increased morbidity in the delayed pushing group. The rate of spontaneous vaginal delivery was 86.0% in the immediate pushing group and 86.1% in the delayed pushing group (absolute difference, −0.2% [95% CI, −3.0% to 2.7%]; RR, 1.00 [95% CI, 0.97 to 1.04]). The estimate of the conditional power to detect a difference in the primary outcome of spontaneous vaginal delivery was 8% at most. In addition, the data and safety monitoring board was concerned about the significantly higher rate of postpartum hemorrhage in the delayed pushing group. Thus, the final sample size was 2414.
Study Participants
A total of 12 088 patients were assessed for eligibility; 9674 were excluded and the remaining 2414 women were randomized to immediate pushing (n = 1204) or delayed pushing (n = 1210; Figure 1). Most patients followed their assigned timing of pushing (92.3% in the immediate pushing group and 80.4% in the delayed pushing group). Ten patients withdrew from the study; 4 (0.3%) in the immediate pushing group and 6 (0.5%) in the delayed pushing group. No patients were lost to follow-up, leaving 2404 patients (1200 in the immediate pushing group and 1204 in the delayed pushing group) included in the primary intention-to-treat analysis. There were no significant differences in baseline maternal or pregnancy characteristics between the groups (Table 1). The mean time from complete cervical dilation to pushing was 18.9 minutes (SD, 15.1 minutes) in the immediate pushing group vs 59.8 minutes (SD, 21.8 minutes) in the delayed pushing group (mean difference, −40.9 minutes [95% CI, −43.1 to −38.9 minutes]).
Table 1. Baseline Participant and Labor Characteristics.
| Immediate Pushing (n = 1200) | Delayed Pushing (n = 1204) | |
|---|---|---|
| Maternal age, mean (SD), y | 26.5 (5.9) | 26.6 (6.2) |
| Gestational age, mean (SD), wk | 39.5 (1.2) | 39.4 (1.2) |
| Body mass index (BMI) at delivery, mean (SD)a | 30.8 (6.2) | 30.7 (6.2) |
| Obese (BMI >30), No./total No. (%) | 557/1182 (47.1) | 559/1188 (47.0) |
| Race, No. (%) | ||
| White | 566 (47.2) | 538 (44.7) |
| Black | 516 (43.0) | 535 (44.4) |
| Other or mixedb | 118 (9.8) | 131 (10.9) |
| Not Hispanic or Latina, No./total No. (%) | 1136/1193 (95.2) | 1131/1191 (95.0) |
| Marital status, No. (%) | ||
| Single | 689 (57.4) | 706 (58.6) |
| Married | 500 (41.7) | 488 (40.5) |
| Other | 11 (0.9) | 9 (0.7) |
| Employment status, No./total No. (%) | ||
| Employed | 611/1021 (59.8) | 611/1012 (60.4) |
| Unemployed | 328/1021 (32.1) | 321/1012 (31.7) |
| Student | 82/1021 (8.0) | 80/1012 (7.9) |
| Insurance, No. (%) | ||
| Private | 603 (50.2) | 621 (51.6) |
| Government | 534 (44.5) | 513 (42.6) |
| Other or mixed | 36 (3.0) | 45 (3.7) |
| Uninsured | 27 (2.2) | 25 (2.1) |
| Type of drug use, No./total No. (%) | ||
| Tobacco | 67/1185 (5.6) | 54/1191 (4.5) |
| Alcohol | 44/1186 (3.7) | 39/1194 (3.3) |
| Recreational | 68/1188 (5.7) | 70/1194 (5.9) |
| Positive for group B Streptococcus, No./total No. (%) | 399/1155 (34.5) | 350/1163 (30.1) |
| Medical comorbidities, No./total No. (%) | ||
| Asthma | 193/846 (22.8) | 189/853 (22.2) |
| Chronic hypertension | 26/846 (3.1) | 25/853 (2.9) |
| Diabetes (type 1 or 2) | 50/844 (5.9) | 56/853 (6.6) |
| Obstetric complications | ||
| Gestational hypertension, No./total No. (%) | 97/846 (11.5) | 96/852 (11.3) |
| Preeclampsia or eclampsia, No. (%) | 25 (2.1) | 23 (1.9) |
| Fetal growth restriction, No. (%) | 60 (5.0) | 62 (5.2) |
| Oligohydramnios, No. (%) | 29 (2.4) | 35 (2.9) |
| Spontaneous labor, No. (%) | 642 (53.5) | 652 (54.2) |
| Oxytocin use, No./total No. (%) | 956/1199 (79.7) | 963/1201 (80.2) |
| Magnesium sulfate use, No./total No. (%) | 29/1200 (2.4) | 29/1199 (2.4) |
| Amnioinfusion, No./total No. (%) | 64/1198 (5.3) | 65/1201 (5.4) |
| Support person during labor, No./total No. (%) | 1160/1171 (99.1) | 1155/1163 (99.3) |
| Fetal station at complete dilation <2 cm, No./total No. (%)c | 869/1178 (73.8) | 863/1185 (72.8) |
| Fetal position at complete dilation, No./total No. (%)d | ||
| Occiput anterior | 784/907 (86.4) | 769/881 (87.3) |
| Occiput posterior | 108/907 (11.9) | 91/881 (10.3) |
| Occiput transverse | 15/907 (1.7) | 21/881 (2.4) |
| Type of personnel delivering the fetus, No. (%) | ||
| Attending physician | 581 (48.4) | 586 (48.7) |
| Resident physician | 503 (41.9) | 516 (42.9) |
| Physician in fellowship | 23 (1.9) | 18 (1.5) |
| Midwife | 82 (6.8) | 78 (6.5) |
| Nurse | 8 (0.7) | 3 (0.2) |
| Student | 3 (0.2) | 2 (0.2) |
| Birth weight, mean (SD), ge | 3285.7 (438.7) | 3262.0 (418.4) |
| Apgar score at 1 min, median (IQR)f,g | 8 (8-9) | 8 (8-9) |
| Apgar score at 5 min, median (IQR)f,h | 9 (9-9) | 9 (9-9) |
| Fetal sex female, No. (%) | 639 (53.2) | 623 (51.7) |
| Approach to pushing, No./total No. (%) | ||
| Guided | 805/1137 (70.8) | 783/1122 (69.8) |
| Spontaneous | 223/1137 (19.6) | 231/1122 (20.6) |
| Both | 109/1137 (9.6) | 108/1122 (9.6) |
Abbreviation: IQR, interquartile range.
Calculated as weight in kilograms divided by height in meters squared. There were missing data for 18 participants in the immediate pushing group and 16 in the delayed pushing group.
Includes American Indian or Alaskan Native, Asian, Hawaiian or Pacific Islander, or other race.
Position of the leading portion of the fetal head relative to maternal pelvic anatomy in centimeters on a scale of −5 (highest) to 5 (lowest).
The direction of the fetal head relative to the maternal position.
There were missing data for 3 participants in the immediate pushing group and 2 in the delayed pushing group.
Summarizes the condition of the newborn after birth. Neonates are given a score of 0, 1, or 2 (best) for 5 items: color, tone, breathing, heart rate, and grimace resulting in a score from 0-10.
There were missing data for 6 participants in the immediate pushing group and 2 in the delayed pushing group.
There were missing data for 6 participants in the immediate pushing group and 5 in the delayed pushing group.
Primary Outcome
The rate of spontaneous vaginal delivery was 85.9% (n = 1031) in the immediate pushing group vs 86.5% (n = 1041) in the delayed pushing group (absolute difference, −0.6% [95% CI, −3.4% to 2.1%]; RR, 0.99 [95% CI, 0.96 to 1.03], P = .67).
Prespecified Secondary Outcomes
Women in the immediate pushing group had a significantly shorter mean duration of the second stage of labor (102.4 minutes) vs women in the delayed pushing group (134.2 minutes) (mean difference, −31.8 minutes [95% CI, −36.7 to −26.9 minutes], P < .001; Table 2). The immediate pushing group had a significantly longer mean duration of active pushing (83.7 minutes) vs the delayed pushing group (74.5 minutes) (mean difference, 9.2 minutes [95% CI, 5.8 to 12.6 minutes], P < .001).
Table 2. Primary and Exploratory Secondary Efficacy Outcomes.
| No. (%)a | Absolute Risk Difference (95% CI)b | Relative Risk (95% CI)c | P Value | ||
|---|---|---|---|---|---|
| Immediate Pushing (n = 1200) |
Delayed Pushing (n = 1204) |
||||
| Primary Outcome | |||||
| Spontaneous vaginal delivery | 1031 (85.9) | 1041 (86.5) | −0.6 (−3.4 to 2.1) | 0.99 (0.96 to 1.03) | .67 |
| Prespecified Secondary Outcomes | |||||
| Total duration of the second stage of labor, min | |||||
| Median (IQR) | 79 (45 to 134) | 113 (84 to 167) | |||
| Mean (SD) | 102.4 (79.6) | 134.2 (76.3) | −31.8 (−36.7 to −26.9) | <.001 | |
| Duration of active pushing, mind | |||||
| Median (IQR) | 58 (29 to 114) | 49 (25 to 100) | |||
| Mean (SD) | 83.7 (76.8) | 74.5 (70.7) | 9.2 (5.8 to 12.6) | <.001 | |
| Type of delivery | |||||
| Operative vaginal | 76 (6.3) | 71 (5.9) | 0.4 (−1.5 to 2.4) | 1.1 (0.7 to 1.7) | .75 |
| Vacuum-assisted vaginal | 55 (4.6) | 56 (4.7) | −0.1 (−1.8 to 1.6) | 1.0 (0.7 to 1.4) | .93 |
| Forceps-assisted vaginal | 21 (1.8) | 15 (1.3) | 0.5 (−0.5 to 1.5) | 1.4 (0.7 to 3.0) | .38 |
| Cesarean | 93 (7.8) | 91 (7.6) | 0.2 (−1.9 to 2.3) | 1.0 (0.9 to 1.1) | .55 |
| Postpartum hemorrhage | 27 (2.3) | 48 (4.0) | −1.7 (−3.1 to −0.4) | 0.6 (0.3 to 0.9) | .03 |
| Chorioamnionitis | 80 (6.7) | 110 (9.1) | −2.5 (−4.6 to −0.3) | 0.70 (0.66 to 0.90) | .005 |
| Endometritis | 7 (0.6) | 4 (0.3) | 0.3 (−0.3 to 0.8) | 1.8 (0.6 to 5.1) | .29 |
| Composite outcome of neonatal morbiditye | 87 (7.3) | 107 (8.9) | −1.6 (−3.8 to 0.5) | 0.8 (0.6 to 1.1) | .16 |
| Perineal laceration (≥second degree) | 551 (45.9) | 558 (46.4) | −0.4 (−4.4 to 3.6) | 1.0 (0.9 to 1.0) | .69 |
| Prespecified Exploratory Outcomes | |||||
| Components of neonatal morbidity | |||||
| Neonatal death | 0 | 0 | |||
| Major birth injury | 6 (0.5) | 3 (0.3) | 0.3 (−0.2 to 0.7) | 2.0 (0.7 to 5.5) | .18 |
| Acidemia (umbilical cord arterial pH <7.1) | 9 (0.8) | 14 (1.2) | −0.4 (−1.2 to 0.4) | 0.7 (0.5 to 0.9) | .01 |
| Respiratory distress | 30 (2.5) | 25 (2.1) | 0.4 (−0.8 to 1.6) | 1.2 (0.8 to 1.8) | .36 |
| Transient tachypnea | 8 (0.7) | 9 (0.8) | −0.1 (−0.8 to 0.6) | 0.9 (0.3 to 2.7) | .84 |
| Meconium aspiration with pulmonary hypertension | 0 | 2 (0.2) | −0.2 (−0.4 to 0.1) | ||
| Hypoxic-ischemic encephalopathy | 3 (0.3) | 2 (0.2) | 0.1 (−0.3 to 0.5) | 1.5 (0.4 to 6.5) | .57 |
| Hypoglycemia | 28 (2.3) | 26 (2.2) | 0.2 (−1.0 to 1.4) | 1.1 (0.6 to 1.9) | .79 |
| Hypothermic treatment (cooling) | 1 (0.1) | 3 (0.3) | −0.2 (−0.5 to 0.2) | 0.3 (0.1 to 2.1) | .24 |
| Suspected sepsis | 38 (3.2) | 53 (4.4) | −1.2 (−2.8 to 0.3) | 0.7 (0.6 to 0.9) | .003 |
| Degree of perineal laceration | |||||
| Second | 483 (40.3) | 503 (41.8) | −1.5 (−5.5 to 2.4) | 1.0 (0.9 to 1.0) | .12 |
| Third | 63 (5.3) | 52 (4.3) | 0.9 (−0. 8 to 2.6) | 1.2 (1.0 to 1.4) | .02 |
| Fourth | 5 (0.4) | 3 (0.3) | 0.2 (−0.3 to 0.6) | 1.7 (0.4 to 6.8) | .44 |
| Post Hoc Outcomes | |||||
| Estimated blood loss, mLf | |||||
| Median (IQR) | 350 (300 to 450) | 350 (300 to 450) | |||
| Mean (SD) | 419.0 (252.8) | 424.4 (298.2) | −5.2 (−24.7 to 14.4) | .60 | |
| Blood transfusion | 14 (1.2) | 15 (1.3) | −0.1 (−1.0 to 0.8) | 0.9 (0.5 to 1.8) | .84 |
| Shoulder dystocia | 40 (3.3) | 27 (2.2) | 1.1 (−0.2 to 2.4) | 1.5 (0.9 to 2.5) | .12 |
| Third- or fourth-degree laceration | 68 (5.7) | 55 (4.6) | 1.1 (−0.7 to 2.9) | 1.2 (1.1 to 1.5) | .01 |
| NICU admission | 63 (5.3) | 78 (6.5) | −1.2 (−3.1 to 0.6) | 0.8 (0.6 to 1.1) | .21 |
Abbreviations: IQR; interquartile range; NICU, neonatal intensive care unit.
Unless otherwise indicated.
Calculated from binomial distribution.
Obtained from generalized estimating equations models, which were used to account for study site.
There were missing data for 2 participants in the immediate pushing group and 4 in the delayed pushing group.
Defined as occurrence of 1 or more of the following: neonatal death, birth injury, umbilical cord arterial acidosis, respiratory distress, transient tachypnea, meconium aspiration with pulmonary hypertension, hypoxic-ischemic encephalopathy, hypoglycemia, hypothermia treatment, or suspected neonatal sepsis.
There were missing data for 37 participants in the immediate pushing group and 30 in the delayed pushing group.
Rates of operative vaginal delivery and cesarean delivery were low and did not differ significantly between the groups (Table 2). Specifically, immediate pushing was not associated with a significant decrease in operative vaginal deliveries (6.3% in the immediate pushing group vs 5.9% in the delayed pushing group; absolute difference, 0.4% [95% CI, −1.5% to 2.4%]; RR, 1.1 [95% CI, 0.7 to 1.7], P = .75) or cesarean deliveries (7.8% vs 7.6%, respectively; absolute difference, 0.2% [95% CI, −1.9% to 2.3%]; RR, 1.0 [95% CI, 0.9 to 1.1], P = .55). There were no significant differences in the distribution of indications for operative vaginal deliveries and cesarean deliveries (eTable 1 in Supplement 2).
The rate of postpartum hemorrhage was significantly lower among women in the immediate pushing group compared with those in the delayed pushing group (2.3% vs 4.0%, respectively; absolute difference, −1.7% [95% CI, −3.1% to −0.4%]; RR, 0.6 [95% CI, 0.3 to 0.9], P = .03). In addition, rates of chorioamnionitis during the second stage of labor were significantly lower among women in the immediate pushing group compared with the delayed pushing group (6.7% vs 9.1%, respectively; absolute difference, −2.5% [95% CI, −4.6% to −0.3%]; RR, 0.70 [95% CI, 0.66 to 0.90], P = .005; Table 2). Rates of endometritis were low and not significantly different between groups (0.6% in the immediate pushing group vs 0.3% in the delayed pushing group; absolute difference, 0.3 [95% CI, −0.3 to 0.8]; RR, 1.8 [95% CI, 0.6 to 5.1]).
The rate of the composite outcome of neonatal morbidity was 8.1% (194/2404), and there was no significant difference between the groups (7.3% in the immediate pushing group vs 8.9% in the delayed pushing group; absolute difference, −1.6% [95% CI, −3.8% to 0.5%]; RR, 0.8 [95% CI, 0.6 to 1.1]; Table 2). Perineal lacerations were common and there was no significant difference in the overall rates between the groups (45.9% in the immediate pushing group vs 46.4% in the delayed pushing group; absolute difference, −0.4% [95% CI, −4.4% to 3.6%]; RR, 0.99 [95% CI, 0.95 to 1.04]; Table 2).
Prespecified Subgroup Analyses
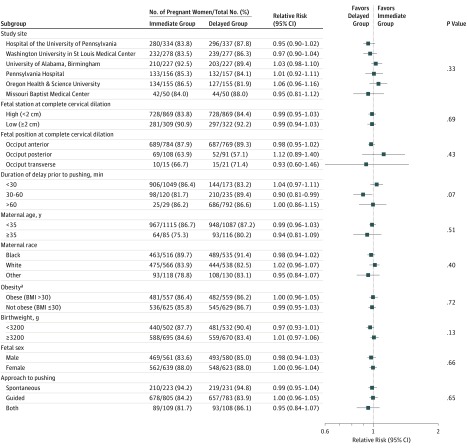
In prespecified subgroup analyses, there were no significant differences in the rates of spontaneous delivery by study site, fetal station at randomization, fetal occiput position, or duration of delay prior to pushing (Figure 2). Similarly, there were no significant differences in the rates of spontaneous vaginal delivery by maternal age or race, maternal obesity, low birthweight, fetal sex, or pushing technique.
Figure 2. Prespecified Subgroup Analysis of the Primary Outcome of Spontaneous Vaginal Delivery.
The P values were calculated using the Breslow-Day test and indicate the interactions between the treatment groups and the subgroup variables.
aBody mass index (BMI) calculated as weight in kilograms divided by height in meters squared.
Prespecified Exploratory Outcomes
For components of the composite outcome of neonatal morbidity, there was a significantly decreased risk of neonatal acidemia in the immediate pushing group compared with the delayed pushing group (0.8% vs 1.2%, respectively; absolute difference, −0.4% [95% CI, −1.2% to 0.4%]; RR, 0.7 [95% CI, 0.5 to 0.9]). There were no neonatal deaths in either group. Compared with the immediate pushing group, there was a significantly higher rate of suspected sepsis in the delayed pushing group (3.2% vs 4.4%, respectively; absolute difference, −1.2% [95% CI, −2.8% to 0.3%]; RR, 0.7 [95% CI, 0.6 to 0.9]; Table 2). Risk of third-degree perineal laceration was significantly higher in the immediate pushing group compared with the delayed pushing group (5.3% vs 4.3%, respectively; absolute difference, 0.9% [95% CI, −0.8% to 2.6%]; RR, 1.2 [95% CI, 1.0 to 1.4], P = .02).
Patients rated their experience highly during the second stage of labor, and there was no significant difference between the immediate and delayed pushing groups on the Mackey Childbirth Satisfaction Rating Scale (median score, 5 vs 5; mean difference, 0.01 [95% CI, −0.04 to 0.06]). Patients in both groups reported feeling in control of their labor management during the second stage of labor, with no significant difference between the groups (median score, 5 vs 5; mean difference, 0.01 [95% CI, −0.03 to 0.05]; eTable 2 in Supplement 2). There was no significant difference in patient preference for the same second stage management plan in a subsequent delivery (91.3% in the immediate pushing group vs 91.0% in the delayed pushing group; absolute difference, 0.3% [95% CI, −1.9% to 2.7%]; RR, 1.1 [95% CI, −0.9 to 1.3]).
Post Hoc Outcomes
There were no significant differences between the groups in either estimated blood loss (419.0 mL in the immediate pushing group vs 424.4 mL in the delayed pushing group; mean difference, −5.2 mL [95% CI, −24.7 to 14.4 mL], P = .60) or rate of blood transfusions (1.2% vs 1.3%, respectively; absolute difference, −0.1% [95% CI, −1.0% to 0.8%]; RR, 0.9 [95% CI, 0.5 to 1.8], P = .84). There was no significant difference in the rates of shoulder dystocia or neonatal intensive care unit admission between the groups (Table 2). Risk of severe perineal laceration (third or fourth degree) was significantly higher in the immediate pushing group compared with the delayed pushing group (5.7% vs 4.6%, respectively; absolute difference, 1.1% [95% CI, −0.7% to 2.9%]; RR, 1.2 [95% CI, 1.1 to 1.5], P = .01).
Adverse Events
The risk of any study-defined adverse event was significantly lower in the immediate pushing group compared with the delayed pushing group (14.1% vs 17.4%, respectively; absolute difference, −3.3% [95% CI, −6.2% to 0.4%]; RR, 0.8 [95% CI, 0.7 to 0.9], P = .001; Table 3). Of the individual adverse events, the rates for neonatal acidemia were significantly lower in the immediate pushing group compared with the delayed pushing group (0.8% vs 1.2%, respectively; absolute difference, −0.4% [95% CI, −1.2% to 0.4%]; RR, 0.7 [95% CI, 0.5 to 0.9]) and for chorioamnionitis (6.7% vs 9.1%; absolute difference, −2.5% [95% CI, −4.6% to −0.3%]; RR, 0.7 [95% CI, 0.6 to 0.9]). The study-defined adverse event of severe postpartum hemorrhage (>1000 mL for vaginal delivery and >2000 mL for cesarean delivery) did not differ significantly between the groups (1.8% in the immediate pushing group vs 2.7% in the delayed pushing group; absolute difference, −0.9% [95% CI, −2.1% to 0.3%]; RR, 0.6 [95% CI, 0.4 to 1.1]). There were no significant differences in the rates of any other individual serious adverse events between the groups.
Table 3. Study-Defined Adverse Events and Serious Adverse Events.
| No. (%) | Absolute Risk Difference (95% CI)a |
Relative Risk (95% CI)b |
P Value | ||
|---|---|---|---|---|---|
| Immediate Pushing (n = 1200) |
Delayed Pushing (n = 1204) |
||||
| Any adverse event | 169 (14.1) | 209 (17.4) | −3.3 (−6.2 to 0.4) | 0.8 (0.7 to 0.9) | .001 |
| Any serious adverse event | 13 (1.1) | 9 (0.8) | 0.3 (−0.4 to 1.1) | 1.4 (0.7 to 2.8) | .29 |
| Type of maternal adverse event | |||||
| Chorioamnionitis | 80 (6.7) | 110 (9.1) | −2.5 (−4.6 to −0.3) | 0.7 (0.6 to 0.9) | .005 |
| Severe postpartum hemorrhagec | 21 (1.8) | 32 (2.7) | −0.9 (−2.1 to 0.3) | 0.6 (0.4 to 1.1) | .11 |
| Death | 0 | 0 | |||
| Life-threatening event | 3 (0.3) | 1 (0.1) | 0.2 (−0.2 to 0.5) | 2.9 (0.5 to 16.2) | .23 |
| Intensive care unit admission | 1 (0.1) | 2 (0.2) | −0.1 (−0.4 to 0.2) | 0.5 (0 to 8.2) | .61 |
| Unplanned hysterectomy | 0 | 1 (0.1) | −0.1 (−0.3 to 0.1) | ||
| Type of neonatal adverse event | |||||
| Acidemia (umbilical cord arterial pH <7.1) | 9 (0.8) | 14 (1.2) | −0.4 (−1.2 to 0.4) | 0.7 (0.5 to 0.9) | .01 |
| NICU admission | 63 (5.3) | 78 (6.5) | −1.2 (−3.1 to 0.6) | 0.8 (0.6 to 1.1) | .22 |
| Serious neonatal birth injury | 9 (0.8) | 7 (0.6) | 0.2 (−0.5 to 0.8) | 1.3 (0.6 to 2.7) | .52 |
Abbreviation: NICU, neonatal intensive care unit.
Calculated from binomial distribution.
Obtained from generalized estimating equations models, which were used to account for study site.
Defined as >1000 mL for vaginal delivery and >2000 mL for cesarean delivery.
Additional Analyses
Predominantly similar results were seen in the per-protocol and as-treated analyses (eTable 3 and eTable 4 in Supplement 2).
Discussion
In this pragmatic, multicenter randomized clinical trial, there was no significant difference in the rate of spontaneous vaginal delivery among women randomized to immediate pushing compared with delayed pushing. Women who delayed pushing had a longer second stage of labor by more than 30 minutes compared with those who pushed immediately, and pushed for a mean of 9 minutes less. Among the prespecified secondary outcomes, rates of postpartum hemorrhage and chorioamnionitis were significantly higher among women in the delayed pushing group, but there was no significant difference in the rate of the composite outcome of neonatal morbidity or the overall rate of perineal laceration between groups. Among the prespecified exploratory outcomes, the rates of neonatal acidemia and suspected neonatal sepsis were significantly higher in the delayed pushing group. In contrast, the rate of third-degree perineal lacerations was significantly higher in the immediate pushing group.
The findings in this trial are consistent with the results of a meta-analysis by Tuuli et al11 of prior randomized clinical trials that found no differences in spontaneous vaginal delivery rates with delayed or immediate pushing among high-quality studies. In contrast, they differ from the results of a meta-analysis that included randomized clinical trials without regard to study quality and found delayed pushing to be a superior strategy to immediate pushing.18 Many of the original randomized clinical trials lacked applicability to current obstetric practices given the historically high rates of operative vaginal deliveries. The present study provides contemporary evidence from a multicenter randomized clinical trial to guide evidence-based management of the initiation of pushing during the second stage of labor among nulliparous women with neuraxial anesthesia. The results from this pragmatic trial conducted in a modern obstetric cohort are more appropriate to guide clinical practice.
The finding of no effect on spontaneous vaginal delivery for pushing timing during the second stage of labor, and the evidence suggesting increased maternal and neonatal complications in the delayed pushing group, support the view that women immediately pushing after complete cervical dilation may be preferred because women without neuraxial analgesia reflexively push immediately. The prior trial by Fraser et al10 found that women reported being more satisfied with their birthing experience when pushing was delayed. In contrast, no significant difference was found in satisfaction scores with delayed pushing compared with immediate pushing, suggesting that patient satisfaction is not a reasonable basis for clinicians to choose delayed pushing over immediate pushing. Because the planned subgroup analyses demonstrated no evidence of any interaction, suggesting that delayed pushing was not superior to immediate pushing in any subgroups, nulliparous patients should not be instructed to delay pushing based on those characteristics.
An important and consistent finding in this and prior trials is that delayed pushing was associated with prolongation of the second stage of labor. A growing body of observational data has suggested that every additional hour spent during the second stage of labor compared with the first hour, regardless of an immediate pushing vs delayed pushing management strategy, is associated with an increase in maternal and neonatal morbidity.19,20,21,22,23 Specifically, a longer second stage of labor has been associated with an increase in the risks of maternal hemorrhage, infection, severe perineal laceration, as well as neonatal acidemia and neonatal intensive care unit admission. Thus, the current finding that delayed pushing prolonged the second stage of labor without increasing spontaneous vaginal delivery rates further argues against routine use of delayed pushing.
This multicenter trial was regionally representative of obstetric patients in the United States, and enrolled patients from both tertiary care and community-based obstetric programs to enhance external validity and generalizability. The pragmatic design of the trial also facilitates generalizability because labor and delivery management (with the exception of the timing of pushing) was at the discretion of the patient’s clinician. The primary analysis adhered to the intention-to-treat principle, producing findings that reflect anticipated outcomes with a strategy of immediate or delayed pushing.
The trial was stopped following a planned interim analysis after 2414 of the planned 3184 patients were recruited, raising the possibility that it is underpowered. However, the decision by the data and safety monitoring board to stop the trial for futility was based on a conditional power analysis showing only an 8% probability of detecting a significant difference in the primary outcome if the total planned sample size was recruited. This suggests that the lack of difference observed is likely due to a true absence of effect on spontaneous vaginal delivery for pushing timing. In addition, the narrow 95% CI (0.96 to 1.03) around the primary outcome effect size of 0.99, suggests that a clinically important effect for pushing timing on the rate of spontaneous vaginal delivery is unlikely. A higher than expected spontaneous vaginal delivery rate in both groups also was observed, which further increased the power of the study to detect a true difference if one existed. Moreover, this study is, to our knowledge, the largest randomized clinical trial of labor management to date, including the second stage.
Limitations
This trial has several limitations. First, the study could not feasibly be blinded, raising the possibility of bias. It is possible that these biases might have influenced individual patient management or clinical diagnoses. However, there were no between-group differences in the management of labor or in the technique of pushing measured in the study. In addition, any bias may likely have been nondirectional because the prestudy rate of delayed pushing at the study centers was about 50%, demonstrating equipoise. However, it is possible that this equipoise overall does not reflect the biases of individual clinicians. To further reduce the potential for bias, data extraction also was performed masked to group assignment.
Second, no adjustment was made for multiple comparisons, raising the possibility that some significant differences in the secondary and exploratory outcomes could have occurred by chance. Third, the study was stopped early, and despite the large sample size, the study may have been underpowered to detect clinically important differences in some of the secondary and exploratory outcomes.
Conclusions
Among nulliparous women receiving neuraxial anesthesia, the timing of second stage pushing efforts did not affect the rate of spontaneous vaginal delivery. These findings may help inform decisions about the preferred timing of second stage pushing efforts, when considered with other maternal and neonatal outcomes.
Trial protocol
eMethods. Definitions of outcome measures
eTable 1. Indications for operative deliveries
eTable 2. Participants’ satisfaction with birthing experience in second stage based on a modified Mackey Childbirth Satisfaction Rating Scale
eTable 3. Primary and secondary efficacy outcomes (per protocol)
eTable 4. Primary and secondary efficacy outcomes (as treated)
Statistical analysis plan
Data sharing statement
References
- 1.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 2.Altman MR, Lydon-Rochelle MT. Prolonged second stage of labor and risk of adverse maternal and perinatal outcomes: a systematic review. Birth. 2006;33(4):315-322. doi: 10.1111/j.1523-536X.2006.00129.x [DOI] [PubMed] [Google Scholar]
- 3.Gilstrap LC III, Hauth JC, Hankins GD, Beck AW. Second-stage fetal heart rate abnormalities and type of neonatal acidemia. Obstet Gynecol. 1987;70(2):191-195. [PubMed] [Google Scholar]
- 4.Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine . Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179-193. doi: 10.1016/j.ajog.2014.01.026 [DOI] [PubMed] [Google Scholar]
- 5.Hansen SL, Clark SL, Foster JC. Active pushing versus passive fetal descent in the second stage of labor: a randomized controlled trial. Obstet Gynecol. 2002;99(1):29-34. [DOI] [PubMed] [Google Scholar]
- 6.Plunkett BA, Lin A, Wong CA, Grobman WA, Peaceman AM. Management of the second stage of labor in nulliparas with continuous epidural analgesia. Obstet Gynecol. 2003;102(1):109-114. [DOI] [PubMed] [Google Scholar]
- 7.Gillesby E, Burns S, Dempsey A, et al. Comparison of delayed versus immediate pushing during second stage of labor for nulliparous women with epidural anesthesia. J Obstet Gynecol Neonatal Nurs. 2010;39(6):635-644. doi: 10.1111/j.1552-6909.2010.01195.x [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick M, Harkin R, McQuillan K, O’Brien C, O’Connell PR, O’Herlihy C. A randomised clinical trial comparing the effects of delayed versus immediate pushing with epidural analgesia on mode of delivery and faecal continence. BJOG. 2002;109(12):1359-1365. doi: 10.1046/j.1471-0528.2002.02109.x [DOI] [PubMed] [Google Scholar]
- 9.Frey HA, Tuuli MG, Cortez S, et al. Does delayed pushing in the second stage of labor impact perinatal outcomes? Am J Perinatol. 2012;29(10):807-814. doi: 10.1055/s-0032-1316448 [DOI] [PubMed] [Google Scholar]
- 10.Fraser WD, Marcoux S, Krauss I, Douglas J, Goulet C, Boulvain M; PEOPLE (Pushing Early or Pushing Late with Epidural) Study Group . Multicenter, randomized, controlled trial of delayed pushing for nulliparous women in the second stage of labor with continuous epidural analgesia. Am J Obstet Gynecol. 2000;182(5):1165-1172. doi: 10.1067/mob.2000.105197 [DOI] [PubMed] [Google Scholar]
- 11.Tuuli MG, Frey HA, Odibo AO, Macones GA, Cahill AG. Immediate compared with delayed pushing in the second stage of labor: a systematic review and meta-analysis. Obstet Gynecol. 2012;120(3):660-668. doi: 10.1097/AOG.0b013e3182639fae [DOI] [PubMed] [Google Scholar]
- 12.Ware JH, Hamel MB. Pragmatic trials—guides to better patient care? N Engl J Med. 2011;364(18):1685-1687. doi: 10.1056/NEJMp1103502 [DOI] [PubMed] [Google Scholar]
- 13.Broglio K. Randomization in clinical trials: permuted blocks and stratification. JAMA. 2018;319(21):2223-2224. doi: 10.1001/jama.2018.6360 [DOI] [PubMed] [Google Scholar]
- 14.Goodman P, Mackey MC, Tavakoli AS. Factors related to childbirth satisfaction. J Adv Nurs. 2004;46(2):212-219. doi: 10.1111/j.1365-2648.2003.02981.x [DOI] [PubMed] [Google Scholar]
- 15.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44(526):793-797. doi: 10.1259/0007-1285-44-526-793 [DOI] [PubMed] [Google Scholar]
- 16.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, II: analysis and examples. Br J Cancer. 1977;35(1):1-39. doi: 10.1038/bjc.1977.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brancato RM, Church S, Stone PW. A meta-analysis of passive descent versus immediate pushing in nulliparous women with epidural analgesia in the second stage of labor. J Obstet Gynecol Neonatal Nurs. 2008;37(1):4-12. doi: 10.1111/j.1552-6909.2007.00205.x [DOI] [PubMed] [Google Scholar]
- 19.Allen VM, Baskett TF, O’Connell CM, McKeen D, Allen AC. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113(6):1248-1258. doi: 10.1097/AOG.0b013e3181a722d6 [DOI] [PubMed] [Google Scholar]
- 20.Rouse DJ, Weiner SJ, Bloom SL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1-357.e7. doi: 10.1016/j.ajog.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Ray C, Audibert F, Goffinet F, Fraser W. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201(4):361.e1-361.e7. doi: 10.1016/j.ajog.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 22.Grobman WA, Bailit J, Lai Y, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network . Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127(4):667-673. doi: 10.1097/AOG.0000000000001354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughon SK, Berghella V, Reddy UM, Sundaram R, Lu Z, Hoffman MK. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124(1):57-67. doi: 10.1097/AOG.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods. Definitions of outcome measures
eTable 1. Indications for operative deliveries
eTable 2. Participants’ satisfaction with birthing experience in second stage based on a modified Mackey Childbirth Satisfaction Rating Scale
eTable 3. Primary and secondary efficacy outcomes (per protocol)
eTable 4. Primary and secondary efficacy outcomes (as treated)
Statistical analysis plan
Data sharing statement