Key Points
Question
Does the use of a pill containing low doses of 3 antihypertensive medications provide improved blood pressure control compared with usual care among patients with mild or moderate hypertension?
Findings
In this randomized clinical trial of 700 patients with hypertension who were untreated or receiving monotherapy, 70% of patients in the triple combination pill therapy group achieved a systolic/diastolic blood pressure of less than 140/90 mm Hg (or <130/80 mm Hg in patients with diabetes or chronic kidney disease) at 6 months compared with 55% of patients in the usual care group (a significant difference).
Meaning
Use of a low-dose triple combination blood pressure–lowering pill for initiation of treatment or escalation from monotherapy increased the proportion of patients with hypertension reaching their blood pressure targets.
Abstract
Importance
Poorly controlled hypertension is a leading global public health problem requiring new treatment strategies.
Objective
To assess whether a low-dose triple combination antihypertensive medication would achieve better blood pressure (BP) control vs usual care.
Design, Setting, and Participants
Randomized, open-label trial of a low-dose triple BP therapy vs usual care for adults with hypertension (systolic BP >140 mm Hg and/or diastolic BP >90 mm Hg; or in patients with diabetes or chronic kidney disease: >130 mm Hg and/or >80 mm Hg) requiring initiation (untreated patients) or escalation (patients receiving monotherapy) of antihypertensive therapy. Patients were enrolled from 11 urban hospital clinics in Sri Lanka from February 2016 to May 2017; follow-up ended in October 2017.
Interventions
A once-daily fixed-dose triple combination pill (20 mg of telmisartan, 2.5 mg of amlodipine, and 12.5 mg of chlorthalidone) therapy (n = 349) or usual care (n = 351).
Main Outcomes and Measures
The primary outcome was the proportion achieving target systolic/diastolic BP (<140/90 mm Hg or <130/80 mm Hg in patients with diabetes or chronic kidney disease) at 6 months. Secondary outcomes included mean systolic/diastolic BP difference during follow-up and withdrawal of BP medications due to an adverse event.
Results
Among 700 randomized patients (mean age, 56 years; 58% women; 29% had diabetes; mean baseline systolic/diastolic BP, 154/90 mm Hg), 675 (96%) completed the trial. The triple combination pill increased the proportion achieving target BP vs usual care at 6 months (70% vs 55%, respectively; risk difference, 12.7% [95% CI, 3.2% to 22.0%]; P < .001). Mean systolic/diastolic BP at 6 months was 125/76 mm Hg for the triple combination pill vs 134/81 mm Hg for usual care (adjusted difference in postrandomization BP over the entire follow-up: systolic BP, −9.8 [95% CI, −7.9 to −11.6] mm Hg; diastolic BP, −5.0 [95% CI, −3.9 to −6.1] mm Hg; P < .001 for both comparisons). Overall, 419 adverse events were reported in 255 patients (38.1% for triple combination pill vs 34.8% for usual care) with the most common being musculoskeletal pain (6.0% and 8.0%, respectively) and dizziness, presyncope, or syncope (5.2% and 2.8%). There were no significant between-group differences in the proportion of patient withdrawal from BP-lowering therapy due to adverse events (6.6% for triple combination pill vs 6.8% for usual care).
Conclusions and Relevance
Among patients with mild to moderate hypertension, treatment with a pill containing low doses of 3 antihypertensive drugs led to an increased proportion of patients achieving their target BP goal vs usual care. Use of such medication as initial therapy or to replace monotherapy may be an effective way to improve BP control.
Trial Registration
anzctr.org.au Identifier: ACTRN12612001120864; slctr.lk Identifier: SLCTR/2015/020
This randomized clinical trial compares the effects of a fixed low-dose triple combination antihypertensive pill vs usual care on high blood pressure control among patients with mild or moderate hypertension treated at urban hospital clinics in Sri Lanka.
Introduction
High blood pressure is the leading cause of mortality and cardiovascular disease globally, and most of the disease burden occurs in low- and middle-income countries.1 Multiple inexpensive blood pressure–lowering drugs prevent cardiovascular events2; however, large gaps remain in identifying and treating people with high blood pressure.3 In low- and middle-income settings, the availability and affordability of medications is a critical issue,4 and only about one-third of individuals with high blood pressure in these settings receive treatment.3 Only approximately half of patients treated in high-income countries and about one-quarter in low- and middle-income countries achieve blood pressure control.3 In most settings, inadequate blood pressure treatment can be principally attributed to persistent use of monotherapy, which has modest efficacy.5 Recent guidelines6,7,8 recommend lower blood pressure targets among high-risk patients, increasing the need for more effective treatment strategies.
A fixed low-dose combination therapy with inexpensive blood pressure–lowering drugs has the potential to address several barriers to improve blood pressure control. Low-dose combinations improve efficacy,9,10 adverse events are minimized at half-standard doses,5 and the benefits are additive across blood pressure–lowering medication classes. Furthermore, fixed-dose combinations can improve medication adherence due to regimen simplification,11,12 thereby reducing patient, physician, and health system barriers related to multiple visits and prolonged titration schedules.13
Triple blood pressure–lowering therapy was first used half a century ago and the potential value of initial low-dose triple therapy was first postulated in 2003.5 Recent trials of triple therapy have demonstrated benefits among patients with severe hypertension not controlled by dual therapy,14 and guidelines currently only recommend triple therapy in this clinical scenario.
To date, there is no evidence on the long-term effectiveness or tolerability of a low-dose triple pill blood pressure–lowering therapy compared with usual care for initial treatment of hypertension or among those with uncontrolled blood pressure while taking monotherapy.
Methods
Study Design
The ethics review committees of the University of Kelaniya, Colombo, Sri Lanka, and the Royal Prince Alfred Hospital, Sydney, Australia, approved the study. All participants gave written informed consent.
The Triple Pill vs Usual Care Management for Patients With Mild-to-Moderate Hypertension (TRIUMPH) pragmatic study evaluated (in the context of usual care) the effectiveness of a strategy for pharmacological initiation or escalation using medications with proven efficacy in the treatment of hypertension. Because the study aim was to provide effectiveness data for the potential adoption of the intervention strategy into routine clinical practice,15 there were relatively few inclusion criteria and study inclusion was primarily at the discretion of the investigators. Study visits were kept to a minimum and investigators and other site staff were encouraged to provide care for hypertension or other conditions consistent with their usual care.
This randomized, open-label trial was conducted at 11 urban hospital outpatient departments in Sri Lanka. The detailed study methods are published elsewhere.16,17 Because the study evaluated an approach designed to potentially replace current practice among patients that required treatment, the comparator was usual care rather than placebo.
The study protocol and statistical analysis plan appear in Supplement 1. The trial was designed by an international steering committee of academic investigators and funded by the Australian National Health and Medical Research Council Global Alliance for Chronic Disease. An independent data and safety monitoring board reviewed the study data twice during the course of the study.
Study Population
Participants were eligible if they were aged 18 years or older and had persistent hypertension (systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg; or, in patients with diabetes mellitus or chronic kidney disease, systolic blood pressure >130 mm Hg and/or diastolic blood pressure >80 mm Hg) requiring initiation of pharmacological treatment (in patients not currently taking drug therapy) or titration of pharmacological treatment (in patients taking single drug therapy).
Patients were excluded if they had (1) current use of 2 or more blood pressure–lowering drugs, (2) severe or uncontrolled blood pressure (systolic blood pressure >180 mm Hg and/or diastolic blood pressure >110 mm Hg), (3) accelerated hypertension or physician-determined need for slower titration of treatment, (4) a contraindication to any of the components of the triple combination pill therapy, (5) an unstable medical condition or a known situation in which the medication regimen might be altered for a significant length of time, or (6) clinically significant abnormal laboratory values judged by the investigator to make study participation unsuitable. Women who were pregnant or breastfeeding and those with childbearing potential who were not using an adequate contraception method also were excluded.
Randomization
Central, computer-based randomization was stratified by study center and use of blood pressure–lowering therapy at baseline. The randomization schedule was generated using SAS Enterprise Guide version 7.15 (SAS Institute Inc) and randomly permuted block sizes of 2 or 4.
Patients were randomized to receive either the triple combination pill therapy or usual care (Figure 1). Medical management of all patients occurred at hypertension clinics. Adherence to national and international guideline-recommended blood pressure targets was encouraged for all study participants. No attempt was made to otherwise influence the treatment of patients assigned to usual care.
Figure 1. Flow of Patients Through the Triple Pill vs Usual Care Management for Patients With Mild-to-Moderate Hypertension Study.
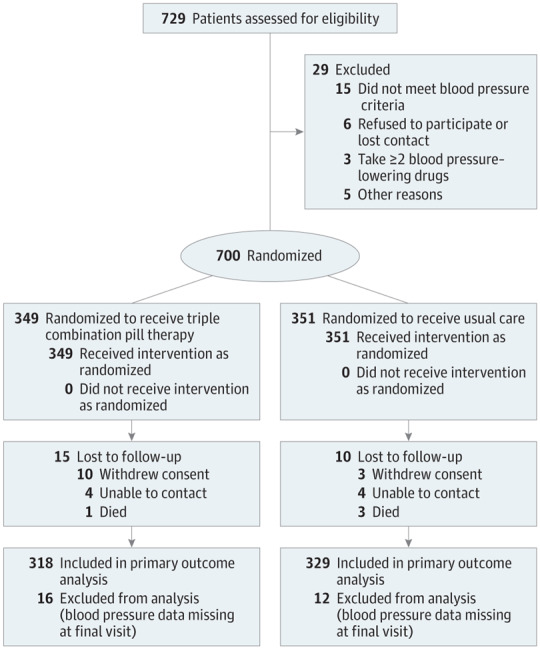
Interventions
The patients randomized to the triple combination pill therapy group who were not previously receiving blood pressure–lowering treatment began taking the low-dose version, which contained half the standard dose of telmisartan (20 mg), amlodipine (2.5 mg), and chlorthalidone (12.5 mg), and were advised to take it once daily (timing during the day was at the discretion of the investigator). For patients in the triple combination pill therapy group who were previously taking monotherapy, that treatment was discontinued at randomization and replaced with the low-dose triple combination pill therapy without any washout period.
During follow-up, the triple combination pill therapy could be discontinued, maintained, or uptitrated at the discretion of the treating physician. A higher-dose version of the triple combination pill therapy, which contained a standard dose of telmisartan (40 mg), amlodipine (5 mg), and chlorthalidone (25 mg), was available for titration. In addition, another blood pressure–lowering therapy could be prescribed in combination with either dose of the triple combination pill.
The low- and high-dose triple combination pills were manufactured using overencapsulation of registered component medications by a current good manufacturing practice–approved facility (Pharmaceutical Packaging Professionals; additional details appear in Supplement 1). Initial batches of the trial medication were overencapsulated manually and then a semiautomated method was subsequently used. At the end of the study, all returned medication was weighed with precision. This procedure confirmed the quality of the trial product (additional details appear in Supplement 2).
All medications (including the triple combination pill therapy) were provided free of charge to participants, which is usual practice in Sri Lankan public hospitals. Patients taking the triple combination pill therapy were provided a sufficient amount of pills to last until the next scheduled trial visit. Other drugs were provided according to usual practice, which in this setting was the dispensing of a 1-month supply.
Study Procedures
All patients attended study visits at registration, at randomization (which could occur concurrently with registration), at 6 weeks (within 14 days before or after), at 12 weeks (within 7 days before or after), and at 6 months (within 14 days before or after). If the patient was unable to attend follow-up visits in person, telephone calls were permitted; however, in accordance with the trial protocol, only blood pressure measurements collected and recorded during in-person visits were included in the analyses.
Blood pressure was measured while the patient was sitting, using a standardized automated sphygmomanometer (Omron T9P) after a 5-minute period of rest at each visit. Three blood pressure readings were taken 1 to 2 minutes apart and the average of the second and third readings was recorded. To reduce outcome ascertainment bias associated with an open trial design, all blood pressure measurements were printed and stored in the patient files for visual inspection during monitoring visits. No systematic bias was identified through this verification process.
Urine and blood samples were obtained at baseline and at 6 months to measure the ratio of urinary albumin to creatinine, blood lipid levels, glucose levels, electrolyte levels, and liver function. Laboratory tests were performed for trial evaluation purposes only. Because this was a pragmatic trial, clinical safety checks could be performed at any time for patients in either group. Investigators were encouraged to follow local guidelines. Data were not collected on laboratory results for routine clinical care.
All study laboratory measurements were analyzed by an independent central laboratory (Lanka Hospitals Diagnostics). Patients completed an EQ-5D quality-of-life questionnaire at baseline and at 6 months.18 Self-reported medication adherence (defined as the number of days medication was taken during the last week) for each prescribed medication was collected at baseline and at all follow-up visits. Data on adverse events also were collected at each visit.
Primary Outcome
The primary study outcome was the proportion of participants achieving target blood pressure at 6 months, which was defined as a systolic blood pressure of less than 140 mm Hg and a diastolic blood pressure of less than 90 mm Hg. For patients diagnosed at baseline or newly diagnosed prior to the final 6-month study visit as having diabetes mellitus, chronic kidney disease, or both, target blood pressure at 6 months was defined as a systolic blood pressure of less than 130 mm Hg and a diastolic blood pressure of less than 80 mm Hg. Newly diagnosed diabetes was defined as a newly documented diagnosis, fasting plasma glucose level of 7.0 mmol/L or greater at the 6-month visit, or a new prescription of blood glucose–lowering drugs during follow-up. New onset chronic kidney disease was defined as a newly documented diagnosis, estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, or a ratio of urinary albumin to creatinine of greater than 30 mg/g at the 6-month visit.
Secondary Outcomes
The prespecified secondary outcomes were the proportion of participants achieving target blood pressure at 6 and 12 weeks, the mean change in systolic and diastolic blood pressure at 6 months, self-reported adherence to blood pressure–lowering medications at 6 months (defined as taking all prescribed blood pressure–lowering drugs for at least 4 of the last 7 days), and intolerance to treatment at 6 months (defined as discontinuation of blood pressure–lowering medications due to adverse events during follow-up).
The frequency of changes in blood pressure–lowering medications (additions, withdrawals, and dose adjustments) also was a prespecified outcome. This included comparisons of mean total standard daily doses of blood pressure–lowering medications with a standard dose defined as the most reported usual maintenance dose recorded by the British National Formulary and the Martindale and Monthly Index of Medical Specialties, which is similar to the method described by Bennett et al19 and Law et al.5
In the absence of consensus between these sources, the World Health Organization–defined daily dose was used as a tiebreaker. If there was still no consensus, the lowest reported dose was considered the standard dose. A detailed listing of standard daily doses appears in eTable 1 in Supplement 2.
Data are presented for the total number of different blood pressure–lowering medication classes that each participant was taking (eg, angiotensin-converting enzyme inhibitor, β-blocker, diuretic). The data presented on the number of pills refers to the number of actual pills or tablets that each participant was taking each day. For example, if a participant was taking the triple combination pill therapy, he or she was taking 3 blood pressure–lowering medication classes but only 1 pill.
Laboratory values for blood lipid levels, electrolyte levels, liver function, and the ratio of urinary albumin to creatinine were reported as mean change from baseline to 6 months. The proportion of patients with values outside conventional ranges at 6 months for serum levels of sodium and potassium, ratio of urinary albumin to creatinine, and estimated glomerular filtration rate also were reported. Data on the quality of life and use of health care services (hospitalizations, medical consultations, and tests) were prespecified to be collected for the cost-effectiveness analyses and those results are not reported herein.
Statistical Analysis
This study was designed with a goal of 700 participants to provide 90% power to detect a 12.4% absolute improvement in the rate of blood pressure control (determined based on the improvements seen in previous trials20,21). This included an assumption of 50% goal attainment in the control (usual care) group at 6 months (relative risk [RR] of 1.25), allowing for a lost to follow-up rate of 5%. A 2-tailed test and an α level of 5% were used.
The proportion of participants achieving target blood pressure control at 6 months was analyzed using log-binomial regression (binomial distribution with a log link) with the treatment group and use of blood pressure–lowering therapy at baseline as fixed effects and trial center as a random effect. The treatment effect was estimated as an adjusted RR and 95% CI. The proportion of patients achieving blood pressure targets at 6 weeks and at 12 weeks were analyzed similarly.
Changes in blood pressure from baseline were analyzed using an analysis of covariance with baseline blood pressure, treatment group, and use of blood pressure–lowering therapy at baseline as fixed effects and trial site as a random effect. Longitudinal linear models were used to estimate the differences in blood pressure over all 3 visits (6 weeks, 12 weeks, and 6 months) combined and the models included treatment group, trial visit as a categorical variable, a treatment × visit interaction, the baseline value (ie, systolic or diastolic blood pressure), and use of blood pressure–lowering therapy at baseline as fixed effects along with trial center as a random effect.
Consistent with the statistical analysis plan (appears in Supplement 1), missing outcome data for blood pressure were not imputed because less than 10% of the data were missing; however, the sensitivity analyses for all the primary and secondary outcomes using blood pressure were conducted using the fully conditional specification method for imputation.22
Self-reported adherence at 6 weeks, 12 weeks, and 6 months was analyzed similarly to the proportion reaching the blood pressure target. A longitudinal log-binomial model using data from all 3 visits was used to estimate overall differences in self-reported adherence including the following fixed effects: treatment group, trial visit as a categorical variable, a treatment × visit interaction, and use of blood pressure–lowering therapy at baseline. Within-patient correlations were modeled using generalized estimating equations with an exchangeable correlation structure.
The changes in laboratory parameters between baseline and 6 months were analyzed using analysis of covariance. Patient registration and randomization could occur at the same visit; therefore, a large proportion (44% in each group) had nonfasting blood samples at baseline. Consequently, a post hoc sensitivity analysis was performed comparing fasting levels of glucose, triglycerides, and low-density lipoprotein cholesterol at the 6-month visit only, without adjustment for baseline values.
The homogeneity of treatment effects across subgroups on the primary outcome was tested by adding interaction terms to the log-binomial model. Prespecified subgroups using baseline characteristics included age (above and below the median at baseline), sex, diabetes, chronic renal disease, education (none or primary school completion vs other), economic stratum (monthly family income <5000, 5000-20 000, or >20 000 Sri Lankan Rupees), systolic blood pressure at baseline (by baseline tertiles), diastolic blood pressure at baseline (by baseline tertiles), and blood pressure–lowering treatment at baseline.
All statistical significance tests were conducted using a 2-sided type I error rate of 5%. Holm-Bonferroni adjustment23 for multiple comparisons relating to all prespecified secondary outcomes was conducted. All analyses were conducted using SAS Enterprise Guide version 7.15 (SAS Institute Inc).
Results
Study Participants
A total of 700 participants were randomized from 11 sites between February 15, 2016, and May 3, 2017, with final study visits completed by October 24, 2017. The mean age of the study population was 56 years, 58% were women, and 29% had diabetes. The mean baseline systolic/diastolic blood pressure was 154/90 mm Hg in both groups. Prior to randomization, 41% of participants were taking blood pressure–lowering treatment.
With incomplete follow-up, deaths, and missing in-clinic blood pressure measurements, final visit data for the primary outcome were available for 91.1% of the patients randomized to the triple combination pill therapy group and 93.7% of the patients randomized to the usual care group.
In-person clinic visits constituted 100% of all baseline visits, 97% of all 6-week visits, 98% of all 12-week visits, and 95% of all 6-month visits for the triple combination pill therapy group; and 100%, 99%, 99%, and 96%, respectively, for the usual care group. At baseline, the 2 groups were comparable (Table 1; baseline characteristics by the stratification variable of antihypertensive medications appear in eTable 2 in Supplement 2).
Table 1. Baseline Characteristics.
| Triple Combination Pill
Therapy (n = 349) |
Usual
Care (n = 351) |
|
|---|---|---|
| Age, mean (SD), y | 56.4 (11.3) | 56.0 (10.7) |
| Females, No. (%) | 207 (59.3) | 196 (55.8) |
| Blood pressure–lowering treatment, No. (%) | ||
| At randomization | 140 (40.1) | 147 (41.9) |
| Treatment classa | ||
| Angiotensin-converting enzyme inhibitor | 22 (15.7) | 21 (14.3) |
| Angiotensin receptor blocker | 97 (69.0) | 104 (70.7) |
| α-Blocker | 1 (0.7) | 1 (0.7) |
| β-Blocker | 8 (5.7) | 12 (8.2) |
| Calcium channel blocker | 12 (8.6) | 10 (6.8) |
| Diuretic | 3 (2.1) | 4 (2.7) |
| Statin use, No. (%) | 97 (27.8) | 84 (23.9) |
| Antiplatelet use, No. (%) | 41 (11.7) | 37 (10.5) |
| Type of antiplatelet, No. (%) | ||
| Aspirin | 36 (10.3) | 29 (8.3) |
| Clopidogrel | 10 (2.9) | 9 (2.6) |
| Current use, No. (%) | ||
| Tobacco (cigarette, cigar, or chewing) | 39 (11.2) | 34 (9.7) |
| Alcoholb | 42 (12.0) | 43 (12.3) |
| Highest level of education, No. (%) | ||
| None | 7 (2.0) | 1 (0.3) |
| Primary school | 151 (43.3) | 142 (40.5) |
| Secondary school | 177 (50.7) | 190 (54.1) |
| University or vocational | 14 (4.0) | 18 (5.0) |
| Medical history, No. (%) | ||
| Coronary artery disease | 30 (8.6) | 22 (6.3) |
| Cerebrovascular disease | 13 (3.7) | 7 (2.0) |
| Chronic kidney disease | 7 (2.0) | 3 (0.9) |
| Type 1 diabetes | 1 (0.3) | 1 (0.3) |
| Type 2 diabetes | 111 (32.0) | 107 (30.0) |
| Gout | 1 (0.3) | 2 (0.6) |
| Systolic blood pressure, mm Hg | ||
| Mean (SD) | 154.2 (11.3) | 154.2 (11.6) |
| No. (%) | ||
| <130 | 4 (1.1) | 2 (0.6) |
| ≥130-<140 | 30 (8.6) | 34 (9.7) |
| ≥140-<150 | 99 (28.4) | 105 (29.9) |
| ≥150 | 216 (61.9) | 210 (59.8) |
| Diastolic blood pressure, mm Hg | ||
| Mean (SD) | 89.5 (9.7) | 90.0 (9.7) |
| No. (%) | ||
| <80 | 57 (16.3) | 59 (16.8) |
| ≥80-<90 | 106 (30.4) | 98 (27.9) |
| ≥90-<100 | 137 (39.3) | 144 (41.0) |
| ≥100 | 49 (14.0) | 50 (14.2) |
| Estimated glomerular filtration rate,c mL/min/1.73 m2 | ||
| Mean (SD) | 90.1 (19.7) | 91.8 (18.6) |
| No. (%) | ||
| <30 | 4 (1.1) | 3 (0.9) |
| ≥30-<50 | 12 (3.4) | 4 (1.1) |
| ≥50 | 333 (95.4) | 344 (98.0) |
| Heart rate, mean (SD), beats/min | 78.1 (12.5) | 77.9 (11.5) |
| Body mass index, No. (%)d | ||
| >25-≤30 (overweight) | 116 (33.2) | 121 (34.5) |
| >30 (obese) | 64 (18.3) | 68 (19.4) |
| Low-density lipoprotein cholesterol level, mean (SD), mg/dL | ||
| All patientse | 123.9 (41.5) | 127.2 (41.3) |
| Patients who fasted | 121.4 (41.0) | 123.1 (38.3) |
| High-density lipoprotein cholesterol level, mean (SD), mg/dL | 47.6 (12.6) | 46.0 (12.5) |
| Triglycerides level, mean (SD), mg/dL | ||
| All patientse | 154.2 (83.2) | 162.1 (82.8) |
| Patients who fasted | 142.0 (71.0) | 142.0 (69.6) |
| Glucose level, mean (SD), mg/dL | ||
| All patientse | 118 (54) | 120 (55) |
| Patients who fasted | 117 (54) | 114 (44) |
| No diabetes | ||
| All patientse | 99 (26) | 100 (27) |
| Patients who fasted | 92 (10) | 93 (10) |
| Type 1 diabetes, No. of patients | 1 | 1 |
| Patients who fasted | 120 (NA) | 123 (NA) |
| Type 2 diabetes, No. of patients | 111 | 107 |
| All patientse | 158 (73) | 166 (73) |
| Patients who fasted | 156 (72) | 147 (56) |
| Creatinine level, mean (SD), mg/dL | 0.88 (0.87) | 0.82 (0.29) |
| Ratio of urine albumin to creatinine, median (IQR), mg/g | 17.0 (9.0-40.0) | 18.0 (9.0-49.5) |
Abbreviations: IQR, interquartile range; NA, not applicable.
SI conversion factors: To convert high-density and low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; creatinine to μmol/L, multiply by 76.25; glucose to mmol/L, multiply by 0.0555; triglycerides to mmol/L, multiply by 0.0113.
The sum of the numbers of participants taking different classes of blood pressure–lowering drugs is greater than the overall number of participants receiving monotherapy because 5 patients (3 in the triple combination pill group and 2 in the usual care group) were identified subsequent to randomization as protocol violations (taking >1 blood pressure–lowering medication at baseline).
Defined as self-reported use of alcohol at least once per week during the past year.
Not presented by presence or absence of chronic kidney disease at baseline because only 10 participants had this disease.
Calculated as weight in kilograms divided by height in meters squared.
Baseline tests were performed on nonfasting blood samples in 44% of participants in each treatment group.
Primary Outcome
At 6 months, 69.5% of participants in the triple combination pill therapy group achieved their blood pressure target compared with 55.3% in the usual care group (adjusted RR, 1.23 [95% CI, 1.09-1.39], P < .001; risk difference, 12.7% [95% CI, 3.2%-22.0%]) (Table 2).
Table 2. Primary and Secondary Outcomes.
| No./Total (%)a | Treatment Effect (95% CI) | P Valueb | ||
|---|---|---|---|---|
| Triple
Combination Pill Therapy (n = 349) |
Usual
Care (n = 351) |
|||
| Primary Outcome | ||||
| Achieving blood pressure target at 6 moc | 221/318 (69.5) | 182/329 (55.3) | RR, 1.23 (1.09 to 1.39)d | <.001 |
| Secondary Outcomes | ||||
| Achieving blood pressure target at 6 wkc | 223/329 (67.8) | 150/344 (43.6) | RR, 1.53 (1.33 to 1.76)d | <.001 |
| Achieving blood pressure target at 12 wkc | 239/329 (72.6) | 161/340 (47.4) | RR, 1.51 (1.32 to 1.72)d | <.001 |
| Change in systolic blood pressure at 6 mo, mm Hg | ||||
| Adjusted mean (95% CI) | −29.1 (−31.4 to −26.8) | −20.3 (−22.6 to −18.0) | MD, −8.8 (−11.2 to −6.4)e | <.001 |
| Crude mean (SD) | −29.3 (18.4) | −20.6 (16.8) | ||
| Change in diastolic blood pressure at 6 mo, mm Hg | ||||
| Adjusted mean (95% CI) | −13.9 (−15.3 to −12.4) | −9.3 (−10.7 to −7.9) | MD, −4.6 (−6.0 to −3.1)e | <.001 |
| Crude mean (SD) | −13.7 (12.1) | −9.5 (10.3) | ||
| Blood pressure–lowering medication | ||||
| Self-reported use at 6 mof | 305/321 (95.0) | 318/336 (94.6) | RR, 1.00 (0.97 to 1.04)d | .82 |
| Withdrawal due to adverse event during follow-up | 23/349 (6.6) | 24/351 (6.8) | RR, 0.97 (0.56 to 1.70)d | .92 |
Abbreviations: MD, mean difference; RR, relative risk.
Unless otherwise indicated.
Holm-Bonferroni adjustment23 for multiple comparisons did not alter the statistical significance of any of the secondary outcomes.
Defined as achieving systolic/diastolic blood pressure of less than 130/80 mm Hg for patients with diabetes or chronic kidney disease and less than 140/90 mm Hg for all other patients.
The treatment effect was estimated from a log-binomial model that included treatment group and use of blood pressure–lowering therapy at baseline as fixed effects and trial center as a random effect.
The treatment effect was estimated from an analysis of covariance including baseline blood pressure, treatment group, and use of blood pressure–lowering therapy at baseline as fixed effects and trial site as a random effect.
Defined as ingestion on at least 4 of the last 7 days. In the triple combination pill therapy group, all prescribed blood pressure–lowering medications includes the triple combination pill and any other blood pressure medication prescribed in addition to or instead of the triple combination pill.
Secondary Outcomes
A greater proportion of participants randomized to the triple combination pill therapy group achieved their blood pressure target at 6 weeks compared with the usual care group (67.8% vs 43.6%, respectively; RR, 1.53 [95% CI, 1.33-1.76], P < .001; risk difference, 23.2% [95% CI, 14.7%-31.0%]) and at 12 weeks (72.6% vs 47.4%; RR, 1.51 [95% CI, 1.32-1.72], P < .001; risk difference, 24.3% [95% CI, 16.1%-31.8%]; Table 2).
At 6 months, mean systolic blood pressure was 125 mm Hg and diastolic blood pressure was 76 mm Hg in the triple combination pill group and was 134 mm Hg and 81 mm Hg, respectively, in the usual care group. Between baseline and 6 months, the mean change in systolic blood pressure was −29.1 mm Hg (95% CI, −31.4 to −26.8 mm Hg) in the triple combination pill therapy group and diastolic blood pressure was −13.9 mm Hg (95% CI, −15.3 to −12.4 mm Hg) compared with −20.3 mm Hg (95% CI, −22.6 to −18.0 mm Hg) and −9.3 mm Hg (95% CI, −10.7 to −7.9 mm Hg), respectively, in the usual care group (adjusted difference in postrandomization blood pressure over the entire follow-up: −9.8 mm Hg [95% CI, −7.9 to −11.6 mm Hg] in mean systolic blood pressure and −5.0 mm Hg [95% CI, −3.9 to −6.1] in mean diastolic blood pressure; P < .001 for both comparisons; Figure 2). The sensitivity analyses incorporating multiple imputation for missing blood pressure data had a minimal effect on the estimates of the effectiveness of the triple combination pill (eTable 3 in Supplement 2).
Figure 2. Mean Blood Pressure in the 2 Treatment Groups During the Course of the Trial.
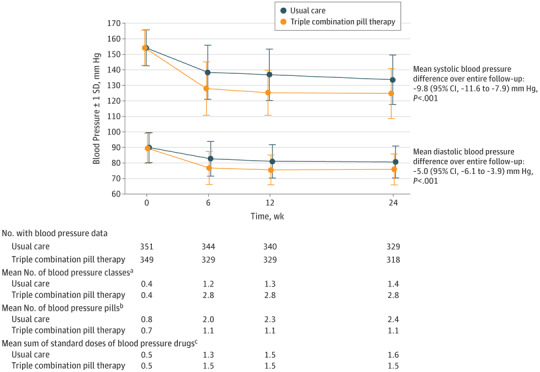
The SD at each visit is shown. Longitudinal linear models were used to estimate the differences in blood pressure during all 3 visits combined and included treatment group, trial visit as a categorical variable, a treatment × visit interaction, the baseline value (ie, baseline systolic or diastolic blood pressure), and use of blood pressure–lowering therapy at baseline as fixed effects along with trial center as a random effect.
aRefers to drug classes (eg, angiotensin-converting enzyme inhibitor, β-blocker, diuretic) the patient was taking. The mean No. of classes of blood pressure–lowering drugs was less than 3 during follow-up in the triple combination pill group because some patients were discontinued from this treatment.
bRefers to the No. of physical tablets or pills that the participant was taking each day. Some patients took more than 1 pill of the same drug to achieve the prescribed dose. The standard dose in milligrams of each medication was defined with reference to usual maintenance doses recorded by major formularies as described by Bennett et al19 and Law et al5 (detailed standard dose listing appears in eTable 1 in Supplement 2).
cThe No. of standard doses in the triple combination pill was 1.5 (calculated as the sum of 0.5 of a standard dose for each of the 3 components).
At the 6-month visit, the proportion of patients reporting adherence to all prescribed blood pressure–lowering medications was not significantly different between the triple combination pill therapy group and the usual care group (95.0% vs 94.6%, respectively; P = .82; Table 2). Similarly, the proportion of participants in the 2 groups who discontinued a blood pressure medication due to an adverse event during follow-up was not significantly different (6.6% in the triple combination pill therapy vs 6.8% in the usual care group, P = .92; Table 2). Details regarding the adverse events leading to discontinuation of any blood pressure–lowering medication appear in eTable 4 in Supplement 2.
Changes in Medication Use
All patients in the triple combination pill therapy group had a change in blood pressure–lowering regimen (commencing or switching to the fixed low-dose combination therapy) at randomization, whereas 75% of patients in the usual care group had a blood pressure–lowering medication change between randomization and the 6-week visit. Subsequent changes in the blood pressure–lowering regimen between weeks 6 and 12 occurred less frequently among patients in the triple combination pill therapy group (11%) compared with those in the usual care group (34%; P < .001) and between weeks 12 and 24 (6% vs 26%, respectively; P < .001).
At 6 months, 292 participants (83.7%) were still prescribed the triple combination pill therapy. Of those receiving the triple combination pill therapy, only 10 (3.4%) had been uptitrated to the higher dose. At the final 6-month study visit, 86.5% of patients in the triple combination pill therapy group were prescribed 2 or more blood pressure–lowering classes (which may or may not have been taken as part of the triple combination pill therapy), whereas this proportion was 33.4% in the usual care group (Figure 3).
Figure 3. Blood Pressure–Lowering Medication Classes by Randomized Group.
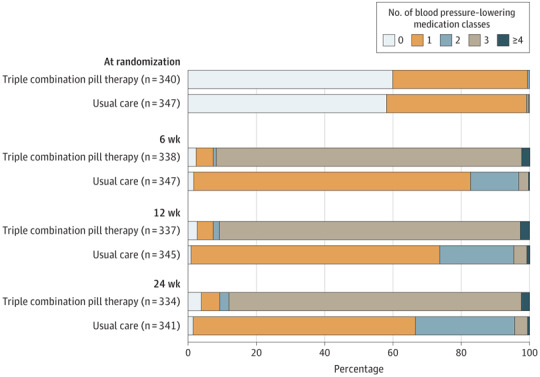
Subsequent to randomization, 5 patients (3 in the triple combination pill group and 2 in the usual care group) were identified as taking more than 1 blood pressure–lowering medication at baseline and these were reported as protocol violations. Patients taking less than 3 blood pressure–lowering classes during follow-up visits had stopped the triple combination pill and commenced an alternate therapy.
At the final 6-month follow-up visit, the mean number of blood pressure classes in the triple combination pill therapy group was 2.8 compared with 1.4 in the usual care group, whereas the mean number of blood pressure–lowering pills taken was 1.1 vs 2.4, respectively (Figure 2). Similar differences were observed when the study population was stratified by use of antihypertensive therapy prior to randomization (eTable 5 in Supplement 2). The mean sum of standard daily doses of blood pressure–lowering medication at the final visit was lower in the triple combination pill therapy group compared with usual care (1.5 vs 1.7, respectively; P = .01).
During follow-up, there were increases in the prescription of antiplatelet drugs and statins in both groups. At the final 6-month study visit, prescription of antiplatelet therapy (16.2% in the triple combination pill therapy group vs 17.0% in the usual care group; P = .75) and statins (57.7% vs 63.8%, respectively; P = .08) were not significantly different between the groups.
Adverse Events
In total, 419 adverse events were reported during the study. In the triple combination pill therapy group, 38.1% of participants reported at least 1 adverse event compared with 34.8% of participants in the usual care group (Table 3). The most common adverse events reported were musculoskeletal pain (6% in the triple combination pill therapy group vs 8% in the usual care group), dizziness, presyncope, or syncope (5.2% vs 2.8%, respectively), and headache (3.7% vs 3.1%).
Table 3. Adverse Events and Serious Adverse Events Reported by Group.
| No. (%) of Events | ||
|---|---|---|
| Triple
Combination Pill Therapy (n = 349) |
Usual
Care (n = 351) |
|
| Total No. of adverse events | 230 | 189 |
| Had ≥1 adverse eventa | 133 (38.1) | 122 (34.8) |
| Type of adverse event | ||
| Musculoskeletal pain | 21 (6.0) | 28 (8.0) |
| Dizziness, syncope, or presyncope | 18 (5.2) | 10 (2.8) |
| Headache | 13 (3.7) | 11 (3.1) |
| Angina pectoris | 8 (2.3) | 6 (1.7) |
| Cough | 8 (2.3) | 18 (5.1) |
| Pyrexia | 6 (1.7) | 12 (3.4) |
| Edema peripheral | 6 (1.7) | 5 (1.4) |
| Upper respiratory tract infection | 6 (1.7) | 11 (3.1) |
| Hypoesthesia | 3 (0.9) | 5 (1.4) |
| All type hypotension | 2 (0.6) | 2 (0.6) |
| Chest pain | 3 (0.9) | 4 (1.1) |
| Had ≥1 serious adverse event | 27 (7.7) | 21 (6.0) |
| Type of serious adverse event | ||
| Infections and infestations | 8 (2.3) | 5 (1.4) |
| General disorders and administration site conditions | 3 (0.9) | 3 (0.9) |
| Renal and urinary disorders | 4 (1.1) | 1 (0.3) |
| Cardiac disorder | 2 (0.6) | 2 (0.6) |
| Nervous system disorder | 2 (0.6) | 2 (0.6) |
| Injury, poisoning, and procedural complications | 2 (0.6) | 1 (0.3) |
| Ear and labyrinth disorders | 0 | 2 (0.6) |
| Gastrointestinal disorder | 2 (0.6) | 0 |
| Immune system disorder | 1 (0.3) | 1 (0.3) |
| Neoplasms (benign, malignant, or unspecified) | 2 (0.6) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 2 (0.6) | 0 |
| Endocrine disorder | 0 | 1 (0.3) |
| Gastrointestinal, renal, and urinary disorders | 1 (0.3) | 0 |
| Investigations | 0 | 1 (0.3) |
| Metabolism and nutrition disorders | 0 | 1 (0.3) |
| Metabolism and nutrition and vascular disorders | 1 (0.3) | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 1 (0.3) |
| Reproductive system and breast disorders | 1 (0.3) | 0 |
| Surgical and medical procedures | 0 | 1 (0.3) |
| Vascular disorders | 0 | 1 (0.3) |
Adverse events occurring in fewer than 1% of all participants are not shown.
Analyses of adverse events after excluding the 7 randomized patients without any postrandomization data recorded did not alter any conclusions (eTable 6 in Supplement 2). Twenty-seven participants in the triple combination pill therapy group reported at least 1 serious adverse event compared with 21 participants in the usual care group (the proportion in each serious adverse event category appears in eTable 7 in Supplement 2).
Laboratory Values
There were statistically significant between-group differences in the changes from baseline to 6 months for low-density lipoprotein cholesterol level (mean difference, 8.1 mg/dL [95% CI, 2.2 to 14.1 mg/dL]), sodium level (mean difference, −0.70 mmol/L [95% CI, −1.07 to −0.33 mmol/L]), potassium level (mean difference, −0.22 mmol/L [95% CI, −0.29 to −0.15 mmol/L]), and uric acid level (mean difference, 0.63 mg/dL [95% CI, 0.44 to 0.83 mg/dL]). There was no significant between-group difference for the change in serum creatinine level between baseline and the final 6-month visit (Table 4). However, the ratio of urinary albumin to creatinine was reduced more among patients in the triple combination pill therapy group compared with the usual care group (mean difference, −18.9 mg/g [95% CI, −34.8 to 3.1 mg/g]).
Table 4. Laboratory Parameters.
| Crude Estimates, Mean (SD)a | Model Estimates, Mean (95% CI) | Mean Difference (95% CI)b | |||
|---|---|---|---|---|---|
| Triple
Combination Pill Therapy (n = 349) |
Usual
Care (n = 351) |
Triple
Combination Pill Therapy (n = 349) |
Usual
Care (n = 351) |
||
| Change From Baseline to 6 mo | |||||
| Cholesterol, mg/dL | |||||
| Low-density lipoprotein | −4.3 (49.0) | −14.2 (47.5) | −4.8 (−10.4 to 0.9) | −12.9 (−18.5 to −7.4) | 8.1 (2.2 to 14.1) |
| High-density lipoprotein | 0.9 (9.5) | 1.4 (9.3) | 1.0 (−0.3 to 2.2) | 1.1 (−0.1 to 2.3) | −0.1 (−1.5 to 1.3) |
| Triglycerides, mg/dL | −27.3 (70.5) | −31.9 (73.0) | −27.2 (−36.8 to −17.7) | −26.9 (−36.3 to −17.4) | −0.3 (−8.1 to 7.4) |
| Creatinine, mg/dL | 0.05 (1.07) | 0.05 (0.20) | 0.1 (0 to 0.1) | 0.03 (0 to 0.1) | 0.04 (−0.06 to 0.14) |
| Uric acid, mg/dL | 0.51 (2.41) | 0.05 (1.02) | 0.6 (0.5 to 0.7) | −0.04 (−0.2 to 0.1) | 0.63 (0.44 to 0.83) |
| Alanine transaminase, U/L | −0.6 (17.4) | −4.2 (23.4) | −1.5 (−3.7 to 0.6) | −3.8 (−5.9 to −1.6) | 2.3 (−0.1 to 4.6) |
| Aspartate transaminase, U/L | −0.7 (11.8) | −2.5 (19.1) | −1.5 (−3.2 to 0.1) | −1.9 (−3.5 to −0.2) | 0.4 (−1.5 to 2.2) |
| Glucose, mg/dL | 0.4 (46.0) | −4.4 (49.6) | 0.5 (−5.7 to 6.8) | −1.8 (−8.0 to 4.3) | 2.4 (−3.8 to 8.5) |
| Sodium, mmol/L | −0.08 (2.87) | 0.69 (2.88) | −0.03 (−0.3 to 0.3) | 0.7 (0.4 to 1.0) | −0.70 (−1.07 to −0.33) |
| Potassium, mmol/L | −0.11 (0.54) | 0.12 (0.56) | −0.1 (−0.2 to 0) | 0.1 (0 to 0.2) | −0.22 (−0.29 to −0.15) |
| Ratio of urinary albumin to creatinine, mg/g | −37.8 (168.4) | −24.3 (146.6) | −40.8 (−52.0 to −29.6) | −21.9 (−33.0 to −10.7) | −18.9 (−34.8 to 3.1) |
| Outside Normal Levels at 6 mo | |||||
| Sodium, mmol/L, No. (%) | |||||
| <135 | 14 (4.4) | 7 (2.1) | |||
| ≥145 | 1 (0.3) | 3 (0.9) | |||
| Potassium, mmol/L, No. (%) | |||||
| <3.5 | 14 (4.4) | 3 (0.9) | |||
| ≥5.0 | 39 (11.2) | 30 (8.5) | |||
| Ratio of urinary albumin to creatinine ≤30 mg/g, No. (%) | 44 (13.9) | 80 (24.6) | |||
| Estimated glomerular filtration rate <60 mL/min/1.73 m2, No. (%) | 33 (10.4) | 24 (7.3) | |||
SI conversion factors: To convert alanine transaminase and aspartate transaminase to μkat/L, multiply by 0.0167; high-density and low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; creatinine to μmol/L, multiply by 76.25; glucose to mmol/L, multiply by 0.0555; triglycerides to mmol/L, multiply by 0.0113; uric acid to μmol/L, multiply by 59.49.
Unless otherwise indicated.
Estimated from an analysis of covariance.
The analyses of the laboratory data after excluding the 7 randomized patients without any recorded data did not alter any of the conclusions (eTable 6 in Supplement 2). There was no evidence of heterogeneity in the effect of the triple combination pill therapy compared with usual care on changes in albuminuria stratified by subgroups defined by age, sex, diabetes status, baseline blood pressure, baseline estimated glomerular filtration rate, or monotherapy at baseline (eTable 8 in Supplement 2).
A post hoc sensitivity analysis of the final 6-month visit values that was restricted to those who were fasting without baseline adjustment did not indicate any significant between-group differences in the levels of triglycerides or glucose. The level of low-density lipoprotein cholesterol was 119 mg/dL for the triple combination pill therapy group compared with 112 mg/dL for the usual care group (P = .05; eTable 9 in Supplement 2).
Primary Outcome in Prespecified Subgroups
There was no evidence of heterogeneity in the treatment effect for the primary outcome in any of the prespecified subgroups (Figure 4). Because only 10 patients had chronic kidney disease, a subgroup analysis based on this condition was not performed.
Figure 4. Primary Outcome by Subgroups.
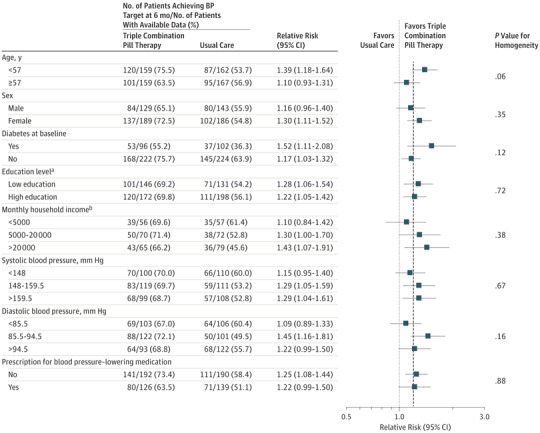
The dashed vertical line represents the relative risk (1.23) of the primary outcome for the overall study population. The proportion of participants achieving target blood pressure control at 6 months was analyzed using log-binomial regression (binomial distribution with a log link) with the treatment group and use of blood pressure–lowering therapy at baseline as fixed effects and trial center as a random effect. Homogeneity of treatment effects across subgroups for the primary outcome was tested by adding interaction terms to the log-binomial model. Subgroups only contain patients for whom blood pressure readings were available at 6 months. BP indicates blood pressure.
aLow education indicates none or primary school completion; high education indicates secondary school, university, or vocational education.
bExpressed as Sri Lankan rupee.
Discussion
In this study of patients with mild or moderate hypertension, initial treatment with or escalation of monotherapy to the low-dose triple combination pill therapy significantly improved achievement of blood pressure targets at 6 months compared with usual care. Improved achievement of blood pressure targets was apparent at the first follow-up visit, which occurred at 6 weeks. The rates were similar between groups for serious adverse events and withdrawal of any blood pressure–lowering medication due to adverse events.
Dual combination therapy is more effective than monotherapy,24 but few data are available on the use of other low-dose combinations. Previous trials have demonstrated that large reductions in blood pressure are possible with low-dose combinations of 3 or more agents compared with placebo9,10 and compared with standard-dose monotherapy.25 However, these were short-term studies that provided few data on longer-term tolerability or effectiveness compared with usual care because the regimens tested were fixed in both groups.
Other trials have shown improved blood pressure control with dual combination therapy compared with monotherapy for initial treatment of hypertension. In the Prevention and Treatment of Hypertension With Algorithm-based Therapy (PATHWAY) trial, treatment with losartan and hydrochlorothiazide was uniformly superior to monotherapy with either component, and there was no difference in the rates of withdrawal due to adverse events.26 In the Simplified Treatment Intervention to Control Hypertension (STITCH) trial,20 a simplified algorithm starting with combination therapy containing an angiotensin-converting enzyme inhibitor and a diuretic provided better blood pressure control than stepped-care titration at 6 months. In the Strategies of Treatment in Hypertension: Evaluation (STRATHE) trial,27 a low-dose combination therapy containing an angiotensin-converting enzyme inhibitor and a thiazide diuretic provided superior blood pressure control without excess adverse events compared with both sequential monotherapy and a stepped-care regimen.
Using a pragmatic design, the TRIUMPH trial is the first, to our knowledge, that extends this evidence to support a management strategy using a fixed low-dose triple combination pill therapy compared with usual care. Previous studies have shown that measured high blood pressure is only followed up with an escalation of treatment in 15% to 38% of encounters.28 The main reasons for not intensifying treatment are (1) an assumption that a patient’s existing treatment has not yet achieved the full effect or (2) clinician satisfaction with a patient’s blood pressure that has decreased or is near goal. Such therapeutic inertia is a major factor in the failure to reach blood pressure goals globally,29,30 and was observed in patients within both groups in this pragmatic trial.
At 6 months, 65% of patients in the usual care group were prescribed monotherapy and 29% were prescribed 2 blood pressure–lowering drugs, whereas in the triple combination pill therapy group, only 3% of patients still prescribed the fixed-dose combination were taking the higher-dose version. Therapeutic inertia is a complex sociological phenomenon involving multiple causal pathways,31 and these data indicate a triple combination pill therapy reduces rather than eliminates its influence.
This study was embedded within the public health system of Sri Lanka, a middle-income country with an estimated prevalence of hypertension of 24%.32 Data on blood pressure control rates in this setting are limited; however, the observation of 55% of participants in the usual care group achieving blood pressure control at 6 months is higher than typically seen in other middle-income countries.3 Usual care treatment was likely better than average as a result of trial participation in a tertiary care hospital setting and likely resulted in the observed narrowing of treatment effectiveness over time. Although it is possible that the differences may have narrowed further with additional follow-up, prolonged time to blood pressure control is associated with poorer outcomes.33
Even though this trial did not show any improvement in self-reported medication adherence or a reduction in adverse events with a low-dose combination therapy, initiation or escalation of treatment with such therapy did not lead to unacceptable increases in adverse events. There were differences in some of the metabolic parameters favoring usual care that were likely related to the greater use of a diuretic in the triple combination pill therapy group (diuretic use has been shown to increase low-density lipoprotein cholesterol level34). The clinical implications of these findings should be considered in light of the clear evidence of improvement in mortality and morbidity with thiazide-based blood pressure–lowering medications, including chlorthalidone.8,35
In this study, there was no significant between-group difference in changes in serum creatinine level. Some trials of blood pressure–lowering strategies in populations without chronic kidney disease have shown an increase in creatinine level, principally in the short-term as a result of hemodynamic changes rather than renal damage.36 Reductions in albuminuria also are commonly seen36 and were observed in this trial. Long-term data are lacking on whether any such changes to renal function and urinary albumin excretion influence the risk of end-stage renal failure in patients with hypertension and without chronic kidney disease.
The clinical and research implications of these findings principally relate to the need for replication and for a global increase in treatment. The scale of blood pressure–attributable disease burden and the scarcity of health resources in low- and middle-income countries warrant urgent adoption of more effective and cost-effective blood pressure–control strategies. An immediate challenge to translating the results of this study in low- and middle-income countries is in incorporating this strategy within systemwide approaches for chronic disease care to enable universal access to treatment. This includes addressing structural, regulatory, pricing, and workforce challenges. Cost and availability currently remain a barrier to effective treatment for the large majority of patients with high blood pressure living around the world.37
The results also may have relevance to high-income countries that have lower control rates for hypertension than the rates seen in this trial despite considerable health service investments.38 The results are also particularly important in light of recent recommendations for lower blood pressure targets in high-risk individuals.8 In the United States, one of the most effective implementation programs for blood pressure control involved organized systems of regular prescription review, with a large component focused on more extensive use of combination therapy at earlier stages within treatment protocols.39 The results of this study strongly reinforce use of a combination therapy approach.
Limitations
This study has several limitations. First, a limitation of open-label designs relates to the potential for differences in study-related procedures. Both triple combination pill therapy and usual care participants were medically managed in outpatient clinics by their usual treating physician.
However, to manage drug supply, participants in the triple combination pill therapy group were dispensed treatment at less frequent intervals than typically done for patients receiving usual care. This may have provided usual care patients greater exposure to opportunities for treatment titration. For study data collection purposes, follow-up was identical between groups. The primary and key secondary blood pressure outcome measures were standardized and objective; however, other outcomes such as adverse event reporting may have been influenced by patient awareness of treatment allocation.
Second, medications in this study for both groups were provided free of charge, which is routine practice in the Sri Lankan public health care system. Although this is not a limitation in the local context, it may affect generalizability to other settings.
Third, as is typical in pragmatic trials, exclusion criteria were primarily left to the judgment of investigators, which may have led to the underrepresentation of certain patient subgroups such as those with chronic kidney disease. Therefore, the outcomes of this study may not be generalizable to such subgroups of the population.
Conclusions
Among patients with mild to moderate hypertension, treatment with a pill containing low doses of 3 antihypertensive drugs led to an increased proportion of patients achieving their target blood pressure goal vs usual care. Use of such medication as initial therapy or to replace monotherapy may be an effective way to improve blood pressure control.
Trial protocol and statistical analysis plan
eText
eTable 1. Listing of standard daily doses of blood pressure medication used in the study
eTable 2. Baseline characteristics by randomized group and blood pressure lowering drug use at baseline
eTable 3. Sensitivity analysis for primary and secondary BP targets outcomes using fully conditional specification for multiple imputation
eTable 4. Reasons for discontinuing any blood pressure lowering medication by group
eTable 5. Number of blood pressure lowering classes and number of blood pressure lowering pills taken by randomized group, and by baseline therapy
eTable 6. Adverse events, serious adverse events and changes in laboratory parameters in the safety population as defined in the statistical analysis plan
eTable 7. Classification of reported serious adverse events
eTable 8. Change in urinary albumin-creatinine ratio from baseline to 6 months by subgroups defined by baseline characteristics
eTable 9. Fasting LDL cholesterol, triglycerides and glucose at the final (6 month) study visit
References
- 1.Gakidou E, Afshin A, Abajobir AA, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016. Lancet. 2017;390(10100):1345-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood-pressure-lowering regimens on major cardiovascular events. Lancet. 2003;362(9395):1527-1535. doi: 10.1016/S0140-6736(03)14739-3 [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control. Circulation. 2016;134(6):441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatib R, McKee M, Shannon H, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries. Lancet. 2016;387(10013):61-69. [DOI] [PubMed] [Google Scholar]
- 5.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs. BMJ. 2003;326(7404):1427-1431. doi: 10.1136/bmj.326.7404.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569-588. [DOI] [PubMed] [Google Scholar]
- 7.Gabb GM, Mangoni AA, Anderson CS, et al. Guideline for the diagnosis and management of hypertension in adults—2016. Med J Aust. 2016;205(2):85-89. doi: 10.5694/mja16.00526 [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults [published correction appears in J Am Coll Cardiol. 2018;71(19):2275-2279]. J Am Coll Cardiol. 2018;71(19):e127-e248. [DOI] [PubMed] [Google Scholar]
- 9.Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7(7):e41297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow CK, Thakkar J, Bennett A, et al. Quarter-dose quadruple combination therapy for initial treatment of hypertension. Lancet. 2017;389(10073):1035-1042. [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents. Hypertension. 2010;55(2):399-407. [DOI] [PubMed] [Google Scholar]
- 12.Webster R, Patel A, Selak V, et al. Effectiveness of fixed dose combination medication (‘polypills’) compared with usual care in patients with cardiovascular disease or at high risk. Int J Cardiol. 2016;205:147-156. doi: 10.1016/j.ijcard.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 13.Okonofua EC, Simpson KN, Jesri A, et al. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47(3):345-351. [DOI] [PubMed] [Google Scholar]
- 14.Kizilirmak P, Berktas M, Uresin Y, Yildiz OB. The efficacy and safety of triple vs dual combination of angiotensin II receptor blocker and calcium channel blocker and diuretic. J Clin Hypertens (Greenwich). 2013;15(3):193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454-463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 16.Salam A, Webster R, Singh K, et al. TRIple pill vs Usual care Management for Patients with mild-to-moderate Hypertension (TRIUMPH). Am Heart J. 2014;167(2):127-132. [DOI] [PubMed] [Google Scholar]
- 17.Webster R. Protocol changes to the TRIUMPH study. Am Heart J. 2017;191:e1. [DOI] [PubMed] [Google Scholar]
- 18.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
- 19.Bennett A, Chow CK, Chou M, et al. Efficacy and safety of quarter-dose blood pressure-lowering agents. Hypertension. 2017;70(1):85-93. [DOI] [PubMed] [Google Scholar]
- 20.Feldman RD, Zou GY, Vandervoort MK, et al. A simplified approach to the treatment of uncomplicated hypertension. Hypertension. 2009;53(4):646-653. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun DA, Lacourcière Y, Chiang YT, Glazer RD. Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide. Hypertension. 2009;54(1):32-39. [DOI] [PubMed] [Google Scholar]
- 22.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 23.Abdi H. Holm’s sequential Bonferroni procedure. In: Encyclopedia of Research Design. Thousand Oaks, CA: SAGE Publications; 2010:1-8. [Google Scholar]
- 24.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure. Am J Med. 2009;122(3):290-300. doi: 10.1016/j.amjmed.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 25.Mahmud A, Feely J. Low-dose quadruple antihypertensive combination. Hypertension. 2007;49(2):272-275. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald TM, Williams B, Webb DJ, et al. Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension. J Am Heart Assoc. 2017;6(11):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mourad JJ, Waeber B, Zannad F, et al. Comparison of different therapeutic strategies in hypertension. J Hypertens. 2004;22(12):2379-2386. [DOI] [PubMed] [Google Scholar]
- 28.Wang YR, Alexander GC, Stafford RS. Outpatient hypertension treatment, treatment intensification, and control in Western Europe and the United States. Arch Intern Med. 2007;167(2):141-147. doi: 10.1001/archinte.167.2.141 [DOI] [PubMed] [Google Scholar]
- 29.Ferrari P; National Coordinators for the Reasons for not Intensifying Antihypertensive Treatment (RIAT) trial12 . Reasons for therapeutic inertia when managing hypertension in clinical practice in non-Western countries. J Hum Hypertens. 2009;23(3):151-159. doi: 10.1038/jhh.2008.117 [DOI] [PubMed] [Google Scholar]
- 30.Egan BM, Zhao Y, Axon RN, et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124(9):1046-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redón J, Coca A, Lázaro P, et al. Factors associated with therapeutic inertia in hypertension. J Hypertens. 2010;28(8):1770-1777. [DOI] [PubMed] [Google Scholar]
- 32.Katulanda P, Ranasinghe P, Jayawardena R, et al. The prevalence, predictors and associations of hypertension in Sri Lanka. Clin Exp Hypertens. 2014;36(7):484-491. [DOI] [PubMed] [Google Scholar]
- 33.Volpe M, Gallo G, Tocci G. Is early and fast blood pressure control important in hypertension management? Int J Cardiol. 2018;254:328-332. [DOI] [PubMed] [Google Scholar]
- 34.Goldman AI, Steele BW, Schnaper HW, et al. Serum lipoprotein levels during chlorthalidone therapy. JAMA. 1980;244(15):1691-1695. [DOI] [PubMed] [Google Scholar]
- 35.Olde Engberink RH, Frenkel WJ, van den Bogaard B, et al. Effects of thiazide-type and thiazide-like diuretics on cardiovascular events and mortality. Hypertension. 2015;65(5):1033-1040. [DOI] [PubMed] [Google Scholar]
- 36.Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease. Ann Intern Med. 2017;167(6):375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attaei MW, Khatib R, McKee M, et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries. Lancet Public Health. 2017;2(9):e411-e419. [DOI] [PubMed] [Google Scholar]
- 38.Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137(2):109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699-705. doi: 10.1001/jama.2013.108769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eText
eTable 1. Listing of standard daily doses of blood pressure medication used in the study
eTable 2. Baseline characteristics by randomized group and blood pressure lowering drug use at baseline
eTable 3. Sensitivity analysis for primary and secondary BP targets outcomes using fully conditional specification for multiple imputation
eTable 4. Reasons for discontinuing any blood pressure lowering medication by group
eTable 5. Number of blood pressure lowering classes and number of blood pressure lowering pills taken by randomized group, and by baseline therapy
eTable 6. Adverse events, serious adverse events and changes in laboratory parameters in the safety population as defined in the statistical analysis plan
eTable 7. Classification of reported serious adverse events
eTable 8. Change in urinary albumin-creatinine ratio from baseline to 6 months by subgroups defined by baseline characteristics
eTable 9. Fasting LDL cholesterol, triglycerides and glucose at the final (6 month) study visit


