Key Points
Question
Is prenatal phthalate exposure associated with language development in children at 30 to 37 months of age?
Findings
In this cohort study of 2 independent studies that included a total of 1333 mother–child pairs, exposure to dibutyl phthalate and butyl benzyl phthalates during pregnancy was significantly associated with language delay in preschool-aged children.
Meaning
These findings suggest that additional examination of the association of phthalates with language delay is warranted.
Abstract
Importance
Prenatal exposure to phthalates has been associated with neurodevelopmental outcomes, but little is known about the association with language development.
Objective
To examine the association of prenatal phthalate exposure with language development in children in 2 population-based pregnancy cohort studies.
Design, Setting, and Participants
Data for this study were obtained from the Swedish Environmental Longitudinal Mother and Child, Asthma and Allergy (SELMA) study conducted in prenatal clinics throughout Värmland county in Sweden and The Infant Development and the Environment Study (TIDES) conducted in 4 academic centers in the United States. Participants recruited into both studies were women in their first trimester of pregnancy who had literacy in Swedish (SELMA) or English or Spanish (TIDES). This study included mothers and their children from both the SELMA study (n = 963) and TIDES (n = 370) who had complete data on prenatal urinary phthalate metabolite levels, language delay, and modeled covariables. For SELMA, the data were collected from November 1, 2007, to June 30, 2013, and data analysis was conducted from November 1, 2016, to June 30, 2018. For TIDES, data collection began January 1, 2010, and ended March 29, 2016, and data analysis was performed from September 15, 2016, to June 30, 2018.
Main Outcomes and Measures
Mothers completed a language development questionnaire that asked the number of words their children could understand or use at a median of 30 months of age (SELMA) and 37 months of age (TIDES). The responses were categorized as fewer than 25, 25 to 50, and more than 50 words, with 50 words or fewer classified as language delay.
Results
In the SELMA study, 963 mothers, 455 (47.2%) girls, and 508 (52.8%) boys were included. In TIDES, 370 mothers, 185 (50.0%) girls, and 185 (50.0%) boys were included in this analysis. The prevalence of language delay was 10.0% in both SELMA (96 reported) and TIDES (37 reported), with higher rates of delay in boys than girls (SELMA: 69 [13.5%] vs 27 [6.0%]; TIDES: 23 [12.4%] vs 14 [7.6%]). In crude analyses, the metabolite levels of dibutyl phthalate and butyl benzyl phthalate were statistically significantly associated with language delay in both cohorts. In adjusted analyses, a doubling of prenatal exposure of dibutyl phthalate and butyl benzyl phthalate metabolites increased the odds ratio (OR) for language delay by approximately 25% to 40%, with statistically significant results in the SELMA study (dibutyl phthalate OR, 1.29 [95% CI, 1.03-1.63; P = .03]; butyl benzyl phthalate OR, 1.26 [95% CI, 1.07-1.49; P = .003]). A doubling of prenatal monoethyl phthalate exposure was associated with an approximately 15% increase in the OR for language delay in the SELMA study (OR, 1.14; 95% CI, 1.00-1.31; P = .05), but no such association was found in TIDES (OR, 0.98; 95% CI, 0.79-1.23).
Conclusions and Relevance
In findings from this study, prenatal exposure to dibutyl phthalate and butyl benzyl phthalate was statistically significantly associated with language delay in children in both the SELMA study and TIDES. These findings, along with the prevalence of prenatal exposure to phthalates, the importance of language development, and the inconsistent results from a 2017 Danish study, suggest that the association of phthalates with language delay may warrant further examination.
This study examines 2 longitudinal studies to investigate the association between a mother’s first-trimester exposure to phthalates and the delay in her young child’s language development.
Introduction
Phthalates comprise a family of high-volume semivolatile synthetic chemicals with the ability to make plastic soft and flexible. They are used in a variety of industrial products, including polyvinyl chloride flooring, food packaging, personal care products, medical supplies, and toys.1,2,3,4 Because phthalates are not covalently bound, they leach into the surrounding environment and are routinely found in indoor air,5,6 dust,7,8,9,10,11 food, and water.12 Global biomonitoring data find most phthalate metabolites ubiquitously in urine from children13 and adults4 as well as in blood14,15 and breast milk.16 These compounds have also been found in amniotic fluid, suggesting that they cross the placental barrier and convey fetal exposure.17
Phthalates have been shown to be environmental endocrine disruptors with antiandrogenic properties and to be associated with androgen-dependent development, including male reproductive tract development.18 In rodents, prenatal exposure to dibutyl phthalate (DBP), butyl benzyl phthalate (BBzP), and di-ethylhexyl phthalate (DEHP) has been associated with a cluster of male genital abnormalities termed the phthalate syndrome.18 Prenatal phthalate exposure has been associated with a shorter male anogenital distance19,20,21 and male genital defects.22 Such changes to the male genitals may arise from phthalate-induced suppression of fetal gonadal testosterone synthesis.23
Antiandrogenic phthalates have been shown to disrupt testosterone-dependent brain development,24 and associations between prenatal exposure and sex-dependent neurodevelopmental end points have been reported.25 Multiple studies found inverse associations between phthalate metabolite level in prenatal urine and child neurodevelopment,26,27,28 behavioral outcomes,29,30,31,32,33 mental and psychomotor development,34,35,36 neonatal and infant neurologic status,31,37 and language development.38 These inverse associations have been reported most often for metabolites of DBP, DEHP and BBzP, with most studies reporting on third-trimester exposure.
Animal studies on prenatal phthalate exposure and neurodevelopment are limited but provide some support for these epidemiologic findings. Associations have been reported between several phthalates and spatial learning and memory,39,40,41,42,43 increased spontaneous motor activity suggestive of potential hyperactive behavior,44,45 and delays in coordinated movements.46,47 Sex-specific associations are often seen, with males typically more affected than females, which is not unexpected given the hormonal activity of phthalates.48
Delays in language development in early childhood, as assessed by validated tests, have been shown to be a factor in later academic achievement and the need for special education.49,50 Therefore, language delay, assessed in early childhood, is an important indicator of later neurodevelopmental impairment.
The goal of the current study is to examine the association between the metabolite phthalate level in first-trimester urine samples and language development in early childhood. In this article, we report on these associations in 2 independent pregnancy cohort studies that share multiple study methods: the Swedish Environmental Longitudinal Mother and Child, Asthma and Allergy (SELMA) study and The Infant Development and the Environment Study (TIDES) in the United States.
Methods
The Regional Ethical Review Board (Uppsala, Sweden) approved the SELMA study. All participants signed informed consent forms before the start of data collection. Data were collected from November 1, 2007, to June 30, 2013, and data analysis was conducted from November 1, 2016, to June 30, 2018. The protocols for TIDES were approved by institutional review boards at the participating institutions, including the Icahn School of Medicine at Mount Sinai, which served as the TIDES coordinating center. Individual participants provided signed informed consent forms before any study activities. Data collection began January 1, 2010, and ended March 29, 2016, and data analysis was performed from September 15, 2016, to June 30, 2018.
Two Pregnancy Cohorts
The SELMA study is designed to investigate the association of early life exposure to environmental chemicals with the health and development of children. The study recruited pregnant women in the county of Värmland, Sweden, from November 1, 2007, through March 31, 2010. Women who could read Swedish were recruited at their first prenatal visit. Of the 8394 pregnant women identified, 6658 were eligible and 2582 (38.8%) agreed to participate, and 1957 children were born into the study. Detailed recruitment selection criteria and sample collection procedures have been published previously.21,51 Language development level was assessed in all children in the SELMA study, but local clinics sent these assessments to the data center (Karlstad University) for only 1113 children. Of these 1113 children, 963 (86.5%) had complete data on all variables.
The TIDES is a US-based cohort study that recruited women in their first trimester of pregnancy at 1 of 4 academic medical centers (University of Minnesota Medical Center [Minneapolis], University of California-San Francisco Clinical Center [San Francisco], University of Rochester Medical Center [Rochester, New York], and Seattle Children's Hospital, University of Washington [Seattle]) between August 1, 2010, and August 31, 2012. The study design, methods, and population have been published previously.19,52 Briefly, all women who were fewer than 13 weeks pregnant, aged 18 years or older, and able to read and write English (or Spanish at the San Francisco medical center) were eligible for inclusion. Of the 969 women who gave consent, 739 (76.3%) had a live birth and were eligible for a follow-up questionnaire in early childhood. Unfortunately, mailing of these questionnaires was poorly executed; the number delivered was unknown and many questionnaires were returned undelivered. Therefore, our analysis included the 370 children in TIDES whose mothers completed and returned the questionnaire when the child was at least 2 years of age.
Phthalate Metabolites in Prenatal Urine
In the SELMA study, first morning void urine samples were obtained at the first prenatal visit (median [range] of 10 [3-27] weeks, with 924 participants [95.4%] enrolled before week 13). Samples were stored at –20°C before being processed at the Laboratory of Occupational and Environmental Medicine at Lund University, Lund, Sweden.21 Aliquots of 0.2 mL of urine were mixed with 0.1 mL of ammonium acetate (1M; pH, 6.5) and 0.01 mL of β-glucoronidase (Escherichia coli) and then incubated at 37°C for 30 minutes. Next, 0.05 mL of a 50 per 50 (vol/vol) water and acetonitrile solution of 3H- or radioactive carbon 13–labeled internal standards of all analyzed compounds were added, and the samples were analyzed by liquid chromatography–tandem mass spectrometry without any further clean up. To adjust for urine concentration, the investigators used an enzymatic method (as described by Mazzachi et al53) to determine the creatinine level, and all samples were corrected for urine dilution by creatinine adjustment. All measured values were reported and used in these analyses.
In TIDES, spot urine samples were collected at the first prenatal visit (median [range] of 10.9 [10.5-11.2] weeks, with 100% of participants enrolled before week 13) and then stored at –80°C before being analyzed at the Division of Laboratory Sciences at the National Center for Environmental Health, Atlanta, Georgia, using previously described methods.20,54 After urine collection, specific gravity was measured using a handheld refractometer (Refractometer Atago PAL-10S; National Instrument Company). The analytical approach involved enzymatic deconjugation of the metabolites from their glucuronidated form, automated online solid-phase extraction, separation with high-performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry.54 Values below the limit of detection were assigned the value limit of detection divided by the square root of 2, as has been recommended when the data are not highly skewed.55
The Laboratory of Occupational and Environmental Medicine and the Division of Laboratory Sciences participate twice yearly in an ongoing quality control program (EQUAAS). This program tests for 6 phthalate metabolites.
Language Delay in Children
Language development is routinely assessed in Sweden when children are 30 months of age to identify children in need of speech therapy or further assessments. This validated assessment consists of a parental questionnaire on language use and a nurse evaluation. The screening questionnaire, which was used in the SELMA study, asks about the number of words the child can use. Responses are categorized as fewer than 25, 25 to 50, and more than 50 words. An English translation of this Swedish questionnaire was mailed to mothers in TIDES when their children were at least 2 years of age (median [range] of 37 [24-63] months). A vocabulary of 50 words or fewer was classified as language delay in both studies.
Statistical Analysis
Phthalate metabolite levels were log transformed using base 2. Three logistic regression models were used to examine the associations between language delay and (continuous) prenatal phthalate metabolite levels overall and stratified by sex. Model 1 controlled only for urinary dilution, which was measured by urinary creatinine level in the SELMA study and specific gravity in TIDES. Model 2 also controlled for several variables previously associated with cognitive development; prematurity (gestational age of <37 weeks); mother’s educational level; and mother’s weight and smoking status at study enrollment; and child sex. Eligibility for the SELMA study required participating mothers to be residents of Värmland County and literate in Swedish; all participants were assumed to be white. In TIDES, race/ethnicity was reported by the mothers and included in these analyses. In the SELMA study, all questionnaires were assumed to be completed when the children were aged 30 months, whereas in TIDES, age at completion of the language development questionnaire varied and was included in the analyses. Model 3 used covariates in model 2 and the interaction term (sex × exposure). The significance threshold was set at P < .05; and significance testing was 2-sided. Statistical analyses were conducted by 2 of us (C.G.B. for SELMA and S.F.E. for TIDES) in SAS, version 9.3 (SAS Institute Inc) and SPSS, version 20 (IBM).
Results
The current study included all mothers and children with complete data on prenatal urinary phthalate metabolite levels, language delay, and modeled covariables. Specifically, 963 mother–child pairs from the SELMA study (963 mothers, 455 [47.2%] girls, and 508 [52.8%] boys) and 370 mother–child pairs (370 mothers, 185 [50.0%] girls, and 185 [50.0%] boys) from TIDES were included (Table 1). When comparing the TIDES and SELMA populations, preterm birth was more common (38 [10.4%] vs 44 [4.6%]), median gestational age at urine collection was slightly later (10.9 weeks vs 10 weeks), and children were somewhat older at language assessment (median age, 37 months vs 30 months). In TIDES, compared with the SELMA study, more mothers completed college- or university-level education (283 [76.5] vs 629 [65.3%]), and mothers were slightly heavier (mean [SD] weight, 71.5 [17.8] kg vs 70.0 [13.9] kg) and slightly more likely to smoke (23 [6.2%] vs 51 [5.3%]) at study enrollment. In TIDES, 274 mothers (74.1%) were white or non-Hispanic, whereas all mothers in the SELMA study were assumed to be white or non-Hispanic.
Table 1. Description of the SELMA and TIDES Study Populations .
| Variable | No. (%) | |
|---|---|---|
| SELMA (N = 963) | TIDES (N = 370) | |
| Child’s sex, male | 508 (52.8) | 185 (50.0) |
| Child’s sex, female | 455 (47.2) | 185 (50.0) |
| Premature birtha | 44 (4.6) | 38 (10.4) |
| Mother’s educational level: completed college or university | 629 (65.3) | 283 (76.5) |
| Mother smoked at study enrollment | 51 (5.3) | 23 (6.2) |
| Mother’s race/ethnicity: white/non-Hispanic | 963 (100) | 274 (74.1) |
| Child’s age at language assessment, median (IQR), mob | 30 (NA) | 37 (29.8-43.0) |
| Mother’s weight at study enrollment, mean (SD), kg | 70.0 (13.9) | 71.5 (17.8) |
| Gestational age at urine collection, median (IQR), wk | 10.0 (9.0-11.0) | 10.9 (9.3-12.3) |
Abbreviations: IQR, interquartile range; NA, not available; SELMA, Swedish Environmental Longitudinal Mother and Child, Asthma and Allergy study; TIDES, The Infant Development and the Environment Study.
Premature birth was defined as gestational age at birth of fewer than 37 weeks.
In the SELMA study, language development was assessed at routine pediatric visit at 30 months of age. In TIDES, language development was assessed by mailed questionnaire at 36 months of age.
Some urinary phthalate metabolite levels were higher in the SELMA study than in TIDES (eTable 1 in the Supplement). This was particularly true for DEP, DBP and BBzP metabolites.
In both populations, the proportion of children who used 50 words or fewer was 10% (SELMA: 96 reported; TIDES: 37 reported) and those who understood or used fewer than 25 words was 2.7% (SELMA: 26 reported; TIDES: 10 reported) (Table 2). Language delay was more common among boys than girls in both studies (SELMA: 69 [13.5%] vs 27 [6.0%]; P = .001; TIDES: 23 [12.4%] vs 14 [7.6%]; P = .12).
Table 2. Language Development Assessment in SELMA and TIDES.
| No. of Words Used by the Age of Assessment | No. (%) | |
|---|---|---|
| SELMA (N = 963) | TIDES (N = 370) | |
| All children | ||
| <25 Words | 26 (2.7) | 10 (2.7) |
| 25-50 Words | 70 (7.3) | 27 (7.3) |
| ≤50 (Language delayed) | 96 (10.0) | 37 (10.0) |
| Boys | ||
| <25 Words | 21 (4.1) | 8 (4.3) |
| 25-50 Words | 48 (9.4) | 15 (8.1) |
| ≤50 (Language delayed) | 69 (13.5) | 23 (12.4) |
| Girls | ||
| <25 Words | 5 (1.1) | 2 (1.1) |
| 25-50 Words | 22 (4.9) | 12 (6.5) |
| ≤50 (Language delayed) | 27 (6.0) | 14 (7.6) |
Abbreviations: SELMA, Swedish Environmental Longitudinal Mother and Child, Asthma and Allergy study; TIDES, The Infant Development and the Environment Study.
Results of the logistic regression analyses using models 1, 2, and 3 are summarized in Table 3 (SELMA) and Table 4 (TIDES). Effect estimates for the total population and within sex were of similar magnitude in both the SELMA study and TIDES, although many statistically significant associations in the SELMA study were no longer statistically significant in TIDES in model 2 analyses. Metabolites of DBP and BBzP were statistically significantly associated with language delay in the total population in both studies in crude analyses. In adjusted analyses, a doubling of prenatal exposure of DBP and BBzP metabolites increased the odds ratio (OR) for language delay by approximately 25% to 40%, with statistically significant results in the SELMA study (dibutyl phthalate OR, 1.29 [95% CI, 1.03-1.63; P = .03]; butyl benzyl phthalate OR, 1.26 [95% CI, 1.07-1.49; P = .003]). A doubling of prenatal monoethyl phthalate exposure was associated with an approximately 15% increase in the OR for language delay in the SELMA study (OR, 1.14; 95% CI, 1.00-1.31; P = .05), but no such association was found in TIDES (OR, 0.98; 95% CI, 0.79-1.23).
Table 3. OR (95% CI) Associations Between Prenatal Urinary Phthalate Metabolite Levels and Language Delay in Children in SELMA.
| Compound | OR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Crudea | Adjustedb | Adjusted for all Covariates and Sex × Exposure | Stratification and Adjustmentd | |||
| Adjusted for All Covariates and Interaction Termc | P Value for Interaction Term | Boys (n = 508)d | Girls (n = 455)d | |||
| MEP | 1.16 (1.02-1.33) | 1.14 (1.00-1.31) | 1.28 (1.04-1.64) | .29 | 1.09 (0.93-1.29) | 1.29 (1.00-1.66) |
| MBP | 1.33 (1.07-1.67) | 1.29 (1.03-1.63) | 1.30 (0.85-2.01) | .99 | 1.31 (1.00-1.71) | 1.23 (0.79-1.92) |
| MBzP | 1.29 (1.10-1.51) | 1.26 (1.07-1.49) | 1.08 (0.81-1.45) | .59 | 1.39 (1.13-1.71) | 1.04 (0.76-1.41) |
| MEHP | 0.91 (0.77-1.09) | 0.90 (0.75-1.09) | 0.80 (0.58-1.10) | .34 | 0.99 (0.78-1.24) | 0.78 (0.56-1.08) |
| MEHHP | 0.91 (0.76-1.10) | 0.89 (0.73-1.09) | 0.79 (0.55-1.13) | .41 | 0.97 (0.76-1.23) | 0.76 (0.51-1.10) |
| MEOHP | 0.97 (0.89-1.18) | 0.95 (0.77-1.18) | 0.78 (0.53-1.15) | .21 | 1.07 (0.83-1.38) | 0.74 (0.50-1.12) |
| MECPP | 0.91 (0.76-1.10) | 0.89 (0.73-1.09) | 0.81 (0.58-1.15) | .50 | 0.95 (0.75-1.21) | 0.77 (0.53-1.12) |
| MCMHP | 0.86 (0.70-1.06) | 0.86 (0.69-1.08) | 0.79 (0.52-1.19) | .60 | 0.90 (0.69-1.18) | 0.76 (0.49-1.17) |
Abbreviations: MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MCMHP, mono(2-carboxymethylhexyl) phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; OR, odds ratio; SELMA, Swedish Environmental Longitudinal Mother and Child, Asthma and Allergy.
Model 1: adjusted for the creatinine level in urine.
Model 2: adjusted for the creatinine level in urine; sex; preterm birth; mother’s educational level; and mother’s smoking status and weight at study enrollment.
Model 3: adjusted for the creatinine level in urine; sex; preterm birth; mother’s educational level; and mother’s smoking status and weight at study enrollment as well as the interaction term (sex × exposure).
Adjusted for the creatinine level in urine; preterm birth; mother’s educational level; and mother’s smoking status and weight at study enrollment.
Table 4. OR (95% CI) Associations Between Prenatal Urinary Phthalate Metabolite Levels and Language Delay in Children in TIDES.
| Compound | OR (95 % CI) | |||||
|---|---|---|---|---|---|---|
| Crudea | Adjustedb | Adjusted for all Covariates and Sex × Exposure | Stratification and Adjustmentd | |||
| Adjusted for All Covariates and Interaction Termc | P Value for Interaction Term | Boys (n = 185)d | Girls (n = 185)d | |||
| MEP | 1.05 (0.88-1.25) | 0.98 (0.79-1.23) | 0.73 (0.38-1.41) | .34 | 0.85 (0.66-1.11) | 1.07 (0.76-1.50) |
| MBP | 1.40 (1.08-1.80) | 1.26 (0.93-1.71) | 1.87 (0.73-4.81) | .38 | 1.15 (1.00-2.10) | 1.15 (0.72-1.84) |
| MiBP | 1.48 (1.12-1.96) | 1.41 (0.99-2.01) | 1.90 (0.63-5.77) | .57 | 1.45 (0.95-2.21) | 0.95 (0.57-1.56) |
| Sum DBP | 1.43 (1.08-1.88) | 1.29 (0.91-1.84) | 1.97 (0.67-5.79) | .42 | 1.47 (0.96-2.24) | 1.06 (0.63-1.78) |
| MBzP | 1.28 (1.04-1.58) | 1.16 (0.90-1.48) | 1.02 (0.49-2.11) | .72 | 1.16 (0.88-1.53) | 1.17 (0.80-1.71) |
| MEHP | 1.10 (0.88-1.39) | 1.07 (0.81-1.41) | 1.95 (0.89-4.26) | .12 | 1.25 (0.93-1.69) | 0.85 (0.55-1.30) |
| MEHHP | 1.14 (0.91-1.43) | 1.15 (0.87-1.51) | 1.56 (0.70-3.48) | .43 | 1.23 (0.92-1.63) | 1.11 (0.72-1.71) |
| MEOHP | 1.08 (0.84-1.37) | 1.10 (0.82-1.46) | 1.51 (0.66-3.48) | .33 | 1.19 (0.88-1.61) | 1.12 (0.72-1.74) |
| MECPP | 1.24 (0.97-1.58) | 1.19 (0.89-1.60) | 1.32 (0.81-2.14) | .63 | 1.19 (0.86-1.65) | 1.16 (0.72-1.86) |
Abbreviations: DBP, dibutyl phthalate; MBiP, monoisobutyl phthalate; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; OR, odds ratio; TIDES, The Infant Development and the Environment Study.
Model 1: adjusted for urinary-specific gravity.
Model 2: adjusted for creatinine urinary-specific gravity; sex; preterm birth; mother’s educational level and race/ethnicity; and mother’s smoking status and weight at study enrollment.
Model 3: adjusted for urinary-specific gravity; sex; preterm birth; mother’s educational level and race/ethnicity; and mother’s smoking status and weight at study enrollment as well as the interaction term (sex × exposure).
Adjusted for urinary-specific gravity; preterm birth; mother’s educational level and race/ethnicity; and mother’s smoking status and weight at study enrollment.
No DEHP metabolite was statistically significantly associated with language delay in either study population. We further examined the interaction between sex and exposure in model 3. The interaction term was not significant for any metabolite, and including an interaction term in the models did not appreciably alter the metabolite effect estimate (Tables 3 and 4).
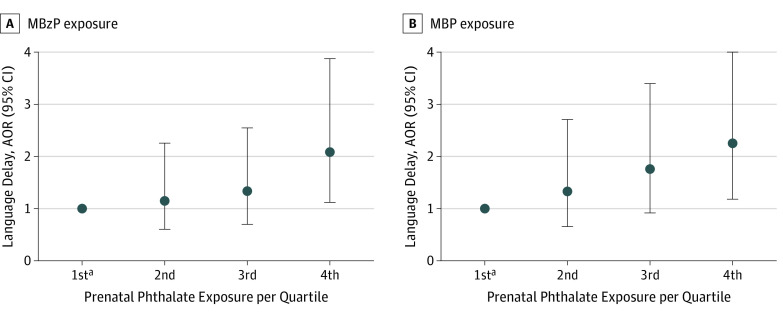
After categorizing monobenzyl phthalate (MBzP) and monobutyl phthalate by quartiles, a dose-response association between metabolite exposure and language delay was seen in the SELMA study (Figure). Risk increased with dose, although the trend was not statistically significant (MbzP, P = .09; monobutyl phthalate, P = .07). An OR of approximately 2.0 (doubling the odds of language delay) reflects exposure in the fourth quartile, compared with that in the first quartile.
Figure. Association Between Prenatal Creatinine Adjusted Phthalate Metabolite Levels and Language Delay in Children in the Swedish Environmental Longitudinal Mother and Child, Asthma and Allergy (SELMA) Study.
Language delay risk increased with monobenzyl phthalate (MBzP) exposure (A) and monobutyl phthalate (MPB) exposure (B), although the trend was not significant (MBzP, P = .09; MBP, P = .07). An adjusted odds ratio (AOR) of approximately 2.0 in the fourth quartile doubles the odds of language delay from the first quartile.
aFirst quartile is the reference and was adjusted for creatinine level; sex; premature birth; mother’s educational level; and mother’s smoking status and weight at study enrollment.
Language delay was available for only a subset of live births in both populations. See eTable 2 in the Supplement for a comparison of the populations included in this current study with the children excluded from the study because of missing data. The mothers included in the SELMA study were better educated than the mothers of the children not included (629 [65.3] vs 416 [57.1%]; P = .001). The mothers included in TIDES, compared with the mothers excluded, were less likely to be white or non-Hispanic (274 [74.1%] vs 303 [82.1%]; P = .009) and more likely to have premature birth (38 [10.4%] vs 20 [5.4%]; P = .01).
Discussion
We conducted similar analyses of prenatal phthalate exposure and language delay in the SELMA study and TIDES. The similarity between the 2 studies in the proportion of children with language delay and the higher rate of language delays in boys than girls suggests that the language development instrument used may not be strongly affected by language and culture.
We found a high degree of consistency in associations between prenatal exposure to phthalates and language delay, despite higher phthalate metabolite levels in the SELMA study and the differences between the 2 cohorts in demographics and age at language assessment. In both studies, statistically significant associations with language delay were seen for DBP and BBzP metabolites, and the associations between language delay and any DEHP metabolite were not statistically significant in either study.
Limited epidemiological data are available on prenatal phthalate exposure and cognitive function in offspring. However, the literature supports the associations between prenatal exposure to some phthalates (most consistently for DBP) and increased risk of impaired mental and psychomotor development. A US study of 328 children reported that a child’s full-scale IQ was inversely associated with third-trimester urinary metabolite levels of DBP and diisobutyl phthalate.26 A Polish study reported an inverse correlation between prenatal DBP metabolite levels and cognitive outcomes in 165 children at 2 years of age.27 In addition, prenatal DBP exposure has been shown to be inversely correlated with the Bayley Scales of Infant and Toddler Development (BSID)-II, Mental Developmental Index (MDI), and Psychomotor Developmental Index (PDI) in 460 children in South Korea at 6 months of age36 and in 319 children in the United States at 3 years of age.34 Factor-Litvak et al26 reported that MBzP was associated with impaired perceptual reasoning at age 7 years, and Téllez-Rojo et al35 and Whyatt et al34 reported inverse associations between prenatal MBzP exposure and the BSID-II, the MDI, and the PDI at age 3 years.
To our knowledge, this current study is one of the first to examine the associations between early language development and first-trimester phthalate exposure. We found statistically significant associations between prenatal DBP and BBzP metabolite levels and language delay for boys in both studies, with no statistically significant associations for girls. However, because of the lower prevalence of language delay in girls, our power to examine this association was limited and the results in girls should be considered inconclusive. On the other hand, a 2017 Danish study38 reported that exposure to DEHP and DEP metabolites in the third trimester was associated with decreased language development in boys at 16 to 36 months of age, but no associations were found in girls. However, the Danish study38 differs from our study in several aspects. It measured exposure in different trimesters, its exposure levels were much lower than those in the SELMA study (although similar to those in TIDES), and it used a different outcome measures; language delay as defined in that study was twice as common as in our study, according to Tina Kold Jensen, MD (oral and email communication, May 2018).
Conversely, Téllez-Rojo et al35 assessed the BSID-II, MDI, and PDI and found a negative association between prenatal DEHP metabolite exposure (mono[2-ethylhexyl] phthalate, mono[2-ethyl-5-hydroxyhexyl] phthalate, mono[2-ethyl-5-oxohexyl] phthalate, and mono[2-ethyl-5-carboxypentyl] phthalate) and the MDI in girls and a positive association between prenatal MBzP exposure and the PDI in boys. However, the different outcomes (language delay in our study and BSID-II subscales in most of the studies we referenced here) may not be directly comparable. The PDI includes an assessment of both gross and fine motor skills. The MDI measures not only early language but also sensory perception, knowledge, memory, and problem solving. Language delay at a young age has been found to be indicative of later neurodevelopmental impairment, but different and heterogeneous domains of early impairments may not have the same origin.
Strengths and Limitations
Our study has several strengths and limitations. Both studies provided a unique opportunity for us to replicate the analysis of the association between language delay and prenatal phthalate exposure, which was initially observed in the SELMA study. The use of the same language assessment instrument in both studies is a strength of our analysis.
Furthermore, phthalate metabolite levels were measured in laboratories that had shown comparable results in ongoing quality control round-robin analyses. However, differences were observed in phthalate metabolite levels between the 2 studies (which were generally higher in the SELMA study) and in some measurement methods between the 2 laboratories. In TIDES, 2 DBP metabolites (mono-n-butyl phthalate and monoisobutyl phthalate) were measured, and in the SELMA study, a single DBP metabolite (monobutyl phthalate) was measured, reflecting the sum of these 2 metabolites. We do not believe that the differences in the method used to adjust for urine dilution would explain these differences, given the extensive literature on the comparability of these methods.56 Rather, these differences may likely be explained by the wide use of DBP- and BBzP-containing polyvinyl chloride products in Swedish homes (eg, flooring materials).1,57,58
The SELMA study, with 963 mother–child pairs, had adequate power to detect overall and sex-specific associations, but TIDES, with language delay data for only 370 children, had much more limited power. One potential problem with both studies is the limited number of children with complete information on language delay, which is a potential source for selection bias. In the SELMA study, mothers included in the analysis were better educated; in TIDES, included mothers were less likely to be white or non-Hispanic and more likely to have premature birth. However, premature birth and the mother’s educational level were included as covariates in all analyses in both studies, and race/ethnicity were included in all TIDES analyses.
In the SELMA study, mothers were invited to the clinic for a 30-month visit during which their children’s language development was assessed; however, the precise age at which this assessment was performed is not known. Therefore, in the absence of other information, we assumed the age at assessment to be 30 months. In TIDES, the mean child age at questionnaire completion was 37 months. On the basis of this variable alone, a lower rate of language delay would be expected in TIDES. However, other factors that differed between the SELMA study and TIDES populations (ie, white or non-Hispanic race/ethnicity, premature birth, and mother’s smoking status at enrollment) would indicate higher rates of language delay in children in TIDES. Our findings suggest that these population differences may be balanced out by the difference in age at assessment.
Conclusions
The SELMA study and TIDES results support our conclusion. First-trimester phthalate exposure (particularly to DBP and possibly to BBzP) appears to be associated with poorer language development in children aged 2.5 to 3 years.
eTable 1. Distribution of Concentration (ng/mL) of Urinary Phthalate Metabolites in SELMA and TIDES
eTable 2. Description of the Study Populations in SELMA and TIDES: Analysis Subset Compared to Not Included Population
References
- 1.Carlstedt F, Jönsson BAG, Bornehag CG. PVC flooring is related to human uptake of phthalates in infants. Indoor Air. 2013;23(1):32-39. doi: 10.1111/j.1600-0668.2012.00788.x [DOI] [PubMed] [Google Scholar]
- 2.Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914-920. doi: 10.1289/ehp.1003170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sathyanarayana S, Karr CJ, Lozano P, et al. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121(2):e260-e268. doi: 10.1542/peds.2006-3766 [DOI] [PubMed] [Google Scholar]
- 4.Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res. 2011;55(1):7-31. doi: 10.1002/mnfr.201000121 [DOI] [PubMed] [Google Scholar]
- 5.Bergh C, Luongo G, Wise S, Ostman C. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Anal Bioanal Chem. 2012;402(1):51-59. doi: 10.1007/s00216-011-5440-2 [DOI] [PubMed] [Google Scholar]
- 6.Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ (1994). 2009;43(1):170-181. doi: 10.1016/j.atmosenv.2008.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abb M, Heinrich T, Sorkau E, Lorenz W. Phthalates in house dust. Environ Int. 2009;35(6):965-970. doi: 10.1016/j.envint.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 8.Bornehag CG, Lundgren B, Weschler CJ, Sigsgaard T, Hagerhed-Engman L, Sundell J. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect. 2005;113(10):1399-1404. doi: 10.1289/ehp.7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornehag CG, Sundell J, Weschler CJ, et al. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect. 2004;112(14):1393-1397. doi: 10.1289/ehp.7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langer S, Weschler CJ, Fischer A, Bekö G, Toftum J, Clausen G. Phthalate and PAH concentrations in dust collected from Danish homes and daycare centers. Atmos Environ. 2010;44(19):2294-2301. doi: 10.1016/j.atmosenv.2010.04.001 [DOI] [Google Scholar]
- 11.Cheng Z, Nie X-P, Wang H-S, Wong M-H. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ Int. 2013;57-58:75-80. doi: 10.1016/j.envint.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Shi W, Hu X, Zhang F, et al. Occurrence of thyroid hormone activities in drinking water from eastern China: contributions of phthalate esters. Environ Sci Technol. 2012;46(3):1811-1818. doi: 10.1021/es202625r [DOI] [PubMed] [Google Scholar]
- 13.Langer S, Bekö G, Weschler CJ, et al. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int J Hyg Environ Health. 2014;217(1):78-87. doi: 10.1016/j.ijheh.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 14.Frederiksen H, Jørgensen N, Andersson A-M. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J Anal Toxicol. 2010;34(7):400-410. doi: 10.1093/jat/34.7.400 [DOI] [PubMed] [Google Scholar]
- 15.Wan HT, Leung PY, Zhao YG, Wei X, Wong MH, Wong CK. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. J Hazard Mater. 2013;261:763-769. doi: 10.1016/j.jhazmat.2013.01.034 [DOI] [PubMed] [Google Scholar]
- 16.Fromme H, Gruber L, Seckin E, et al. ; HBMnet . Phthalates and their metabolites in breast milk–results from the Bavarian Monitoring of Breast Milk (BAMBI). Environ Int. 2011;37(4):715-722. doi: 10.1016/j.envint.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 17.Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, et al. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26(1):91-99. doi: 10.1097/EDE.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 18.Gray LE, Foster PM. Significance of experimental studies for assessing adverse effects of endocrine-disrupting chemicals. Pure Appl Chem. 2003;75(11-12):2125-2141. doi: 10.1351/pac200375112125 [DOI] [Google Scholar]
- 19.Swan SH, Sathyanarayana S, Barrett ES, et al. ; TIDES Study Team . First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015;30(4):963-972. doi: 10.1093/humrep/deu363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swan SH, Main KM, Liu F, et al. ; Study for Future Families Research Team . Decrease in anogenital distance among male infants with prenatal phthalate exposure [published correction appears in Environ Health Perspect. 2005;113(9):A583]. Environ Health Perspect. 2005;113(8):1056-1061. doi: 10.1289/ehp.8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bornehag CG, Carlstedt F, Jönsson BA, et al. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect. 2015;123(1):101-107. doi: 10.1289/ehp.1408163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sathyanarayana S, Butts S, Wang C, et al. ; TIDES Team . Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab. 2017;102(6):1870-1878. doi: 10.1210/jc.2016-3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathyanarayana S, Grady R, Barrett ES, et al. First trimester phthalate exposure and male newborn genital anomalies. Environ Res. 2016;151:777-782. doi: 10.1016/j.envres.2016.07.043 [DOI] [PubMed] [Google Scholar]
- 24.Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227(3):185-192. doi: 10.1016/j.tox.2006.07.022 [DOI] [PubMed] [Google Scholar]
- 25.Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res. 2015;142:51-60. doi: 10.1016/j.envres.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 26.Factor-Litvak P, Insel B, Calafat AM, et al. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One. 2014;9(12):e114003. doi: 10.1371/journal.pone.0114003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polanska K, Ligocka D, Sobala W, Hanke W. Phthalate exposure and child development: the Polish mother and child cohort study. Early Hum Dev. 2014;90(9):477-485. doi: 10.1016/j.earlhumdev.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 28.Huang H-B, Chen H-Y, Su P-H, et al. Fetal and childhood exposure to phthalate diesters and cognitive function in children up to 12 years of age: Taiwanese maternal and infant cohort study. PLoS One. 2015;10(6):e0131910. doi: 10.1371/journal.pone.0131910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lien Y-J, Ku H-Y, Su P-H, et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan maternal and infant cohort study. Environ Health Perspect. 2015;123(1):95-100. doi: 10.1289/ehp.1307154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobrosly RW, Evans S, Miodovnik A, et al. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6-10 years of age. Environ Health Perspect. 2014;122(5):521-528. doi: 10.1289/ehp.1307063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33(5):558-566. doi: 10.1016/j.ntt.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118(4):565-571. doi: 10.1289/ehp.0901470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261-267. doi: 10.1016/j.neuro.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whyatt RM, Liu X, Rauh VA, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120(2):290-295. doi: 10.1289/ehp.1103705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Téllez-Rojo MM, Cantoral A, Cantonwine DE, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461-462:386-390. doi: 10.1016/j.scitotenv.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Ha E-H, Kim E-J, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119(10):1495-1500. doi: 10.1289/ehp.1003178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel SM, Zhu C, Berkowitz GS, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522-528. doi: 10.1016/j.neuro.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olesen TS, Bleses D, Andersen HR, et al. Prenatal phthalate exposure and language development in toddlers from the Odense Child Cohort. Neurotoxicol Teratol. 2018;65:34-41. doi: 10.1016/j.ntt.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhuang M, Li T, Shi N. Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J Appl Toxicol. 2009;29(7):603-611. doi: 10.1002/jat.1447 [DOI] [PubMed] [Google Scholar]
- 40.Boberg J, Christiansen S, Axelstad M, et al. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011;31(2):200-209. doi: 10.1016/j.reprotox.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 41.Chen T, Yang W, Li Y, Chen X, Xu S. Mono-(2-ethylhexyl) phthalate impairs neurodevelopment: inhibition of proliferation and promotion of differentiation in PC12 cells. Toxicol Lett. 2011;201(1):34-41. doi: 10.1016/j.toxlet.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 42.Tang J, Yuan Y, Wei C, et al. Neurobehavioral changes induced by di (2-ethylhexyl) phthalate and the protective effects of vitamin E in Kunming mice. Toxicol Res (Camb). 2015. doi: 10.1039/C4TX00250D [DOI] [Google Scholar]
- 43.Ma P, Liu X, Wu J, et al. Cognitive deficits and anxiety induced by diisononyl phthalate in mice and the neuroprotective effects of melatonin. Sci Rep. 2015;5:14676. doi: 10.1038/srep14676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept. 2004;123(1-3):225-234. doi: 10.1016/j.regpep.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 45.Masuo Y, Ishido M, Morita M, Oka S. Effects of neonatal treatment with 6-hydroxydopamine and endocrine disruptors on motor activity and gene expression in rats. Neural Plast. 2004;11(1-2):59-76. doi: 10.1155/NP.2004.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T. Reproductive and neurobehavioural toxicity study of bis(2-ethylhexyl) phthalate (DEHP) administered to mice in the diet. Food Chem Toxicol. 2002;40(10):1499-1506. doi: 10.1016/S0278-6915(02)00073-X [DOI] [PubMed] [Google Scholar]
- 47.Tanaka T. Reproductive and neurobehavioural effects of bis(2-ethylhexyl) phthalate (DEHP) in a cross-mating toxicity study of mice. Food Chem Toxicol. 2005;43(4):581-589. doi: 10.1016/j.fct.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 48.Weiss B. The intersection of neurotoxicology and endocrine disruption. Neurotoxicology. 2012;33(6):1410-1419. doi: 10.1016/j.neuro.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McIntyre LL, Pelham WE III, Kim MH, Dishion TJ, Shaw DS, Wilson MN. A brief measure of language skills at 3 years of age and special education use in middle childhood. J Pediatr. 2017;181:189-194. doi: 10.1016/j.jpeds.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson HD, Nygren P, Walker M, Panoscha R. Screening for speech and language delay in preschool children: systematic evidence review for the US Preventive Services Task Force [published correction appears in Pediatrics. 2006;117(6):2336-2337]. Pediatrics. 2006;117(2):e298-e319. doi: 10.1542/peds.2005-1467 [DOI] [PubMed] [Google Scholar]
- 51.Bornehag CG, Moniruzzaman S, Larsson M, et al. The SELMA study: a birth cohort study in Sweden following more than 2000 mother-child pairs. Paediatr Perinat Epidemiol. 2012;26(5):456-467. doi: 10.1111/j.1365-3016.2012.01314.x [DOI] [PubMed] [Google Scholar]
- 52.Barrett ES, Sathyanarayana S, Janssen S, et al. ; TIDES Study Team . Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;176:119-125. doi: 10.1016/j.ejogrb.2014.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzachi BC, Peake MJ, Ehrhardt V. Reference range and method comparison studies for enzymatic and Jaffé creatinine assays in plasma and serum and early morning urine. Clin Lab. 2000;46(1-2):53-55. [PubMed] [Google Scholar]
- 54.Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106-112. doi: 10.1016/j.jchromb.2007.10.023 [DOI] [PubMed] [Google Scholar]
- 55.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46-51. doi: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- 56.Chadha V, Garg U, Alon US. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol. 2001;16(4):374-382. doi: 10.1007/s004670000551 [DOI] [PubMed] [Google Scholar]
- 57.Larsson M, Hägerhed-Engman L, Kolarik B, et al. PVC–as flooring material–and its association with incident asthma in a Swedish child cohort study. Indoor Air. 2010;20(6):494-501. doi: 10.1111/j.1600-0668.2010.00671.x [DOI] [PubMed] [Google Scholar]
- 58.Shu H, Jönsson BA, Larsson M, Nånberg E, Bornehag CG. PVC flooring at home and development of asthma among young children in Sweden, a 10-year follow-up. Indoor Air. 2014;24(3):227-235. doi: 10.1111/ina.12074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Distribution of Concentration (ng/mL) of Urinary Phthalate Metabolites in SELMA and TIDES
eTable 2. Description of the Study Populations in SELMA and TIDES: Analysis Subset Compared to Not Included Population