This population-based cohort study compares surgical outcomes of Dutch patients diagnosed as having colorectal cancer through the fecal immunochemical test–based screening program (screen-detected) and patients with non–screen-detected colorectal cancer.
Key Points
Question
Do patients referred through the fecal immunochemical test–based colorectal cancer screening program have better surgical outcomes compared with non–screen-detected patients?
Findings
In this population-based cohor study of 18 826 patients, those with screen-detected colon cancer had significantly lower odds on different postoperative complications, even when extensive case-mix adjustment is performed.
Meaning
Future studies on surgical outcomes of colorectal cancer treatment should be aware of the differences between patients detected through the screening program and non–screen-detected patients and consequently take this into account in their comparison models.
Abstract
Importance
The nationwide fecal immunochemical test–based screening program has influenced surgical care for patients with colorectal cancer (CRC) in the Netherlands, although these implications have not been studied in much detail so far.
Objective
To compare surgical outcomes of patients diagnosed as having CRC through the fecal immunochemical test–based screening program (screen detected) and patients with non–screen-detected CRC.
Design, Setting, and Participants
This was a population-based comparative cohort study using the Dutch ColoRectal Audit and analyzed all Dutch hospitals performing CRC resections. Patients who underwent elective resection for CRC between January 2011 to December 2016 were included.
Interventions
Colorectal cancer surgery.
Main Outcomes and Measures
Postoperative nonsurgical complications, postoperative surgical complications, postoperative 30-day or in-hospital mortality, and complicated course (postoperative complication resulting in a hospital stay >14 days and/or a reintervention and/or mortality). A risk-stratified comparison was made for different postoperative outcomes based on screening status (screen detected vs not screen detected), cancer stage (I-IV), and for cancer stage I to III also on age (aged ≤70 years and >70 years) and American Society of Anesthesiologists score (I-II and III-IV). To determine any residual case-mix–corrected differences in outcomes between patients with screen-detected and non–screen-detected cancer, univariable and multivariable logistic regression analyses were performed.
Results
In total, 36 242 patients with colon cancer and 17 416 patients with rectal cancer were included for analysis. Compared with patients with non–screen-detected CRC, screen-detected patients were younger (mean [SD] age, 68 [5] vs 70 [11] years), more often men (3777 [60%] vs 13 506 [57%]), and had lower American Society of Anesthesiologists score (American Society of Anesthesiologists score III+: 838 [13%] vs 5529 [23%]). Patients with stage I to III colon cancer who were screen detected had a significantly lower mortality and complicated course rate compared with non–screen-detected patients. For patients with rectal cancer, only a significant difference was found in mortality rate in patients with a cancer stage IV disease, which was higher in the screen-detected group. Compared with non–screen-detected colon cancer, an independent association was found for screen-detected colon cancer on nonsurgical complications (adjusted odds ratio, 0.81; 95% CI, 0.73-0.91), surgical complications (adjusted odds ratio, 0.80; 95% CI, 0.72-0.89), and complicated course (adjusted odds ratio, 0.80; 95% CI, 0.71-0.90). Screen-detected rectal cancer had significantly higher odds on mortality.
Conclusions and Relevance
Postoperative outcomes were significantly better for patients with colon cancer referred through the fecal immunochemical test–based screening program compared with non–screen-detected patients. These differences were not found in patients with rectal cancer. The outcomes of patients with screen-detected colon cancer were still better after an extensive case-mix correction, implying additional underlying factors favoring patients referred for surgery through the screening program.
Introduction
With an estimated number of 15 800 new cases and 5100 deaths in 2015, colorectal cancer (CRC) is the second most common cause of cancer-related death in the Netherlands.1 To increase CRC-specific survival, organized screening programs have been endorsed by the European Commission.2 A national CRC screening program was introduced in 2014 in the Netherlands. The program is gradually implemented with a complete rollout by 2019. By then, all men and women aged 55 to 75 years will be invited to participate in the program by a biennial fecal immunochemical test (FIT).
Because the FIT has a sensitivity of around 75% for CRC, screening is an iterative process.3 In the Netherlands, participation rates are high compared with other countries4 from 71.3% in 20145 to 73% in 2016.6 Colonoscopy participation after a positive screening FIT was 77.8% in 20145 and 82.8% in 2016.6
To allow a comprehensive appreciation of the CRC screening program targeting a supposedly asymptomatic population, an integrated view of the harms and benefits is necessary, including those of surgical treatment. However, literature on morbidity and mortality after surgical treatment of CRC detected through a screening program is limited.7
The primary aim of this study was to examine whether patients undergoing surgery for CRC following diagnosis through the FIT-based screening program have different surgical outcomes compared with nonscreening patients and to what extent an extensive case-mix correction can adjust for any differences found. In addition, an overview is given of patient, tumor, and treatment characteristics of the surgically treated screen-detected CRCs in the Netherlands, based on the data registered in the Dutch ColoRectal Audit (DCRA).
Methods
Data from the DCRA, formerly known as the Dutch Surgical Colorectal Audit (ie, DSCA), were extracted for this study.8 In this nationwide and disease-specific audit, data on various patient, tumor, treatment, and short-term (30-day) outcome characteristics are collected of every patient undergoing a resection for primary CRC in the Netherlands.
Patient Selection
The DCRA is an obligatory audit from the inspectorate of health care, which required no informed consent from patients for data collection. Data analyses were performed on an anonymized dataset and do not need ethical approval according to Dutch law. Eligibility criteria required patients to have undergone surgical treatment for primary CRC between January 1, 2011, and December 31, 2016, and be registered in the DCRA before March 31, 2017 (n = 63 370). Minimal data requirements were information on tumor location, date of surgery, and 30-day or in-hospital mortality (n = 63 136). For the objective of this study, only patients in whom the surgery took place in an elective setting were selected (n = 55 531). Furthermore, the heterogenous group of patients with multiple synchronous colorectal tumors (n = 1873) were excluded.9 This resulted in 53 658 patients eligible for analyses. For trend analysis, all patients (2011-2016) were selected (eFigure in the Supplement). For the comparison of the outcomes of screen-detected vs non–screen-detected patients, all patients were selected who underwent surgery since the start of the nationwide CRC screening program in 2014.
Data
The following data were retrospectively extracted from the DCRA database: patient characteristics, disease characteristics, (pre)procedural characteristics, postoperative outcomes within 30 days after resection or in hospital, and whether the patients were referred through the screening program. Invited birth cohorts for the screening program in the 3 years were 1938 to 1941, 1945 to 1955, and 1957. Only patients who were referred through the screenings program after a positive FIT and were diagnosed as having a CRC that was surgically resected were marked as screen-detected CRC. All missing values were 10% or less and no imputation was conducted (eTable 1 and eTable 2 in the Supplement).
Outcome Parameters
Outcome parameters were nonsurgical postoperative complications (pulmonary, cardiac, thromboembolic, infectious, neurologic, other), surgical postoperative complications, complicated course (postoperative complication leading to a hospital stay of >14 days and/or a reintervention and/or mortality), and postoperative mortality (≤30 days or in hospital during the same admission).
Data Analysis
Colon and rectal cancer were analyzed separately. To evaluate trends over time and the impact of the implementation of the nationwide screening program on the DCRA, data on complicated course and mortality were evaluated for all included patients, according to year of registration. Differences in baseline characteristics were compared between non–screen-detected patients during 2011 to 2013 and 2014 to 2016 and between screen-detected and non–screen-detected patients during 2014 to 2016.
Patients registered between 2014 to 2016 were stratified into homogenous subgroups based on known risk factors (age, American Society of Anesthesiologists [ASA] classification, cancer stage), and differences in outcomes (complicated course and mortality) of screen-detected vs non–screen-detected patients were assessed.
Absolute risk differences with corresponding 95% CIs were compared between screen-detected and non–screen-detected patients. Differences in categorical variables were analyzed using a χ2 test and for nonnormally distributed continuous variables (eg, length of stay), a nonparametric Mann-Whitney U test was used. To evaluate differences in outcomes between screen-detected and non–screen-detected patients from 2014 to 2016, univariable and multivariable logistic regression analyses were performed, and the results were expressed as odds ratios with corresponding 95% CIs. To adjust for differences in case mix, factors included in the multivariable analysis consisted of age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), ASA score, Charlson comorbidity score, any tumor-related complication, previous abdominal surgery (not further specified), pathological (p)T-classification, presence of metastasis, additional resection due to tumor invasion, and additional resection due to metastasis. For colon cancer, the location of the tumor within the colon (cecum, appendix, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid) was added to the case mix. For case-mix correction in rectal cancer, tumor distance from the anal verge, clinical (c)T-classification, preoperative radiotherapy, and surgical procedure (low anterior resection, abdominoperineal resection, or other procedure) were added to the model. Preoperative radiotherapy was categorized as no radiotherapy, short-course radiotherapy with immediate (≤3 week) surgery, short-course radiotherapy with delayed (>3 week) surgery, or chemoradiotherapy/long-course radiotherapy. A P value less than .05 was considered statistically significant. SPSS 24.0 Statistics for Windows (IBM Corp) was used for all analyses.
Results
Baseline Characteristics
In total, 36 242 patients with colon cancer and 17 416 patients with rectal cancer were included for analysis. Table 1 provides a comprehensive overview of patient and tumor characteristics of 23 508 patients prior to the start of the screening program (2011-2013) and for 23 872 non–screen-detected and 6278 screen-detected patients since the start of the screening program (2014-2016). Of all patients undergoing surgery for CRC since the moment of introduction of the screening program, 4696 patients (22.8%) with colon cancer and 1582 patients (16.6%) with rectal cancer were screen detected, respectively.
Table 1. Patient and Tumor Characteristics of Non–Screen-Detected and Screen-Detected Colorectal Cancera,b.
| Characteristic | Colon | Rectum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not Screen Detected, No. (%) | P Value: Not Screen Detected 2014-2016 vs 2011-2013 | Screen Detected, 2014-2016, No. (%) | P Value: Screen Detected (2014-2016) vs Not Screen Detected (2014-2016) | Not Screen Detected, No. (%) | P Value: Not Screen Detected 2014-2016 vs 2011-2013 | Screen Detected, 2014-2016, No. (%) | P Value: Screen Detected (2014-2016) vs Not Screen Detected (2014-2016) | |||
| 2011-2013 | 2014-2016 | 2011-2013 | 2014-2016 | |||||||
| Total patients, No. | 15 610 | 15 936 | NA | 4696 | NA | 7898 | 7936 | NA | 1582 | NA |
| Age, y | ||||||||||
| ≤60 | 2625 (17) | 2678 (17) | .96 | 160 (3) | <.001c | 2025 (26) | 2040 (26) | .64 | 48 (3) | <.001c |
| 61-70 | 4572 (29) | 4621 (29) | 3009 (64) | 2693 (34) | 2667 (34) | 1068 (68) | ||||
| 71-80 | 5452 (34) | 5596 (35) | 1527 (33) | 2335 (30) | 2326 (29) | 466 (30) | ||||
| ≥81 | 2957 (19) | 3029 (19) | 0 | 843 (11) | 895 (11) | 0 | ||||
| Men | 8227 (53) | 8464 (53) | .44 | 2706 (58) | <.001 | 4928 (62) | 5042 (64) | .12 | 1071 (68) | .002 |
| American Society of Anesthesiologists score III+d | 3653 (23) | 4120 (26) | <.001 | 638 (14) | <.001 | 1309 (17) | 1409 (18) | .05 | 200 (13) | <.001 |
| Charlson score 3+d | 1857 (12) | 2332 (15) | <.001 | 362 (8) | <.001 | 687 (9) | 758 (10) | .001 | 112 (7) | .003 |
| Body mass index, ≥30d,e | 2547 (16) | 2959 (19) | <.001 | 1175 (25) | <.001 | 1193 (15) | 1351 (17) | .01 | 332 (21) | <.001 |
| Previous abdominal surgery | 5597 (36) | 5788 (36) | .42 | 1432 (31) | <.001 | 2427 (31) | 2426 (31) | .80 | 395 (25) | <.001 |
| Location of tumor | ||||||||||
| Ascending colon up to and including hepatic flexure | 7217 (46) | 7370 (46) | .24 | 1523 (32) | <.001 | NA | NA | NA | NA | NA |
| Transverse colon up to and including splenic flexure | 1487 (10) | 1592 (10) | 494 (11) | NA | NA | NA | NA | NA | ||
| Descending colon | 869 (6) | 935 (6) | 346 (7) | NA | NA | NA | NA | NA | ||
| Sigmoid colon | 6037 (39) | 6039 (38) | 2333 (50) | NA | NA | NA | NA | NA | ||
| Distance from anal verge, cm | ||||||||||
| ≤5 | NA | NA | NA | NA | NA | 2849 (38) | 2971 (38) | .02 | 436 (28) | <.001 |
| 6-10 | NA | NA | NA | 3008 (40) | 3027 (39) | 627 (40) | ||||
| >10 | NA | NA | NA | 1576 (21) | 1789 (23) | 501 (32) | ||||
| Preoperative tumor complications | 5128 (33) | 5105 (32) | .06 | 197 (4) | <.001 | 2010 (26) | 1636 (21) | <.001 | 66 (4) | <.001 |
| cT stage | ||||||||||
| cT1 | NA | NA | NA | NA | NA | 318 (4) | 411 (5) | <.001 | 233 (15) | <.001 |
| cT2 | NA | NA | NA | NA | NA | 1826 (24) | 1835 (23) | 541 (34) | ||
| cT3 | NA | NA | NA | NA | NA | 4471 (58) | 4617 (58) | 690 (44) | ||
| cT4 | NA | NA | NA | NA | NA | 674 (9) | 818 (10) | 42 (3) | ||
| cTX/unknown | NA | NA | NA | NA | NA | 439 (6) | 253 (3) | 75 (5) | ||
| pT stage | ||||||||||
| (y)pT0-1 | 1409 (9) | 1646 (10) | <.001 | 1211 (26) | <.001 | 1469 (19) | 1619 (21) | .02 | 500 (32) | <.001 |
| (y)pT2 | 2768 (18) | 2807 (18) | 1184 (25) | 2463 (31) | 2374 (30) | 555 (35) | ||||
| (y)pT3 | 9205 (59) | 9018 (57) | 2009 (43) | 3606 (46) | 3560 (45) | 486 (31) | ||||
| (y)pT4 | 2144 (14) | 2422 (15) | 287 (6) | 323 (4) | 343 (4) | 24 (2) | ||||
| M-stage tumor | ||||||||||
| M0 | 13970 (89) | 14287 (90) | .65 | 4489 (96) | <.001 | 7255 (92) | 7281 (92) | .80 | 1544 (98) | <.001 |
| M1 | 1640 (11) | 1649 (10) | 207 (4) | 643 (8) | 655 (8) | 38 (2) | ||||
| Cancer staged | ||||||||||
| I | 3207 (21) | 3518 (22) | <.001 | 1847 (39) | <.001 | 1410 (18) | 1639 (21) | .001 | 644 (41) | <.001 |
| II | 5707 (37) | 5701 (36) | 1209 (26) | 1469 (19) | 1500 (19) | 293 (19) | ||||
| III | 4766 (31) | 5024 (32) | 1372 (29) | 3622 (46) | 3911 (49) | 525 (33) | ||||
| IV | 1617 (10) | 1570 (10) | 191 (4) | 530 (7) | 556 (7) | 28 (2) | ||||
| 0/X | 313 (1) | 123 (1) | 77 (1) | 867 (11) | 330 (4) | 92 (6) | ||||
Abbreviations: cT, clinical tumor; NA, not applicable; pT, pathologic tumor.
Missing per category are reported in eTable 1 in the Supplement. All variables had 10% or less missing values.
χ2 Test was used for all categorical variables.
Analysis by χ2 was done for different subgroups than shown in this Table (because of low number [<5] of cases in ≥1 subcategory) for age (≤70 vs >70 years).
Pathologic stage was used for colon cancer, and clinical stage was used for rectum. Stage 0 to X includes stage 0 or stage X (unknown or not judgeable).
Calculated as weight in kilograms divided by height in meters squared.
Compared with the patients with colon cancer diagnosed before the start of the screening program (2011-2013), the non–screen-detected patients between 2014 and 2016 had a higher ASA score, BMI, and Charlson score. For patients with rectal cancer, only BMI and Charlson score were significantly different. Comparing non–screen-detected patients with screen-detected patients between 2014 to 2016, almost all patient and tumor characteristics differed significantly. This was also found for the different workup and surgery characteristics and length of stay (Table 2). For patients with rectal cancer, no significant differences were found between non–screen-detected patients compared with screen-detected patients for the proportion of patients being discussed in a multidisciplinary team meeting and the proportion of patients being converted after an initial laparoscopic approach.
Table 2. Workup and Surgery Characteristics and Length of Stay of Non–Screen-Detected and Screen-Detected Colorectal Cancera,b.
| Characteristic | Colon | Rectum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Not Screen Detected, No. (%) | P Value: Not Screen Detected 2014-2016 vs 2011-2013 | Screen Detected, 2014-2016, No. (%) | P Value: Screen Detected (2014-2016) vs Not Screen Detected(2014-2016) | Not Screen Detected, No. (%) | P Value: Not Screen Detected 2014-2016 vs 2011-2013 | Screen Detected, 2014-2016, No. (%) | P Value: Screen Detected (2014-2016) vs Not Screen Detected (2014-2016) | |||
| 2011-2013 | 2014-2016 | 2011-2013 | 2014-2016 | |||||||
| Total patients, No. | 15 610 | 15 936 | NA | 4696 | NA | 7898 | 7936 | NA | 1582 | NA |
| Workup | ||||||||||
| Entire visualization of colon | 12202 (79) | 13221 (83) | <.001 | 4354 (93) | <.001 | 6707 (86) | 6864 (87) | .11 | 1494 (95) | <.001 |
| Discussed in MDT | 13386 (87) | 15053 (95) | <.001 | 4537 (97) | <.001 | 7715 (98) | 7828 (99) | .001 | 1563 (99) | .65 |
| Neoadjuvant chemotherapy | 308 (2) | 374 (2) | .02 | 27 (0.6) | <.001 | NA | NA | NA | NA | NA |
| Neoadjuvant radiotherapyc | ||||||||||
| No | 15481 (99) | 15850 (100) | .02 | 4684 (100) | .01 | 1401 (18) | 2926 (37) | <.001 | 1005 (64) | <.001 |
| SCRT-IS | 37 (0.2) | 17 (0.1) | 5 (0.1) | 2924 (37) | 1354 (17) | 286 (18) | ||||
| SCRT-DS | 77 (0.5) | 65 (0.4) | 7 (0.1) | 528 (7) | 769 (10) | 46 (3) | ||||
| CRT/long course | 15 (0.1) | 4 (0) | 0 | 3045 (39) | 2887 (36) | 245 (16) | ||||
| Procedure | ||||||||||
| Ileocecal resection | 169 (1) | 101 (0.6) | <.001 | 12 (0.3) | <.001 | NA | NA | <.001 | NA | <.001 |
| Right hemicolectomy | 7251 (48) | 7713 (49) | 1656 (36) | NA | NA | NA | ||||
| Transversectomy | 404 (3) | 359 (2) | 83 (2) | NA | NA | NA | ||||
| Left hemicolectomy | 1588 (10) | 1606 (10) | 623 (13) | NA | NA | NA | ||||
| Sigmoid resection | 5815 (38) | 5834 (37) | 2271 (49) | NA | NA | NA | ||||
| (Low) anterior resection | NA | NA | NA | 5197 (66) | 5214 (66) | 1148 (73) | ||||
| Abdominoperineal resection | NA | NA | NA | 2289 (29) | 2165 (27) | 214 (14) | ||||
| Other | NA | NA | NA | 353 (5) | 511 (7) | 213 (14) | ||||
| Surgical approach | ||||||||||
| Open | 6849 (44) | 3732 (24) | <.001 | 527 (11) | <.001 | 3365 (43) | 1450 (18) | <.001 | 136 (9) | <.001 |
| Laparoscopic | 8735 (56) | 12142 (76) | 4150 (89) | 4278 (54) | 6034 (76) | 1247 (79) | ||||
| Otherd | 11 (0) | 9 (0) | 6 (0) | 249 (3.2) | 433 (6) | 196 (12) | ||||
| No laparoscopic conversion | 7184 (86) | 10454 (88) | .004 | 3719 (92) | <.001 | 3499 (86) | 5236 (91) | <.001 | 1087 (92) | .230 |
| Additional resection due to tumor invasion | ||||||||||
| No | 14107 (90) | 14441 (91) | .74 | 4589 (98) | <.001 | 7380 (93) | 7283 (92) | <.001 | 1551 (98) | <.001 |
| Yes, limited | 859 (6) | 860 (5) | 66 (1) | 240 (3) | 317 (4) | 22 (1) | ||||
| Yes, extensive | 644 (4) | 635 (4) | 41 (0.9) | 278 (4) | 336 (4) | 9 (0.6) | ||||
| Additional resection due to metastasis | 585 (4) | 661 (4) | .07 | 83 (2) | <.001 | 226 (3) | 253 (3) | .23 | 11 (0.7) | <.001 |
| Stomac | ||||||||||
| No | 13947 (90) | 14572 (92) | <.001 | 4534 (97) | <.001 | 1316 (17) | 2066 (27) | <.001 | 609 (43) | <.001 |
| End colostomy | 778 (5) | 754 (5) | 56 (1) | 3442 (45) | 3065 (41) | 331 (23) | ||||
| Other | 739 (5) | 562 (4) | 90 (2) | 2864 (38) | 2422 (32) | 473 (34) | ||||
| Unknown | 16 (0.1) | 13 (0.1) | 3 (0.1) | 9 (0.1) | 5 (0.1) | 0 (0) | ||||
| Completeness of Resection | ||||||||||
| Radical resectionc | ||||||||||
| R0 | 14944 (98) | 15620 (98) | <.001 | 4658 (100) | <.001 | 7273 (96) | 7199 (95) | .03 | 1380 (98) | <.001 |
| R1 | 258 (2) | 215 (1) | 21 (0.4) | 266 (4) | 335 (4) | 31 (2) | ||||
| R2 | 121 (0.8) | 42 (0.3) | 3 (0.1) | 27 (0.4) | 11 (0.1) | 0 (0) | ||||
| Circumferential margin, positive (≤1 mm) | NA | NA | NA | NA | NA | 464 (7) | 406 (5) | .006 | 37 (2) | <.001 |
| Median lymphe nodes removed, median (IQR) | 15 (12-21) | 18 (13-24) | <.001f | 16 (12-22) | <.001f | 12 (9-17) | 15 (11-20) | <.001f | 15 (11-19) | <.001f |
| Positive lymph node ratio, %c | 9.0 | 7.8 | <.001 | 5.7 | <.001 | 8.6 | 6.8 | <.001 | 4.9 | <.001 |
| Length of stay, median (IQR), d | 6 (5-10) | 6 (4-9) | <.001f | 5 (4-7) | <.001f | 8 (6-13) | 7 (5-11) | <.001f | 5 (4-9) | <.001f |
Abbreviations: CRT, chemoradiotherapy; IQR, interquartile range; MDT, multidisciplinary team meeting; NA, not applicable; SCRT-DS, short-course radiotherapy with delayed surgery; SCRT-IS, short-course radiotherapy with immediate surgery.
Missing per category are reported in eTable 2 in the Supplement. All missing were 10% or less.
χ2 Test was used for all categorical variables.
Analysis by χ2 was done for different subgroups than shown in this Table (because of low number [<5] of cases in 1 or more subcategory) for neoadjuvant radiotherapy (categorized into yes vs no neoadjuvant radiotherapy), stoma (unknown was excluded for analysis), and radical resection (R0 vs R1-2).
Other surgical approach (eg, local excision, transanal endoscopic microsurgery, single-port transanal surgery).
Excluded for rectum were the local excisions (total patients analyzed: non–screen-detected rectum, 2011 to 2013, n = 7652; 2014 to 2016, n = 7565; and screen-detected rectum, 2014-2016, n = 1415).
Mann-Whitney U test.
Adverse Outcome Over Time
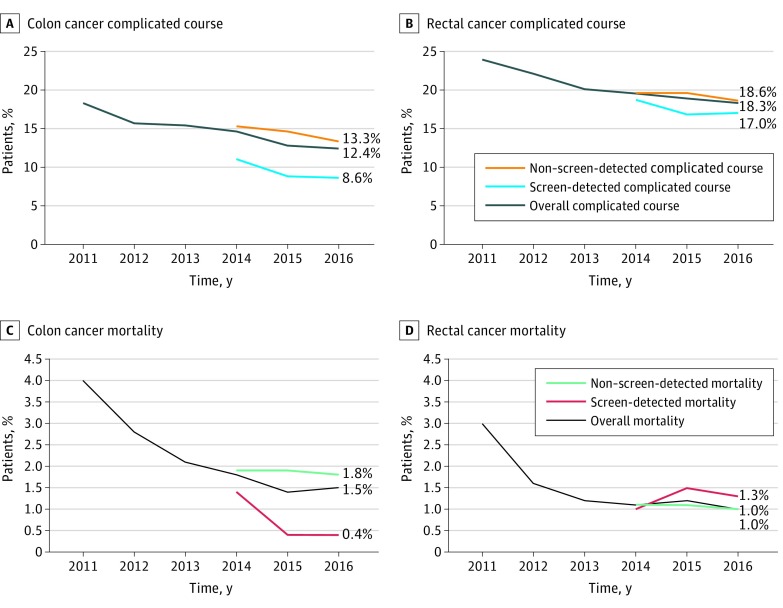
Figure 1 shows the crude trend of complicated course and mortality of patients with primary CRC between 2011 and 2016 for colon (Figure 1A) and rectal cancer (Figure 1B). Patients with colon cancer diagnosed through the screening program had a complicated course rate ranging from 11% (2014) to 8.6% (2016) and a mortality rate declining from 1.4% (2014) to 0.4% (2015 and 2016). In the same time (2014-2016), complicated course for patients with non–screen-detected CRC ranged from 15.3% (2014) to 13.3% (2016) and mortality from 1.9% (2014) to 1.8% (2016). Both postoperative complicated course (screen detected: 434 [9.2%] and not screen detected: 2293 [14.4%]; P < .001) and mortality (screen detected: 30 [0.6%] and not screen detected: 295 [1.9%]; P < .001) differed significantly between patients with screen-detected and non–screen-detected colon cancer undergoing surgery between 2014 and 2016.
Figure 1. Trends of Postoperative Adverse Outcomes for Non–Screen-Detected, Screen-Detected, and Overall Colorectal Cancer.
Trends of different outcomes (complicated course and mortality), separately shown for colon and rectal cancer. From 2014 and on, the outcomes are shown separately for 3 subgroups: (1) overall (all patients), (2) non–screen-detected patients, and (3) screen-detected patients.
For patients with rectal cancer diagnosed through the screening program, postoperative complication rate ranged from 18.7% (2014) to 16.8% (2015), and mortality rate ranged from 1.5% (2015) to 1.0% (2014). For patients with non–screen-detected rectal cancer, this postoperative complication rate varied from 29.6% (2014 and 2015) to 18.6% (2016) and mortality rate declined from 1.1% in 2014 and 2015 to 1.0% in 2016. For patients with rectal cancer, no significant differences were found for complicated course (screen detected: 266 [17.2%] and not screen detected: 1511 [19.2%]; P = .06) and mortality (screen detected: 19 [1.2%] and not screen detected: 81 [1.1%]; P = .33) between screen-detected and non–screen-detected patients during 2014 to 2016.
Stratified Comparison of Screen-Detected vs Non–Screen-Detected CRC
In Figure 2, patients with screen-detected and non–screen-detected CRC are compared regarding complicated course and mortality. Patients diagnosed as having colon cancer through the screening program had a significantly lower postoperative complication rate and mortality compared with non–screen-detected patients for stage I to III, with a similar (nonsignificant) result for stage IV (Figure 2A).
Figure 2. Risk-Stratified Comparison of Postoperative Adverse Outcomes for Non–Screen-Detected and Screen-Detected Colorectal Cancer.
Risk-stratified comparison on outcomes (complicated course and mortality) between screen-detected and non–screen-detected patients for colon and rectal cancer separately. A, Colon cancer, differences in outcomes for pathologic (p) tumor stage I to IV (and other) between screening and nonscreening patients. B, Rectal cancer, differences in outcomes for clinical (c) tumor stage I to IV (and other) between screening and nonscreening patients. C, Colon cancer, differences in outcomes of patients with tumor stage I to III (pT1-3N0-2M0) stratified on age (≤70 y vs >70 y) and American Society of Anesthesiologists (ASA) score (I-II vs III-IV). D, Rectal cancer, differences in outcomes of patients with tumor stage I to III (cT1-3N0-2M0) stratified on age (≤70 y vs >70 y) and ASA score (I-II vs III-IV). Missing values in Figure 2C not screen detected, n = 14; screen detected, n = 1. Missing values in Figure 2D not screen detected, n = 9; screen detected, n = 0.
aSignificant difference (χ2) between screen-detected and non–screen-detected patients.
For patients with rectal cancer, higher stage was associated with an increase in complication rate in screen-detected patients, and this was more pronounced compared with non–screen-detected patients (Figure 2B). No significant differences of complication rates between screen-detected and non–screen-detected patients were found for each of the cancer stages. Similar mortality rates were found for stage I to III, with a significantly higher mortality rate after resection of screen-detected compared with non–screen-detected stage IV rectal cancer.
In Figure 2C, complicated course and mortality are shown for stage I to III colon cancer with a stratified comparison based on operative risk using age (≤70 years and >70 years) and ASA score (I-II and III-IV). Lower complication and mortality rates in the screen-detected compared with non–screen-detected populations were observed for any of the operative risk groups except for mortality in young and fit patients (≤70 years with ASA score I-II). These effects reached statistical significance for complicated course in all risk groups, except for patients older than 70 years with ASA score III to IV. For patients with rectal cancer, none of the stratified risk groups revealed a significant difference in complicated course or mortality (Figure 2D). A nonsignificant but noteworthy trend was found toward a higher risk of complicated course and mortality after resection of screen-detected rectal cancer in frail elderly patients (age >70 years with ASA score III-IV).
Case Mix–Adjusted Comparison of Screen-Detected vs Non–Screen-Detected CRC
For colon cancer, surgery of screen-detected patients was independently associated with lower odds on nonsurgical complications (adjusted odds ratio [AOR], 0.81; 95% CI, 0.73-0.91), surgical complications (AOR, 0.80; 95% CI, 0.72-0.89), and complicated course (AOR, 0.80; 95% CI, 0.71-0.90) compared with surgery for patients with colon cancer that were not screen detected (Table 3). Whether colon cancer was detected through screening was not associated with mortality in multivariable analysis. Referral through the screening program was not independently associated with any postoperative complication after rectal cancer surgery. However, surgery in patients with screen-detected rectal cancer was associated with a significantly higher risk of mortality compared with patients with non–screen-detected rectal cancer (AOR, 2.27; 95% CI, 1.31-3.96).
Table 3. Differences in Postoperative Outcomes Between Non–Screen-Detected and Screen-Detected Colorectal Cancera.
| Operation Year 2014-2016 | No. (%) | Absolute Risk Reduction, % (95% CI) | Univariable vs Multivariableb | Screen Detected vs Not Screen Detected Odds Ratio (95% CI) | |
|---|---|---|---|---|---|
| Screen Detected | Not Screen Detected | ||||
| Colonc,d | |||||
| Total No. | 4696 | 15 936 | NA | NA | NA |
| Nonsurgical postoperative complication | 555 (11.8) | 2941 (18.5) | 6.7 (5.6-7.8) | Univariable | 0.59 (0.54-0.65)e |
| Multivariable | 0.81 (0.73-0.91)e | ||||
| Surgical postoperative complication | 563 (12.0) | 2714 (17.0) | 5.0 (3.9-6.1) | Univariable | 0.66 (0.60-0.73)e |
| Multivariable | 0.80 (0.72-0.89)e | ||||
| Complicated course | 434 (9.2) | 2293 (14.4) | 5.2 (4.2-6.2) | Univariable | 0.61 (0.54-0.68)e |
| Multivariable | 0.80 (0.71-0.90)e | ||||
| Mortality | 30 (0.6) | 295 (1.9) | 1.3 (1.0-1.6) | Univariable | 0.34 (0.23-0.50)e |
| Multivariable | 0.74 (0.49-1.12) | ||||
| Rectumc,f | |||||
| Total No. | 1582 | 7936 | NA | NA | NA |
| Nonsurgical postoperative complication | 293 (18.5) | 1733 (21.8) | 3.3 (1.1-5.4) | Univariable | 0.81 (0.71-0.93)e |
| Multivariable | 0.99 (0.85-1.15) | ||||
| Surgical postoperative complication | 323 (20.4) | 1837 (23.1) | 2.7 (0.4-4.8) | Univariable | 0.85 (0.75-0.97)e |
| Multivariable | 0.99 (0.86-1.15) | ||||
| Complicated course | 266 (17.2) | 1511 (19.2) | 2.0 (−0.1 to 4.0) | Univariable | 0.93 (0.80-1.07) |
| Multivariable | 1.03 (0.88-1.21) | ||||
| Mortality | 19 (1.2) | 81 (1.0) | −0.2 (−0.9 to 0.2) | Univariable | 1.27 (0.79-2.06) |
| Multivariable | 2.27 (1.31-3.96)e | ||||
Abbreviation: NA, not applicable.
Univariable and multivariable analysis for the odds on different preoperative and postoperative outcomes for 2014 to 2016 for screen-detected vs non–screen-detected patients undergoing surgery for primary colorectal cancer.
Frequency of missing values in multivariable analysis colon: 49 (0.2%) (missing: sex, n = 10; age, n = 12; American Society of Anesthesiologists score, n = 7; previous abdominal surgery, n = 21). Frequency of missing values rectum: 191 (2%) (missing: sex, n = 8; age, n = 8; American Society of Anesthesiologists score, n = 2; tumor distance from anal verge, n = 167).
The following factors were included in the multivariable model to correct for differences in case mix between patients: age, sex, body mass index, American Society of Anesthesiologists score, Charlson comorbidity score, any tumor-related complication, previous abdominal surgery, pathologic tumor classification, presence of metastasis, additional resection due to tumor invasion, and additional resection due to metastasis.
Added for the colon: location of tumor within colon.
Significant values.
Added for the rectum: received radiotherapy (no short-course radiotherapy with immediate surgery, short-course radiotherapy with delayed surgery, or chemoradiation/long-course radiotherapy), procedure (lower anterior resection, abdominal perineal resection, or different), clinical tumor classification, and tumor distance from anal verge.
Discussion
Surgery for screen-detected colon cancer was associated with better postoperative outcomes compared with non–screen-detected patients, even when an extensive case-mix adjustment was applied. This was not observed for rectal cancer. Most patient, tumor, and surgical treatment characteristics of the group of screen-detected CRC were significantly different compared with the group of non–screen-detected CRC in the same period. Besides a shift toward lower stages, patients with screen-detected cancers had fewer preoperative tumor-related complications such as bleeding or ileus. American Society of Anesthesiologists and Charlson scores were also more favorable in patients with screen-detected CRC, although more pronounced in colon cancer than in rectal cancer. However, significantly more patients with screen-detected CRC had a BMI more than 30. Also in line with expectations, treatment differed between the screen-detected and non–screen-detected group with less need for preoperative radiotherapy, more laparoscopic procedures, fewer stomas, less extensive resections for local ingrowth, and fewer simultaneous resections of metastases in the patients with screen-detected tumors.
The question remains whether extensive case-mix correction can sufficiently adjust for differences between characteristics of screen-detected and non–screen-detected patients, or if the variable screening represents factors that are unmeasured or unadjusted for. However, despite extensive case-mix correction, we still observed significant differences in outcomes of screen-detected compared with non–screen-detected patients for colon cancer. Therefore, one might consider adding screening as a variable in future case-mix models.
For patients with rectal cancer, screening did not reveal any statistical association for postoperative complications in the multivariable model. Although the case-mix–adjusted odds ratio on postoperative mortality was surprisingly higher in patients with screen-detected rectal cancer, an important remark has to be made interpreting this finding. Owing to the low event rate of mortality (n = 100) relative to the df used in the model (df = 29), the model could be less stable, thereby possibly affecting the reliability of the outcome. Also, there might be a chance of a type I statistical error in this analysis since we do not have a plausible explanation for this finding. This aside, analysis of the stratified subgroup did reveal a few additional events among the frail elderly patients and stage IV screen-detected rectal cancer. Stage IV screen-detected cancer may consist of a specific category of patients, with either aggressive tumor biology or relatively small asymptomatic primaries that eventually will develop metastases at an asymptomatic stage or patients who neglect initial symptoms and retrospectively should have been diagnosed earlier.
It is generally agreed that screening will eventually result in earlier stage at diagnosis and that this is associated with a better prognosis.10,11,12,13 However, the impact of fecal occult blood tests screening on a surgical CRC audit is less clear with several potential influences. First, earlier cancer stage will enable more nonsurgical treatment using endoscopic removal (with or without laparoscopic assistance), and these patients are not included in the DCRA. Second, more patients might be candidates for minimally invasive procedures, such as laparoscopic surgery or local excision, with a positive impact on postoperative outcomes.14,15 Third, screening will diagnose a group of patients at an earlier cancer stage, which is oncologically relevant, but will not have a significant impact on short-term morbidity and mortality in the DCRA. For example, a shift from T1-3N1M0 (stage III) to T1-3N0M0 (stage II) colon cancer will reduce the need for adjuvant chemotherapy and is associated with better long-term survival, but the type of surgery (segmental colonic resection) remains identical and there might not be any benefit visible in the DCRA for the in-hospital/30-day period. Finally, a (possibly small) negative effect on the overall outcomes in the DCRA could even exist if patients with locally advanced or metastatic tumors are diagnosed somewhat earlier by screening, making them eligible for resection, while they would otherwise have been treated by systemic or supportive therapy and therefore would not be registered in the DCRA.
Amri et al16 compared long-term outcomes in colon cancer surgery of non–screen-detected patients with screen-detected patients but with the important difference that screen-detected patients were referred through screening colonoscopy. They found patients with screen-detected colon cancer to have better outcomes independent of their cancer stage. A possible contributing factor for this observation, also observed by Saraste et al,17 is that patients in the screening program had a more extensive workup with optimized preoperative multidisciplinary team meeting discussion and preoperative visualization of the entire colon. Tumor biology may also be different in screen-detected cancers,18,19 such as the speed of tumor growth, tissue invasiveness, and the ease of the tumor of causing symptoms (eg, bleeding). Additionally, healthy user bias might play a role. For example, it is known that people with a low socioeconomic status are less likely to participate in a CRC screening program20,21,22,23 but have a higher risk of developing CRC and more coexisting morbidities compared with people with a high socioeconomic status.24 The present data and the study by Amri et al16 suggest that screen-detected colon cancer represents a different population of patients undergoing surgical resection. In the transition phase toward a fully implemented colorectal screening program, this might have implications for benchmarking surgical outcomes, possibly urging us to add screening to the case-mix model.
For rectal cancer, outcomes between screen-detected and non–screen-detected patients did not differ. One of the potential explanations might be that rectal cancer is becoming symptomatic at a relatively early stage compared with colon cancer, which reduces the differences between screen-detected and non–screen-detected cancers.
Limitations
Besides the strength of the present study, such as the usage of population-based data, which reflect daily practice and the large numbers of patients, several limitations have to be taken into account. A certain extent of missing data are unavoidable in population-based studies. As also mentioned before, one might argue that some potential contributing factors to the difference observed were not included in the case-mix correction, such as substance abuse (eg, smoking), nutritional status prior to surgery, or other (unknown) factors. Moreover, stage distributions might also change over time independent of the screening program, making the current findings less consistent over time. Also, this study lacks information on people not participating in the screening program, in whom the FIT was false negative, or people not receiving a colonoscopy after a positive FIT owing to patient preferences. In addition, some patients with screen-detected cancers do not undergo surgical resection. These patients may undergo endoscopic removal of low-risk T1 tumors, be unfit for surgery, or have irresectable disease. Finally, although impossible to prove or quantify, the start of the screening may have already affected characteristics of the non–screen-detected CRC population through earlier identification and the creation of more awareness about the disease.
Conclusions
From a surgical perspective, patients diagnosed as having a CRC detected through the national FIT-based CRC screening program represent a different population. Surgery for screen-detected colon cancer was associated with better postoperative outcomes compared with non–screen-detected patients, even when an extensive case-mix adjustment was applied. Future studies on surgical outcomes of CRC treatment should be aware of these differences and consequently take this into account in their comparison models.
eFigure. Flowchart of the study patient selection
eTable 1. Missing values of Table 1 per category
eTable 2. Missing values of Table 2 per category
References
- 1.Nederland Kankerregistratie. Cijfers over Kanker. https://www.cijfersoverkanker.nl/. Accessed August 20, 2018.
- 2.Council of the European Union Council recommendation of 2 December 2003 on cancer screening. Off J Eur Union. 2003:-. [Google Scholar]
- 3.van der Vlugt M, Grobbee EJ, Bossuyt PMM, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology. 2017;153(2):439-447. doi: 10.1053/j.gastro.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Klabunde C, Blom J, Bulliard JL, et al. Participation rates for organized colorectal cancer screening programmes: an international comparison. J Med Screen. 2015;22(3):119-126. doi: 10.1177/0969141315584694 [DOI] [PubMed] [Google Scholar]
- 5.National monitor and evaluation colorectal cancer screening program 2014. https://www.rivm.nl/dsresource?objectid=28200e8e-51e6-46b9-8543-66f0c370114b&type=pdf&disposition=inline. Accessed August 21, 2018.
- 6.National monitor and evaluation colorectal cancer screening program 2015. https://www.rivm.nl/dsresource?objectid=73a29577-66b8-471f-b0a5-2cdbfd6f27c0&type=pdf&disposition=inline. Accessed August 21, 2018.
- 7.Vermeer NC, Snijders HS, Holman FA, et al. Colorectal cancer screening: systematic review of screen-related morbidity and mortality. Cancer Treat Rev. 2017;54:87-98. doi: 10.1016/j.ctrv.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 8.Van Leersum NJ, Snijders HS, Henneman D, et al. ; Dutch Surgical Colorectal Cancer Audit Group . The Dutch surgical colorectal audit. Eur J Surg Oncol. 2013;39(10):1063-1070. doi: 10.1016/j.ejso.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 9.van Leersum NJ, Aalbers AG, Snijders HS, et al. Synchronous colorectal carcinoma: a risk factor in colorectal cancer surgery. Dis Colon Rectum. 2014;57(4):460-466. doi: 10.1097/DCR.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 10.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(suppl 1):S108-S115. doi: 10.1038/bjc.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mengual-Ballester M, Pellicer-Franco E, Valero-Navarro G, Soria-Aledo V, García-Marín JA, Aguayo-Albasini JL. Increased survival and decreased recurrence in colorectal cancer patients diagnosed in a screening programme. Cancer Epidemiol. 2016;43:70-75. doi: 10.1016/j.canep.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev. 2007;(1):CD001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toes-Zoutendijk E, Kooyker AI, Elferink MA, et al. ; LECO working group . Stage distribution of screen-detected colorectal cancers in the Netherlands. Gut. 2018;67(9):1745-1746. doi: 10.1136/gutjnl-2017-315111 [DOI] [PubMed] [Google Scholar]
- 14.Gietelink L, Wouters MW, Bemelman WA, Dekker JW, Tollenaar RA, Tanis PJ; Dutch Surgical Colorectal Cancer Audit Group . Reduced 30-day mortality after laparoscopic colorectal cancer surgery: a population based study from the Dutch Surgical Colorectal Audit (DSCA). Ann Surg. 2016;264(1):135-140. doi: 10.1097/SLA.0000000000001412 [DOI] [PubMed] [Google Scholar]
- 15.Veereman G, Vlayen J, Robays J, et al. Systematic review and meta-analysis of local resection or transanal endoscopic microsurgery versus radical resection in stage I rectal cancer: a real standard? Crit Rev Oncol Hematol. 2017;114:43-52. doi: 10.1016/j.critrevonc.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 16.Amri R, Bordeianou LG, Sylla P, Berger DL. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg. 2013;148(8):747-754. doi: 10.1001/jamasurg.2013.8 [DOI] [PubMed] [Google Scholar]
- 17.Saraste D, Martling A, Nilsson PJ, Blom J, Törnberg S, Janson M. Screening vs non-screening detected colorectal cancer: differences in pre-therapeutic work up and treatment. J Med Screen. 2017;24(2):69-74. doi: 10.1177/0969141316656216 [DOI] [PubMed] [Google Scholar]
- 18.Kalady MF, Sanchez JA, Manilich E, Hammel J, Casey G, Church JM. Divergent oncogenic changes influence survival differences between colon and rectal adenocarcinomas. Dis Colon Rectum. 2009;52(6):1039-1045. doi: 10.1007/DCR.0b013e31819edbd4 [DOI] [PubMed] [Google Scholar]
- 19.Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148(1):77-87. doi: 10.1053/j.gastro.2014.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansouri D, McMillan DC, Grant Y, Crighton EM, Horgan PG. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLoS One. 2013;8(6):e66063. doi: 10.1371/journal.pone.0066063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Wagner C, Good A, Wright D, et al. Inequalities in colorectal cancer screening participation in the first round of the national screening programme in England. Br J Cancer. 2009;101(suppl 2):S60-S63. doi: 10.1038/sj.bjc.6605392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss SM, Campbell C, Melia J, et al. Performance measures in three rounds of the English bowel cancer screening pilot. Gut. 2012;61(1):101-107. doi: 10.1136/gut.2010.236430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Klerk CM, Gupta S, Dekker E, Essink-Bot ML; Expert Working Group ‘Coalition to reduce inequities in colorectal cancer screening’ of the World Endoscopy Organization . Socioeconomic and ethnic inequities within organised colorectal cancer screening programmes worldwide. Gut. 2018;67(4):679-687. [DOI] [PubMed] [Google Scholar]
- 24.Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. 2012;118(14):3636-3644. doi: 10.1002/cncr.26677 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of the study patient selection
eTable 1. Missing values of Table 1 per category
eTable 2. Missing values of Table 2 per category