Key Points
Question
In children with uncomplicated Kawasaki disease with previously normal coronary arteries, what is the benefit of follow-up echocardiography 6 weeks after an initial examination?
Findings
In this study of 464 patients with Kawasaki disease who had normal coronary artery z scores at baseline and 2 weeks, 456 (98.3%) continued to have normal dimensions at 6 weeks. Of the 8 patients (1.7%) whose coronary arteries were larger than normal, dimensions were minimally dilated and ultimately regressed to normal in 7 patients and nearly normal in 1 patient.
Meaning
In patients with Kawasaki disease who had initially normal coronary artery measurements, new abnormalities were rare at 6 weeks, which suggests limited benefit of 6-week echocardiographic imaging in patients with uncomplicated Kawasaki disease.
This cohort study assesses whether echocardiographic imaging of pediatric patients with Kawasaki diseaes 6 weeks after onset of illness adds value for patients whose earlier examinations showed no coronary artery abnormalities.
Abstract
Importance
American Heart Association guidelines recommend echocardiography in Kawasaki disease at baseline, 1 to 2 weeks, and 4 to 6 weeks after treatment to detect coronary artery abnormalities. However, these examinations are expensive and may require sedation in young children, which is burdensome and carries some risk.
Objective
To assess the benefit of additional echocardiographic imaging at 6 weeks in patients with uncomplicated Kawasaki disease who had previously normal coronary arteries.
Design, Setting, and Participants
This is a retrospective review of patients with Kawasaki disease who were cared for between 1995 and 2014 in 2 academic pediatric referral practices Eligibility criteria included receiving intravenous immunoglobulin treatment for acute Kawasaki disease at a center; the absence of significant congenital heart disease; available echocardiographic measurements of both the right and left anterior descending coronary arteries at 10 days or less after diagnosis (baseline), 2 (±1) weeks, and 6 (±3) weeks of illness; and normal coronary arteries at baseline and 2 weeks, defined as maximum coronary artery z scores less than 2.0 and no distal aneurysms. Data analysis was completed from March 2015 to November 2015.
Main Outcomes and Measures
The number of patients with right coronary artery or left anterior descending coronary artery z scores of 2.0 or more at 6 weeks.
Results
The median age of the 464 included patients was 3.3 years (interquartile range, 1.8-5.4 years); 264 (56.9%) were male, 351 of 414 for whom data were available (84.8%) had complete Kawasaki disease, and 66 (14.2%) received additional intravenous immunoglobulin treatment. At 6 weeks of illness, 456 patients (98.3%) who had had normal coronary artery z scores at baseline and 2 weeks continued to have normal z scores. Of the remaining 8 patients (1.7%), the maximum z score within 6 weeks was 2.0 to 2.4 in 5 patients (1.2%), 2.5 to 2.9 in 1 patient (0.2%), and 3.0 or more in 2 patients (0.4% [95% CI, 0.1%-1.5%]). Coronary artery dimensions ultimately normalized in all but 1 patient, who had minimal dilation at 6 weeks (right coronary artery z score, 2.1). Sensitivity analyses using less restrictive cut points (eg, a maximum z score <2.5) or less restrictive timing windows (eg, considering patients with incomplete echocardiographic data within 21 days) gave similar results; in these analyses, 454 to 463 of 464 patients (98% to 99.7%) had coronary artery z scores of less than 2.5 at 6 weeks.
Conclusions and Relevance
New abnormalities in coronary arteries are rarely detected at 6 weeks in patients with Kawasaki disease who have normal measurements at baseline and 2 weeks of illness, suggesting that the 6-week echocardiographic imaging may be unnecessary in patients with uncomplicated Kawasaki disease and z scores less than 2.0 in the first 2 weeks of illness.
Introduction
Kawasaki disease (KD)1,2 is an acute vasculitis that affects medium-sized muscular extraparenchymal arteries, with a predilection for the coronary arteries (CA).3,4 Echocardiography is recommended at KD diagnosis (baseline), 1 to 2 weeks after treatment, and 4 to 6 weeks after treatment (generally corresponding to 2 weeks and 4 to 6 weeks after fever onset) to maximize detection of CA abnormalities and to guide therapy.5 Imaging of the CAs at 2 weeks is particularly important, because new abnormalities in the early weeks of illness should prompt the consideration of additional antiinflammatory and anticoagulant therapies that are time-sensitive.6 Although inclusion of echocardiographic imaging at 6 weeks maximizes sensitivity for detection of coronary abnormalities, its benefits in a low-risk group must be balanced against financial cost, inconvenience to the family, and potential (albeit rare) adverse effects of sedation required to obtain the necessary coronary images in many infants and toddlers. In particular, in the patient with uncomplicated KD with a maximum CA z score less than 2.0 in the first 2 weeks after diagnosis and the absence of distal aneurysms at both baseline and 2 weeks, we hypothesized that subsequent echocardiographic imaging would demonstrate normal CAs.
In this retrospective review, we evaluated whether findings on echocardiography performed more than 3 weeks after illness onset altered management of children treated for KD within the first 10 days of illness and who had normal CA dimensions both at diagnosis and 2 weeks of illness.
Methods
Patient Population
We conducted a retrospective medical record review of patients who received intravenous immunoglobulin (IVIG) as treatment for complete or incomplete KD within 10 days of onset of illness at Boston Children’s Hospital or Rady Children’s Hospital San Diego from 1995 through 2014. Permission to conduct the study was granted by the Boston Children’s Hospital institutional review board. Waiver of informed consent was granted because of the use of deidentified data from existing medical records.
We restricted our analyses to patients who had at least 1 available echocardiographic measurement of the proximal right coronary artery (RCA) and the left anterior descending (LAD) CA at 3 times: baseline (≤10 days), 2 weeks (±1 week; 7 to 21 days), and 6 weeks (±3 weeks; 22 to 63 days) from the onset of fever. (eTable 1 in the Supplement presents details on excluded patients.) For patients who had multiple CA measurements during the relevant period, we used the largest CA z score during that period. We did not include left main CA dimensions in our analyses because of the known anatomic variability of this CA segment compared with the LAD and RCA.7 We excluded patients coded in our clinical database as having distal coronary aneurysms in the absence of dilation (z score ≥2.0) of proximal CA z scores at either the baseline or 2-week echocardiographic imaging, patients evaluated as a second opinion, or experiencing a second episode of KD. Patients were also excluded if they had congenital heart disease, if the date of onset of illness was unknown, or if documentation of IVIG treatment was unavailable in the medical record.
Clinical Characteristics
Using the electronic medical records, paper records, and quality improvement databases, we collected the following sociodemographic variables: age at onset, race/ethnicity, and sex. We extracted clinical characteristics including the date of onset of illness, date of initial IVIG treatment, KD criteria, and types of KD treatments (including IVIG, retreatment with IVIG, and additional therapies). For patients with abnormal CA imaging at 6 weeks, defined by a z score of 2.0 or more, we reviewed clinical records.
To assess CA z scores, we used CA dimensions, height, and weight recorded in the original echocardiography readings to calculate z scores of the proximal RCA and the LAD.8,9 We used the z score calculator currently in use at Boston Children’s Hospital,7 using these formulas (dimensions in millimeters; BSA indicates body surface area):
Left main coronary artery (LMCA): z = (LMCA − [0.31471 × (BSA0.36783) − 0.02449]) / (0.02974 + 0.01649 × BSA)
LAD: z = (LAD − [0.31471 × (BSA0.36783) − 0.02449]) / (0.02974 + 0.01649 × BSA)
RCA: z = (RCA − [0.2475 × BSA0.44618] − 0.01082) / (0.02572 + 0.01336 × BSA)
We also collected absolute dimensions and relevant z scores for other echocardiographic parameters, including aortic root and left ventricular ejection fraction.
Statistical Methods
Patient characteristics, including CA z scores and other echocardiographic parameters, were described as either mean (SD) or median (interquartile range [IQR]), as appropriate. We compared characteristics for the Boston, Massachusetts, and San Diego, California, sites using the Fisher exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. For the primary analysis, we examined patients who had normal CA dimensions, defined as proximal RCA and LAD z scores less than 2.0 at baseline and 2 weeks, and who were without known distal aneurysms. We estimated the proportion of patients who had progression of either their proximal RCA or LAD z score from normal (<2.0) to abnormal. Abnormal was defined as CA z score of 2.0 or more. We calculated 95% CIs to assess the upper bound of the proportion of patients who may have a CA z score that enlarges at values greater than 2.0.
We performed sensitivity analyses using higher CA cut points to define normal CA dimensions at baseline and 2 weeks. For these additional analyses, patients met the same inclusion and exclusion criteria but had maximum z scores at baseline and 2 weeks of 2.5 or less (eg, including CA dilation), and separately 3.0 or less (eg, including small aneurysms). Because many patients were excluded because of missing echocardiographic data at either the baseline or 2-week points, we conducted an additional sensitivity analysis including all patients with any available echocardiographic image within 21 days of illness onset with CA z scores less than 2.0, even if they only had 1 such echocardiogram, to determine whether patients with more limited echocardiographic data had similar CA outcomes at 6 weeks.
Analyses were performed using Stata version 14 (StataCorp) from March 2015 to November 2015. Two-tailed P values were used, and values less than .05 were considered significant.
Results
Characteristics of Patient Population
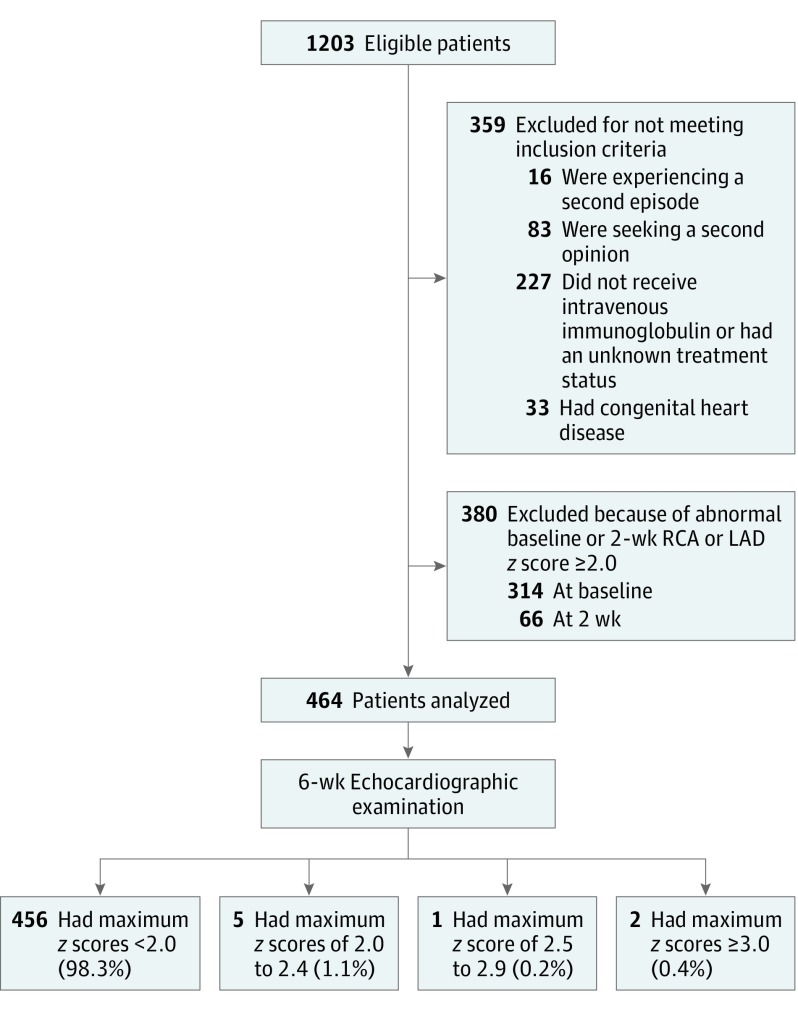
At the 2 centers between 1995 and 2014, there were 1203 patients who had been treated for KD and had available RCA and LAD z scores at baseline, 2 weeks, and 6 weeks (Figure 1). Exclusion criteria were present in 359 patients (29.8%), and 380 patients (31.6%) had abnormal CAs at baseline or 2 weeks. Thus, 464 patients met entry criteria and formed the cohort for the primary analysis.
Figure 1. Cohort for Analysis.
LAD indicates left anterior descending coronary artery; RCA, right coronary artery.
The patients included in our analysis are described in Table 1. In our cohort, 264 patients (56.9%) were male, the median age was 3.3 years (IQR, 1.8-5.4 years), and 351 of 414 for whom data was available (84.8%) had complete KD. Based on inclusion criteria, all were initially treated with IVIG; 66 (14.2%) received IVIG retreatment. No patients received additional antiinflammatory treatments for KD, aside from medications administered to participants in randomized clinical trials; in these, 26 of 370 patients in Boston (7.0%) received steroids, and 49 of 94 patients in San Diego (52.1%) received infliximab as primary adjunctive therapy. When patient characteristics were compared between sites, a greater proportion of the San Diego patients were Hispanic (38 of 94 [40.4%]) than patients in Boston were (33 of 370 [8.9%]; P < .001). In addition, a greater percentage were diagnosed in 2010 through 2014 (64 of 94 [68.1%]) rather than in 1995 through 2009 (97 of 370 [26.2%]). More Boston patients had 3 or fewer clinical criteria (n = 54 of 370 [17%]), compared with San Diego patients (n = 9 of 94 [9.6%]; P = .004). The cohorts were combined for analysis.
Table 1. Baseline Patient Characteristics.
| Patient Characteristic | No./Total No. (%) (N = 464) |
|---|---|
| Age, median (IQR), y | 3.3 (1.8-5.4) |
| Male | 264/464 (56.9) |
| Race | |
| White | 319/464 (68.8) |
| Black | 42/464 (9.1) |
| Asiana | 67/464 (14.4) |
| Otherb | 26/464 (5.6) |
| Unknown/not reported | 10/464 (2.2) |
| Hispanic ethnicity, No. (%) | 71/464 (15.3) |
| Days of fever, median (IQR) (n = 448) | 6 (5-8) |
| Clinical criteriac | |
| ≤3 | 63/414 (15.2) |
| 4 | 228/414 (55.1) |
| 5 | 123/414 (29.7) |
| Treated with IVIG | 464/464 (100) |
| Day of illness on which IVIG was given, median (IQR) | 6 (5-8) |
| Retreated with IVIGd | 66/464 (14.2) |
Abbreviations: IQR, interquartile range; IVIG, intravenous immunoglobulin.
Includes patients recorded as Asian and patients in the category of more than 1 race if the reported races included Asian.
Includes patients described as other and patients in the category of more than 1 race if the reported races did not include Asian.
Data are missing for some clinical criteria for 50 patients; therefore, only the 414 (89.2%) with complete data are included in these categories.
For 1 patient in the cohort, IVIG treatment or the lack thereof was not documented.
Echocardiographic Measurements
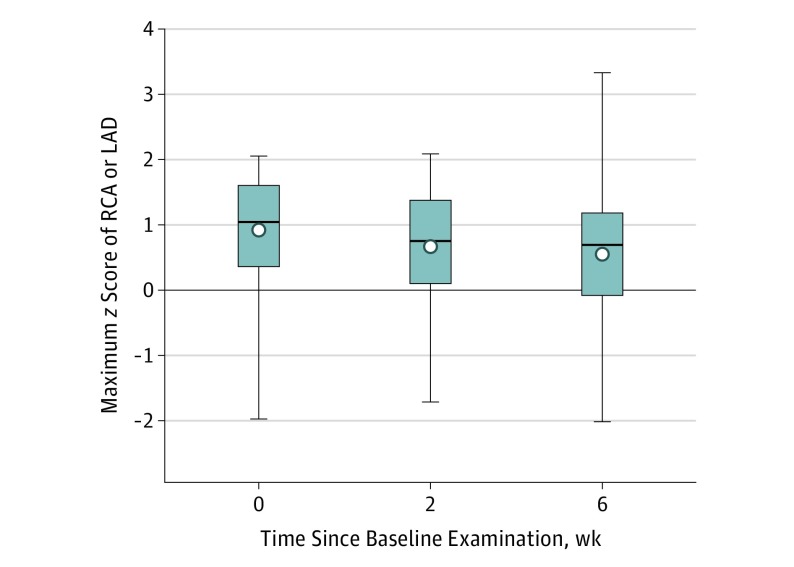
By entry criteria, echocardiographic measurements were normal at baseline and 2 weeks. At 6-week follow-up (Table 2 and Figure 2), CA z scores were normal in 456 patients (98.3%; Table 3). Of the remaining 8 patients, the maximum z score was 2.0 to 2.4 in 5 patients (1.1%); the z scores normalized in all 5 patients by the 1-year echocardiographic follow-up examination. The maximum z score was 2.5 to 2.9 in 1 patient (0.2%), who continued to receive low-dose aspirin until the next echocardiographic examination at 6 months, which showed normal CA dimensions. The maximum z score was 3.0 or greater in 2 patients (0.4% [95% CI, 0.1%-1.5%]); z scores measured in the normal range at the next echocardiographic imaging, which occurred 3 weeks later in 1 patient and 3 years later in the other. At late follow-up, all except for 1 patient had normal CA z scores; 1 patient had an RCA z score of 2.1 at 6 weeks after onset of illness, with no subsequent imaging available.
Table 2. Acute and Subacute Echocardiographic Findings.
| Characteristic | Mean (SD) | |||||
|---|---|---|---|---|---|---|
| Patients, No. | Baseline Values | Patients, No. | Values at 2-wk Follow-up | Patients, No. | Values at 6-wk Follow-up | |
| Proximal right coronary artery anterior-posterior diameter | ||||||
| Dimension, cm | 464 | 0.23 (0.19) | 464 | 0.23 (0.23) | 464 | 0.23 (0.23) |
| z Score | 464 | 0.43 (0.94) | 464 | 0.28 (0.92) | 464 | 0.15 (0.91) |
| Proximal left anterior descending coronary artery | ||||||
| Dimension, cm | 464 | 0.22 (0.18) | 464 | 0.23 (0.24) | 464 | 0.23 (0.26) |
| z Score | 464 | 0.50 (0.92) | 464 | 0.28 (0.27) | 464 | 0.07 (0.94) |
| Left main coronary artery anterior-posterior diametera | ||||||
| Dimension, cm | 358 | 0.27 (0.05) | 362 | 0.26 (0.05) | 369 | 0.26 (0.05) |
| z Score | 358 | 0.51 (0.93) | 362 | 0.35 (0.85) | 369 | 0.19 (0.89) |
| Anterior-posterior diameters of coronary arteries, cm | ||||||
| Posterior descendinga | 205 | 0.14 (0.12) | 202 | 0.15 (0.13) | 203 | 0.12 (0.08) |
| Distal anterior descending a | 348 | 0.18 (0.04) | 352 | 0.17 (0.04) | 351 | 0.18 (0.12) |
| Circumflexa | 315 | 0.16 (0.04) | 324 | 0.17 (0.11) | 326 | 0.16 (0.10) |
| Aortic root anterior-posterior diametera | ||||||
| Dimension, cm | 333 | 1.78 (0.33) | 338 | 1.81 (0.34) | 342 | 1.83 (0.33) |
| z Score | 333 | 0.49 (0.96) | 338 | 0.61 (0.89) | 342 | 0.60 (0.88) |
| Left ventricular ejection fraction, mean (SD)a | 152 | 0.62 (0.06) | 172 | 0.65 (0.05) | 169 | 0.64 (0.05) |
These data were available for Boston, Massachusetts, patients only.
Figure 2. Maximum Coronary Artery z Scores.
Maximum coronary artery z scores of right coronary artery (RCA) and the left anterior descending coronary artery (LAD) are shown as means (circles), medians (lines), interquartile ranges (boxes), and minimum to maximum ranges (whiskers).
Table 3. Maximum z Scores of the Right Coronary Artery or Left Anterior Descending Coronary Artery at 6 Weeks.
| Characteristics | Patients, No. | 6-wk Maximum z Scores, No. (%) [95% CI] | |||
|---|---|---|---|---|---|
| <2.0 | 2.0-2.4 | 2.5-2.9 | ≥3.0 | ||
| Baseline and 2-wk z scores | |||||
| <2.0 | 464 | 456 (98.3) | 5 (1.1) | 1 (0.2) | 2 (0.4) [0.1-1.5] |
| <2.5 | 557 | 540 (96.9) | 11 (2.0) | 3 (0.5) | 3 (0.5) [0.1-1.6] |
| <3.0 | 640 | 604 (94.4) | 21 (3.3) | 8 (1.3) | 7 (1.1) [0.4-2.2] |
| Any echocardiogram within 21 d with z scores <2.0 | 769 | 752 (97.8) | 10 (1.3) | 5 (0.7) | 2 (0.3) [0.03-0.9] |
There were 2 patients included in this cohort with normal CAs at baseline, 2 weeks, and 6 weeks who had maximum z scores greater than 3.0 at a later measurement. One patient had an LAD z score of 3.12 at 1 year and 14 days after fever onset. The last available coronary imaging 4 years after onset of illness showed an LAD z score of 2.12. The second patient had an RCA z score of 3.25 at 1.3 years after fever onset; however, the report summary described normal coronary arteries, and the last available echocardiographic imaging 6 years after the onset of illness reported a normal RCA (0.26 cm; z score, 0.9) and normal left ventricular function. At last follow-up, neither patient had cardiac symptoms, was under any activity restrictions, or took any cardiac medications.
We performed sensitivity analyses to explore whether patients with mildly abnormal CAs at baseline and 2 weeks had similar results using maximum z score cut points of 2.5 and 3.0 (instead of 2.0). The 6-week echocardiography results were similar to those obtained using the more stringent z score cut point (Table 3). When rather than requiring 2 separate echocardiographic images at baseline and 2 weeks, we instead included any patient in whom all echocardiograms obtained within 21 days of illness onset had coronary maximum z scores of 2.0 or less (even if there was only 1 such echocardiogram), an additional 305 patients were available for analysis. The results were similar, with 752 of 769 patients (97.8%) having a maximum z score less than 2.0 at 6 weeks (Table 3).
We compared clinical characteristics of those patients included in the analysis cohort with those excluded from the cohort because of incomplete echocardiographic data (n = 1204). Patients included in the study were slightly older (median [IQR], 3.3 [1.8-5.4] years vs 2.8 [1.4-4.9] years; P = .001), more likely to be non-Hispanic white (Hispanic individuals included in study, n = 71 of 464 [15.3%] vs those excluded from study, n = 356 of 1204 [29.6%]; P < .001), and had slightly fewer days of fever (median [IQR], 6 [5-8] days vs 7 [5-9] days; P < .001; eTable 2 in the Supplement).
Discussion
Frequent echocardiographic follow-up of patients with KD is recommended because coronary enlargement can progress rapidly in the acute phase of the illness, aneurysms carry significant risk, and adjunctive KD therapies should be administered as early as possible.5 Fortunately, longitudinal follow-up has shown that children with CA dimensions that are always normal have an excellent prognosis, and recent guidelines recommend that patients who have had no coronary enlargement in the first 6 weeks of illness may be discharged from follow-up.5 A single study published in 1999 showed minimal added benefit of echocardiographic imaging beyond 6 weeks.10 In our review of patients with KD evaluated and treated at 2 large clinical centers, we found nearly all of the patients (98.3%) with completely normal RCA and LAD coronary dimensions at baseline and 2 weeks (with maximum z scores <2.0) had normal CA dimensions at 6 weeks, and 99.8% of the children with maximum z scores of less than 2.5 had subsequently normal CA dimensions, using the cut point for aneurysms set by the 2017 Kawasaki American Heart Association Statement.5 Our findings are consistent with a report suggesting that baseline coronary z scores greater than 2.5 are predictive of coronary aneurysms.11 Furthermore, those few children who began with normal CAs in the first 2 time windows and whose CA z scores became abnormal at 6 weeks had only mild CA dilation or small aneurysms and did not require additional antiinflammatory therapies or anticoagulation. Indeed, virtually all patients (n = 463 of 464 [99.7%]), even those with mild abnormalities, had a return of dimensions to within normal limits on available late follow-up imaging. Thus, this study suggests that there is limited usefulness to performing a 6-week echocardiographic examination in the patient with otherwise uncomplicated KD in whom coronaries are visualized and normal at diagnosis and 2 weeks after illness onset.
Our analysis highlights the degree to which, despite relatively good reproducibility of CA measurement, the use of a dichotomous cutoff as a basis for important clinical decision making has important limitations. Small differences in dimension (fractions of millimeters) may tip a finding higher than or lower than such a dichotomous z score cutoff.7 Measurement variation has the largest effect on z scores when coronary dimensions are small, as in infants, and may be of clinical significance in patients with z scores just below the threshold of 2.0. Rarely, patients with KD can have normal proximal RCA and LAD z scores with distal aneurysms; these study results would not apply to such individuals, because patients coded in the clinical database with aneurysms at baseline or 2 weeks were excluded from our cohort. None of the patients with normal baseline and 2-week CA proximal z scores were noted to have isolated distal aneurysms at late follow-up.
Limitations
Although we had reasonable medium-term longitudinal follow-up for many of the patients, we were unable to assess the consequences of very mild dilation of the coronaries (ie, with largest dimensions in the range of z scores of 2.0 to 2.5) with remodeling to normal on outcomes in later life; to our knowledge, the association of CA remodeling with long-term outcomes is unknown.
This study did not formally assess cost-effectiveness. However, eliminating 1 echocardiographic examination for approximately 70% of the 6000 new cases of KD in the United States annually12 could translate into substantial savings through eliminating approximately 4200 follow-up echocardiograms per year.
This study did not solicit parental perspectives on echocardiographic imaging in children with previously normal CA dimensions. It is possible that parents are reassured by additional imaging, minimizing the potential for mislabeling children with cardiac nondisease. However, parents may view the benefit of such reassurance as being outweighed by the risks of sedation.
Our study has additional limitations. As this was a retrospective medical record review of clinical care, the timing and extent of the echocardiographic images was determined by clinical practice. Sensitivity analyses including patients with any available echocardiographic data (eg, a single echocardiographic examination or incomplete imaging of all CAs) within the first 21 days of onset of illness showed similar results. Comparison of the children included with those excluded from the analysis because of incomplete echocardiographic data showed patients included in the study were slightly older (median [IQR], 3.3 [1.8-5.4] years vs 2.8 [1.4-4.9] years; P = .001), were more likely to be non-Hispanic white individuals (Hispanic individuals included in study, n = 71 of 464 [15.3%] vs those excluded from study, n = 356 of 1204 [29.6%]; P < .001), and had fewer days of fever (median [IQR], 6 [5-8] days vs 7 [5-9] days; P < .001). The difference in age may be associated with ease of imaging, and the difference in ethnicity might be associated with differences in imaging practice by site (in Boston vs San Diego); the small difference in days of fever is likely to be of low clinical significance.
Whereas CA z scores used in this analysis were calculated using current z score formulas in use at Boston Children’s Hospital, clinical decisions were made based on the measurements and z scores available at the time that the echocardiographic examination was performed. A comparison of commonly used CA z score calculators showed low variability in the mildly dilated range.7 For these reasons, a few patients with abnormal z scores using current algorithms were described in past medical records as having normal coronary dimensions. Treatment and follow-up decisions were made based on contemporaneously recommended cut points,3 rather than based on the 2017 American Heart Association Guidelines.
Our findings may have limited generalizability because they represent the experience of 2 academic medical centers with large KD programs. Despite small differences in clinical practice between the 2 centers (eg, institutional experience, caregiver subspecialty, and treatment approach beyond IVIG), clinical and sociodemographic factors of patients from each center were generally similar.
These findings cannot be applied to patients with KD who have congenital heart disease,13 in whom coronary z scores may differ from those without structural heart disease. These findings may not apply to infants with KD younger than 6 months, who may have clinically significant variability in z scores with even small differences in absolute measurements and who are at higher risk for adverse CA outcomes.14 Finally, our study findings should not be used to eliminate echocardiograms in children whose initial CA dimensions are abnormal; these children often require even greater frequency of coronary imaging.5
Conclusions
We found that, among patients with uncomplicated KD treated within the first 10 days of illness and with z scores of the proximal RCA and LAD less than 2.0 at baseline and 2 weeks, only 1% had mild enlargement on the 6-week echocardiographic examination; using a cut point of 2.5, we found that only 0.2% had abnormal dimensions at 6 weeks. No significant CA dilation was found on available late follow-up imaging. With an initial CA z score cut point of 2.5, 99.5% of included patients were normal at 6 weeks. Future work should examine whether our findings are validated in other settings and populations, and if confirmed, consideration should be given to a cost-benefit analysis on eliminating the 6-week echocardiographic examination in patients with low-risk KD with initially normal coronaries.
eTable 1. Patients excluded for incomplete echo data. Any given patient may have incomplete data at multiple time points therefore the categories are not exclusive.
eTable 2. Comparison of patients meeting study criteria entry versus those meeting criteria but without complete echo data. Values shown are number (percent) or median [25th, 75th percentile].
References
- 1.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):-. [PubMed] [Google Scholar]
- 2.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children [published in Japanese]. Arerugi. 1967;16(3):178-222. [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Gerber MA, et al. ; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association . Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. doi: 10.1542/peds.2004-2182 [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67(14):1738-1749. doi: 10.1016/j.jacc.2015.12.073 [DOI] [PubMed] [Google Scholar]
- 5.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927-e999. doi: 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 6.Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131(6):888-893. doi: 10.1016/S0022-3476(97)70038-6 [DOI] [PubMed] [Google Scholar]
- 7.Ronai C, Hamaoka-Okamoto A, Baker AL, et al. . Coronary artery aneurysm measurement and z score variability in Kawasaki disease. J Am Soc Echocardiogr. 2016;29(2):150-157. doi: 10.1016/j.echo.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 8.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW; de ZA . Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133(2):254-258. doi: 10.1016/S0022-3476(98)70229-X [DOI] [PubMed] [Google Scholar]
- 9.McCrindle BW, Li JS, Minich LL, et al. ; Pediatric Heart Network Investigators . Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174-179. doi: 10.1161/CIRCULATIONAHA.107.690875 [DOI] [PubMed] [Google Scholar]
- 10.Scott JS, Ettedgui JA, Neches WH. Cost-effective use of echocardiography in children with Kawasaki disease. Pediatrics. 1999;104(5):e57. doi: 10.1542/peds.104.5.e57 [DOI] [PubMed] [Google Scholar]
- 11.Son MBF, Gauvreau K, Kim S, et al. . Predicting coronary artery aneurysms in Kawasaki disease at a North American center: an assessment of baseline z scores. J Am Heart Assoc. 2017;6(6):e005378. doi: 10.1161/JAHA.116.005378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. doi: 10.1097/01.inf.0000069765.43405.3b [DOI] [PubMed] [Google Scholar]
- 13.Ronai C, Baker AL, Friedman KG, Fulton DR, Newburger JW, Lang P. Prevalence of undiagnosed structural heart disease in children undergoing echocardiography for Kawasaki disease. Clin Pediatr (Phila). 2016;55(6):557-559. doi: 10.1177/0009922815594588 [DOI] [PubMed] [Google Scholar]
- 14.Salgado AP, Ashouri N, Berry EK, et al. . High risk of coronary artery aneurysms in infants younger than 6 months of age with Kawasaki disease. J Pediatr. 2017;185:112-116. doi: 10.1016/j.jpeds.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Patients excluded for incomplete echo data. Any given patient may have incomplete data at multiple time points therefore the categories are not exclusive.
eTable 2. Comparison of patients meeting study criteria entry versus those meeting criteria but without complete echo data. Values shown are number (percent) or median [25th, 75th percentile].