This cohort study of 392 participants with obesity and severe obstructive sleep apnea evaluates whether the prescription of positive airway pressure treatment is associated with lower mortality.
Key Points
Question
Is the prescription of positive airway pressure (PAP) associated with lower all-cause mortality?
Findings
In this cohort study of 392 patients with obesity and severe obstructive sleep apnea with 11 years of follow-up, we found that prescription of PAP therapy was associated with a 62% lower risk of all-cause mortality compared with nonprescription of PAP, independent of major confounders.
Meaning
Positive airway pressure therapy prescription is associated with lower all-cause mortality, beginning several years after PAP prescription.
Abstract
Importance
The association of positive airway pressure (PAP) with reduced mortality in patients with obstructive sleep apnea (OSA) remains uncertain.
Objective
To investigate the association between PAP prescription and mortality.
Design, Setting, and Participants
This multicenter, population-based cohort study evaluated data from the Sleep Heart Health Study (SHHS), a long-term observational cohort study that included participants between 1995 and 1998, with a mean follow-up of 11.1 years. Analyses were performed in September 2018. Within the SHHS, we compared patients with obesity and severe OSA with (n = 81) and without (n = 311) prescription of PAP therapy, after matching patients from each group by age, sex, and apnea-hypopnea index.
Exposures
Self-reported use of PAP.
Main Outcomes and Measures
All-cause mortality.
Results
Of 392 study participants, 316 (80.6%) were men, and mean (SD) age was 63.1 (11.0) years. Ninety-six deaths occurred; 12 among the prescribed-PAP group and 84 among the nonprescribed-PAP group, yielding crude incidence rates of 12.8 vs 24.7 deaths per 1000 person-years. In Cox multivariate analysis, the hazard ratio (HR) of all-cause mortality for prescribed PAP therapy was 0.38 (95% CI, 0.18-0.81). After propensity matching, the HR of all-cause mortality for prescribed PAP therapy was 0.58 (95% CI, 0.35-0.96). According to survival curves, the difference in mortality appears 6 to 7 years after initiation of PAP therapy.
Conclusions and Relevance
Positive airway pressure prescription is associated with reduced all-cause mortality, and this association appears several years after PAP initiation. If replicated, these findings may have strong clinical implications.
Introduction
Sleep disturbances, including obstructive sleep apnea (OSA), are 1 of the top 10 modifiable cardiovascular risk factors.1,2 Widely recognized as a major public health burden,3 the prevalence of OSA may be as high as 49% in men and 23% in women older than 40 years old.4 Among several other adverse medical conditions such as cognitive impairment5 or incident diabetes,6 OSA is associated with an elevated risk of coronary artery disease, heart failure, and stroke, and with up to a 3-fold increased risk of all-cause mortality.3,7,8,9,10
To date, the most effective treatment of OSA relies on nocturnal positive airway pressure (PAP) treatment, which has been consistently shown to reduce markers of OSA severity, such as the apnea-hypopnea index (AHI), the oxygen desaturation index, and daytime sleepiness.11 In recent meta-analyses, PAP treatment has also been shown to be positively associated with intermediate end points such as decreased blood pressure.3,12,13 However, the benefit of PAP on hard end points, including all-cause and cardiovascular (CV) mortality, remains unproven3,14 and raises recurrent controversies.15,16
Indeed, randomized clinical trials (RCTs), including the largest so far, to our knowledge, the SAVE trial, have not established whether PAP therapy reduces mortality. However, RCTs usually have short follow-up times and thus a small number of events.2,3,14,17,18,19,20,21 On the other hand, evidence provided by observational studies has remained weak, because most have been of small sample size, focused on selected populations (men,8 women,22 or very elderly patients23), and/or have not taken into account important confounders such as age or body mass index (BMI).24,25,26,27 Moreover, the majority of observational studies have not controlled for the severity of OSA (eg, using the AHI), and for comorbidities often associated with OSA, raising the issue of residual confounding.9,28,29
In this context, our aim was to quantify the association between PAP prescription and mortality in an observational study taking advantage of a long follow-up duration.
Methods
Study Design
We used data from the Sleep Heart Health Study (SHHS, ClinicalTrials.gov identifier: NCT00005275).30,31,32 Between 1995 and 1998, adult men and women 40 years and older were recruited from 9 existing population-based studies (parent cohorts). Of the 6441 participants included in the SHHS, 5804 were available for the present analysis, as participants of the Strong Heart Study are not included in the shared SHHS data (more information available at https://sleepdata.org/datasets/shhs). Of the 5804 available participants, this study analyzed 392 of them, 81 of which were prescribed PAP and 311 of which were not prescribed PAP. This study was conducted in accordance with the amended Declaration of Helsinki. Each participant provided written consent and the institutional review board of each participating field site approved the study protocol. Data analysis took place in September 2018.
Polysomnography Recordings
At baseline, all participants had a clinic visit and underwent a complete overnight polysomnography. Along with other measurements, inductance plethysmography was used to measure chest and abdominal excursion, and airflow was recorded by thermal sensor. A complete or near-complete cessation of airflow lasting for at least 10 seconds defined an apnea; hypopnea was identified by a clear decrease in airflow or chest or abdominal plethysmograph amplitude for at least 10 seconds. Both apnea and hypopnea had to be associated with a 4% or greater oxyhemoglobin desaturation. The AHI was defined as the average number of apneas plus hypopneas per hour of sleep. Sleep duration was obtained through the polysomnography recording.
PAP Treatment and All-Cause and Cardiovascular Mortality
Two to 3 years after baseline, participants had an interim clinic visit or a telephone call. In particular, participants were asked if their physician had prescribed PAP as a treatment for sleep apnea since baseline. The date of PAP prescription and/or PAP initiation was, however, unknown. Overall, 91 participants were prescribed PAP and constituted our exposed population.
All participants were under ongoing surveillance for CV and all-cause mortality by parent cohorts. Events were tracked from 2008 to 2011, depending on the parent cohort, and follow-up was available for 5802 participants.
Covariates at Baseline
Prevalent cardiovascular disease (CVD) at baseline was defined as the self-report of a previous history of stroke, myocardial infarction, and/or angina pectoris. Hypertension status was based on the second and third blood pressure measurements taken during the clinic visit, or being treated with blood pressure–lowering medications. Diabetes was reported from parent cohorts. Weights and heights were used to calculate BMI. Education level was self-reported and categorized into 10 years or less, 11 to 15 years, 16 to 20 years, or more than 20 years of education. Smoking status was categorized as never, current, or former smoker. Alcohol consumption was categorized according to the daily number of glasses of alcohol: 0, 1 to 3, or more than 3 glasses per day.
Statistical Analyses
We performed a matched cohort study within the SHHS. Exposed participants were those who were prescribed PAP therapy. From the remaining participants, we selected up to 4 unexposed participants (nonprescribed PAP) for each prescribed-PAP participant, matched by age, sex, and AHI. Overall, 81 participants prescribed PAP could be matched with 311 participants not prescribed PAP (4:1 ratio in 74 cases, 3:1 in 2 cases, 2:1 in 4 cases, and 1:1 in 1 case), resulting in a study sample of 392 participants. We compared descriptive characteristics between groups (prescribed vs nonprescribed PAP) using univariate conditional logistic regression to account for the matching variables.
Kaplan-Meier survival curves between prescribed and nonprescribed PAP groups were compared using the log-rank test. We then used Cox regression modeling to estimate hazard ratio (HR) and 95% confidence intervals (CIs) of all-cause and CV mortality associated with PAP compared with no PAP prescription. Regression models were adjusted for the following baseline covariates: prevalent CVD, hypertension, diabetes, BMI, education level, smoking status, and alcohol consumption. The proportional hazards assumption was graphically checked.
To limit indication bias of being prescribed PAP therapy, a propensity score–matched analysis was performed. We calculated the propensity of being prescribed PAP therapy using variables related to the outcome (all-cause mortality)33 including age, sex, ethnicity, education level, BMI, AHI, smoking, driving a car, the respiratory disturbance index, the total sleep time, neck circumference, hypertension, diabetes, history of myocardial infarction and angina, history of stroke, heart failure, history of a pacemaker, cholesterol and high-density lipoprotein levels, and the Epworth sleepiness score. Then, for each participant prescribed PAP therapy, we selected 4 participants with no prescription for PAP but who had a similar propensity score as the participants prescribed PAP. Fifty-one participants who were prescribed PAP could be matched with 204 participants who were not prescribed PAP (the propensity score mean [SD] was 0.09 [0.06] in both groups after matching). Kaplan-Meier survival curves of mortality and Cox regression modeling were then performed.
To test the robustness of our results, we conducted several sensitivity analyses. First, we reestimated 95% CIs of adjusted HRs using 10 000 iterations of bootstrap resampling. Second, because information on exposure to PAP therapy was available 2 to 3 years after baseline, we reran the previous analysis considering the third year of follow-up as the starting point of mortality follow-up.
All analyses were 2-sided and were conducted using R software, version 3.3.3 (http://www.r-project.org).
Results
Baseline Characteristics of the Population
Of the 392 study participants, 319 (80.6%) were men and the mean (SD) age was 63.1 (11.0) years. The main characteristics of the 81 participants prescribed PAP and the 311 not prescribed PAP are summarized in Table 1. Participants with PAP prescription had a higher BMI and were more educated than participants not prescribed PAP.
Table 1. Baseline Characteristics of the Study Participantsa.
| Characteristics | PAP Not Prescribed (n = 311) | PAP Prescribed (n = 81) |
|---|---|---|
| Matching variables | ||
| Male sex | 256 (82.3) | 60 (74) |
| Age, mean (SD), y | 63.4 (11.3) | 61.6 (9.68) |
| AHI, mean (SD) | 34.2 (19.4) | 37.9 (19.3) |
| Demographics | ||
| BMI, mean (SD) | 30.1 (5.4) | 32.5 (5.8) |
| Education, y | ||
| ≤10 | 26 (8.93) | 5 (7) |
| 11-15 | 155 (53.3) | 36 (48) |
| 16-20 | 104 (35.7) | 26 (35) |
| >20 | 6 (2.06) | 8 (11) |
| Smoking status | ||
| Never | 132 (43.0) | 35 (43) |
| Current | 26 (8.47) | 4 (5) |
| Former | 149 (48.5) | 42 (52) |
| Alcohol consumption, drinks/d | ||
| 0 | 140 (49.3) | 36 (49) |
| 1-3 | 57 (20.1) | 21 (28) |
| >3 | 87 (30.6) | 17 (23) |
| Comorbidities | ||
| Hypertension | 154 (49.5) | 45 (56) |
| Diabetes | 33 (11.4) | 10 (13) |
| Prevalent CVD | 58 (19.0) | 19 (24) |
| Mortality rates | ||
| All-cause mortality | 84 (27.0) | 12 (15) |
| Cardiovascular mortality | 26 (9.5) | 1 (2) |
Abbreviations: AHI, apnea-hypopnea index; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVD, cardiovascular disease; PAP, positive airway pressure.
Unless otherwise noted, units are expressed as No. (%).
Follow-up
The Matched Cohort Study
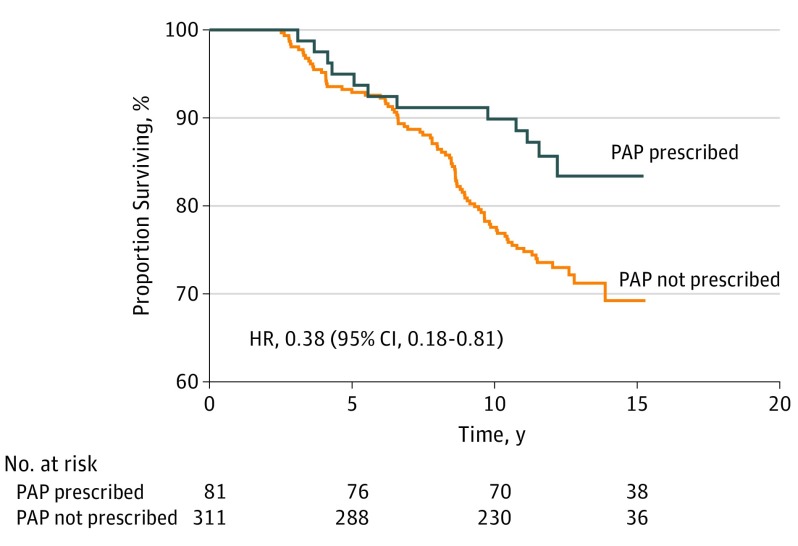
After a mean follow-up of 11.6 years in the prescribed-PAP group and 10.9 years in the nonprescribed group, 96 deaths occurred: 12 in the prescribed-PAP group and 84 in the nonprescribed-PAP group. Accordingly, the crude incidence rate of mortality was twice as high in the nonprescribed PAP group compared with the PAP-prescribed group: 24.7 and 12.8 deaths per 1000 person-years, respectively (P = .03, Figure 1). Visual inspection of survival curves further indicates that the difference in survival between the PAP-prescribed and nonprescribed groups appears after 6 to 7 years of follow-up (Figure 1). Results of the Cox analysis are presented in Table 2. The adjusted HR of all-cause mortality for participants prescribed PAP relative to those who were not was 0.38 (95% CI, 0.18-0.81). Prevalent CVD, diabetes, and former smoking status (higher risk), together with 11 to 15 years of education (lower risk), were the other significant covariates (supporting data provided in eTable 1 in the Supplement).
Figure 1. Kaplan-Meier Curves of All-Cause Mortality for Participants With and Without Prescribed PAP .
HR indicates hazard ratio; PAP, positive airway pressure.
Table 2. Incidence Rates and Adjusted HRs of Mortality for Prescription of PAPa.
| Characteristic | Patients, No. | Deaths, No. | Incidence, per 1000 Person-Years | Adjusted HR (95% CI) |
|---|---|---|---|---|
| All-cause death | ||||
| PAP not prescribed | 311 | 84 | 24.7 | 1 [Reference] |
| PAP prescribed | 81 | 12 | 12.8 | 0.38 (0.18-0.81)b |
| CV death | ||||
| PAP not prescribed | 311 | 26 | 7.6 | 1 [Reference] |
| PAP prescribed | 81 | 1 | 1.1 | 0.06 (0.01-0.68)c |
Abbreviations: CV, cardiovascular; HR, hazard ratio; PAP, positive airway pressure.
Results are from Cox regression analysis.
Adjusted for prevalent cardiovascular disease, hypertension, diabetes, body mass index, education, smoking status, and alcohol consumption.
Adjusted for prevalent cardiovascular disease only.
There were 27 deaths of CV origin: 1 in the prescribed-PAP group and 26 in the nonprescribed-PAP group (1.1 and 7.6 deaths per 1000 person-years, respectively). Exploratory analysis shows that after adjusting for prevalent CVD (no further adjustments were made owing to the small number of events in the prescribed-PAP group), the HR of CV mortality for participants with prescribed PAP relative to those without was 0.06 (95% CI, 0.01-0.68). No significant association was observed between PAP therapy and non-CVD deaths (HR, 0.55; 95% CI, 0.25-1.20).
The Propensity Score–Matched Analysis
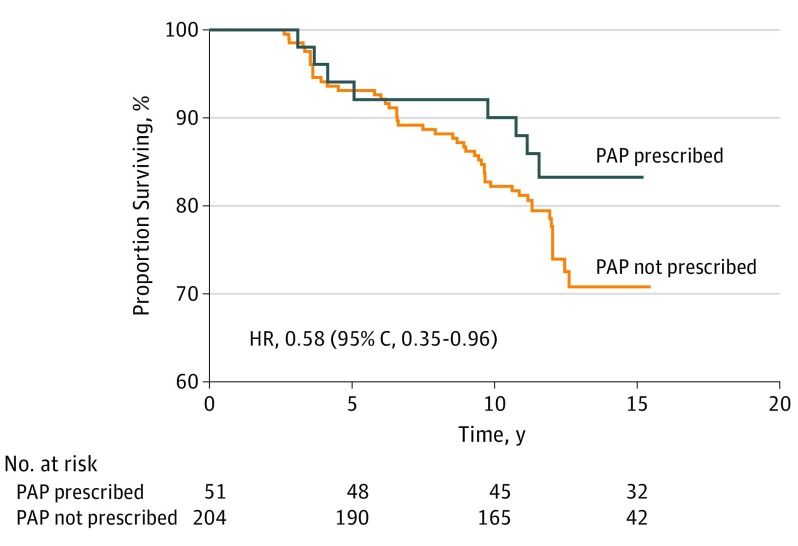
The characteristics of the PAP-prescribed group and nonprescribed group after propensity score matching are presented in the eTable 2 in the Supplement. Survival curves for all-cause mortality in PAP and nonPAP-prescription groups are presented in Figure 2, and visual inspection confirmed a delayed association of PAP prescription with reduced mortality of approximately 6 to 7 years. The HR of all-cause mortality associated with PAP prescription was 0.58 (95% CI, 0.35-0.96).
Figure 2. Kaplan-Meier Curves of All-Cause Mortality for Participants With and Without Prescribed PAP in the Propensity-Matched Cohort.
HR indicates hazard ratio; PAP, positive airway pressure.
Sensitivity Analyses
Sensitivity analyses were conducted for all-cause mortality. After bootstrapping, the 95% CIs of the HRs for prescribed PAP subjects ranged from 0.04 to 0.78, using the fully adjusted Cox regression analysis. When considering the third year of follow-up as baseline, consistent associations were found between PAP prescription and all-cause mortality (HR, 0.41; 95% CI, 0.19-0.90).
Discussion
Within the SHHS, prescription of PAP therapy was associated with a 62% reduced risk of all-cause mortality, independent of major confounders such as prevalent CVD, BMI, diabetes, and hypertension. This association appeared 6 to 7 years after PAP prescription. Exploratory analysis further suggests that prescription of PAP therapy might also be associated with a lower risk of CV mortality.
The benefit on mortality associated with PAP therapy prescription in the present study contrasts with the null findings repeatedly reported in RCTs. A first explanation could be that the benefit on mortality appears several years after PAP initiation (6 to 7 years in the present study), a mean length of follow-up usually not reached in RCTs. For instance, in the latest and largest RCT to our knowledge, the SAVE trial, the mean duration of follow-up was 3.7 years.20 A second related explanation is the small number of fatal events observed in RCTs, usually fewer than 3.3,14
Meanwhile, the results of observational studies provide a low level of evidence. A recent meta-analysis of observational studies regarding PAP therapy and all-cause mortality found only 8 relevant studies.34 Among these, some were specifically conducted in populations of elderly patients,35,36 some did not take into account confounders such as AHI or CVD,28,29 and most recorded few events during follow-up.24,27,35 Moreover, in most studies, PAP users were compared with a nonapneic control group,24,26,27,36 maximizing the contrast between the groups and raising the issue of residual confounding. Hence, the potential benefit associated with PAP was suggested indirectly. For instance, in the Wisconsin Sleep Cohort (n = 1522 participants), Young et al9 found a higher risk of mortality for participants with sleep-disordered breathing than those without, and this risk increased after excluding participants using PAP (n = 126). From this observation, the authors suggested that PAP might reduce all-cause mortality.
The study by Marti et al37 assessing 124 PAP users and 98 PAP nonusers is the sole observational study that considered major confounding factors including BMI, hypertension, and AHI. In that study, PAP therapy was associated with a significant risk reduction of all-cause mortality (adjusted HR, 0.23; 95% CI, 0.09-0.58). The present results are consistent with those of Marti et al, but also expand the findings in the following aspects. First, while patients were followed up for OSA and referred to a sleep clinic in the study by Marti et al, the present study was community-based and therefore less selected. Second, the mean follow-up time in Marti et al was 2 times shorter than in the present study (5.2 years vs 11.6 years in the prescribed PAP group).
The lower risk of mortality associated with PAP therapy could have several explanations. Systemic or pulmonary hypertension, insulin resistance, and hyperglycemia have been shown to decrease after PAP therapy.38,39 Similarly, PAP therapy has been associated with fewer arrhythmias, including atrial fibrillation, improved endothelial function (probably by reducing oxidative stress), and lower concentrations of circulating inflammatory mediators (eg, C-reactive protein and interleukin-6).40 Furthermore, the lower risk of mortality associated with PAP therapy in the present study might be mediated in part by a lower risk of vascular events, although further adjustment for stroke or myocardial infarction did not offset the benefit of PAP therapy.
Clinical Implications
Despite the limited evidence currently provided by the literature, and pending further evidence from more contemporary observational studies, prescribing PAP in patients with OSA should be pursued and encouraged given its potential major public health implication, in particular, in patients with severe OSA and comorbid obesity. In addition to the critical importance of adherence to PAP through the night, the importance of adherence over the years may be crucial, given that significant association with decreased mortality appeared several years (according to our survival curves) after PAP prescription, although this should be further investigated.
Limitations
Our study has several limitations. First, as an ancillary study from the SHHS, the results should be considered as exploratory and must be confirmed by future studies. Second, initiation and interruption of PAP therapy during follow-up could not be accounted for. Moreover, information on adherence to PAP treatment was not available. In RCTs and observational studies, mean adherence to PAP is between 3 to 6 hours per night.14,24,37 Therefore, we were not able to study the extent to which the benefit of PAP therapy prescription increased with the level of adherence to PAP therapy. Third, PAP therapy was not randomly assigned, but to limit confounding bias, we matched participants with prescribed PAP with participants not prescribed PAP of comparable age, sex, and AHI. Furthermore, we tried to minimize indication bias on PAP prescription by using propensity score matching analysis. Fourth, additional residual confounding cannot be excluded, since participants prescribed PAP might be more compliant with pharmacological treatments, might be more likely to adhere to health recommendations, and therefore might be less likely to die. Fifth, the recording methods and scoring criteria used in the SHHS do not correspond to the current standard and may underestimate AHI severity. For instance, the mean AHI of 37.9 per hour in the PAP-treated participants in the present study would correspond to much higher values using current scoring criteria and recording methods. Finally, the study population was mainly composed of participants with obesity and severe OSA. Therefore, our study results may not be generalizable to patients who are not obese and/or patients with mild to moderate OSA.
Conclusion
In this observational cohort study with 11 years follow-up, prescription of PAP therapy was associated with a substantial reduced risk of all-cause mortality, and possibly CV mortality. If confirmed in more contemporary observational studies, these findings may have strong clinical implications given the burden and the poor prognosis associated with OSA.
eTable 1. Multivariable hazard ratios and their 95% confidence intervals of the covariates included in the Cox regression analysis evaluating association between positive airway pressure and all-cause mortality.
eTable 2. Description of the population after propensity score matching.
References
- 1.Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124(19):2049-2051. doi: 10.1161/CIRCULATIONAHA.111.062190 [DOI] [PubMed] [Google Scholar]
- 2.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) . Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840-1850. doi: 10.1161/CIRCULATIONAHA.117.029400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(4):415-433. doi: 10.1001/jama.2016.19635 [DOI] [PubMed] [Google Scholar]
- 4.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haba-Rubio J, Marti-Soler H, Tobback N, et al. Sleep characteristics and cognitive impairment in the general population: the HypnoLaus study. Neurology. 2017;88(5):463-469. doi: 10.1212/WNL.0000000000003557 [DOI] [PubMed] [Google Scholar]
- 6.Nagayoshi M, Punjabi NM, Selvin E, et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med. 2016;25:156-161. doi: 10.1016/j.sleep.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somers VK, White DP, Amin R, et al. ; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing, in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080-1111. doi: 10.1161/CIRCULATIONAHA.107.189420 [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046-1053. doi: 10.1016/S0140-6736(05)74229-X [DOI] [PubMed] [Google Scholar]
- 9.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071-1078. [PMC free article] [PubMed] [Google Scholar]
- 10.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iftikhar IH, Bittencourt L, Youngstedt SD, et al. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med. 2017;30:7-14. doi: 10.1016/j.sleep.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Guo J, Sun Y, Xue L-J, et al. Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2016;20(3):965-974. doi: 10.1007/s11325-016-1319-y [DOI] [PubMed] [Google Scholar]
- 13.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314(21):2280-2293. doi: 10.1001/jama.2015.16303 [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156-166. doi: 10.1001/jama.2017.7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plante DT, Hoyt WT. Outcomes of positive airway pressure for sleep apnea. JAMA. 2017;318(20):2042. doi: 10.1001/jama.2017.16287 [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb DJ. Does obstructive sleep apnea treatment reduce cardiovascular risk?: it is far too soon to say. JAMA. 2017;318(2):128-130. doi: 10.1001/jama.2017.7966 [DOI] [PubMed] [Google Scholar]
- 17.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. ; Spanish Sleep And Breathing Network . Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161-2168. doi: 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- 18.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2012;35(12):1593-1602. doi: 10.5665/sleep.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMillan A, Bratton DJ, Faria R, et al. ; PREDICT Investigators . Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804-812. doi: 10.1016/S2213-2600(14)70172-9 [DOI] [PubMed] [Google Scholar]
- 20.McEvoy RD, Antic NA, Heeley E, et al. ; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 21.Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J. 2018;39(24):2291-2297. doi: 10.1093/eurheartj/ehx597 [DOI] [PubMed] [Google Scholar]
- 22.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115-122. doi: 10.7326/0003-4819-156-2-201201170-00006 [DOI] [PubMed] [Google Scholar]
- 23.López-Padilla D, Alonso-Moralejo R, Martínez-García MÁ, De la Torre Carazo S, Díaz de Atauri MJ. Continuous positive airway pressure and survival of very elderly persons with moderate to severe obstructive sleep apnea. Sleep Med. 2016;19:23-29. doi: 10.1016/j.sleep.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 24.Anandam A, Patil M, Akinnusi M, Jaoude P, El-Solh AA. Cardiovascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: an observational study. Respirology. 2013;18(8):1184-1190. doi: 10.1111/resp.12140 [DOI] [PubMed] [Google Scholar]
- 25.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076-2084. doi: 10.1378/chest.127.6.2076 [DOI] [PubMed] [Google Scholar]
- 26.Molnar MZ, Mucsi I, Novak M, et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax. 2015;70(9):888-895. doi: 10.1136/thoraxjnl-2015-206970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan X, Fang J, Wang L, et al. Adequate continuous positive airway pressure therapy reduces mortality in Chinese patients with obstructive sleep apnea. Sleep Breath. 2015;19(3):911-920. doi: 10.1007/s11325-014-1091-9 [DOI] [PubMed] [Google Scholar]
- 28.Jennum P, Tønnesen P, Ibsen R, Kjellberg J. All-cause mortality from obstructive sleep apnea in male and female patients with and without continuous positive airway pressure treatment: a registry study with 10 years of follow-up. Nat Sci Sleep. 2015;7:43-50. doi: 10.2147/NSS.S75166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrone O, Lo Bue A, Salvaggio A, Dardanoni G, Insalaco G. Comorbidities and survival in obstructive sleep apnoea beyond the age of 50. Eur J Clin Invest. 2013;43(1):27-33. doi: 10.1111/eci.12011 [DOI] [PubMed] [Google Scholar]
- 30.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077-1085. [PubMed] [Google Scholar]
- 31.Dean DA II, Goldberger AL, Mueller R, et al. Scaling up scientific discovery in sleep medicine: the national sleep research resource. Sleep. 2016;39(5):1151-1164. doi: 10.5665/sleep.5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redline S, Sanders MH, Lind BK, et al. ; Sleep Heart Health Research Group . Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21(7):759-767. doi: 10.1093/sleep/21.7.759 [DOI] [PubMed] [Google Scholar]
- 33.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2017;21(1):181-189. doi: 10.1007/s11325-016-1393-1 [DOI] [PubMed] [Google Scholar]
- 35.Ou Q, Chen Y-C, Zhuo S-Q, et al. Continuous positive airway pressure treatment reduces mortality in elderly patients with moderate to severe obstructive severe sleep apnea: a cohort study. PLoS One. 2015;10(6):e0127775. doi: 10.1371/journal.pone.0127775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez-García M-A, Campos-Rodríguez F, Catalán-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909-916. doi: 10.1164/rccm.201203-0448OC [DOI] [PubMed] [Google Scholar]
- 37.Marti S, Sampol G, Muñoz X, et al. Mortality in severe sleep apnoea/hypopnoea syndrome patients: impact of treatment. Eur Respir J. 2002;20(6):1511-1518. doi: 10.1183/09031936.02.00306502 [DOI] [PubMed] [Google Scholar]
- 38.Martínez-Cerón E, Barquiel B, Bezos A-M, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. a randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476-485. doi: 10.1164/rccm.201510-1942OC [DOI] [PubMed] [Google Scholar]
- 39.Barbé F, Durán-Cantolla J, Capote F, et al. ; Spanish Sleep and Breathing Group . Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718-726. doi: 10.1164/rccm.200901-0050OC [DOI] [PubMed] [Google Scholar]
- 40.Baessler A, Nadeem R, Harvey M, et al. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers–a meta-analysis. J Inflamm (Lond). 2013;10:13. doi: 10.1186/1476-9255-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Multivariable hazard ratios and their 95% confidence intervals of the covariates included in the Cox regression analysis evaluating association between positive airway pressure and all-cause mortality.
eTable 2. Description of the population after propensity score matching.