Abstract
Importance
Intake of dietary docosahexaenoic acid (DHA) among toddlers is low. Supplementation may benefit developmental outcomes of toddlers who were born preterm.
Objective
To determine whether 6 months of daily DHA supplementation improves developmental outcomes of toddlers who were born preterm.
Design, Setting, and Participants
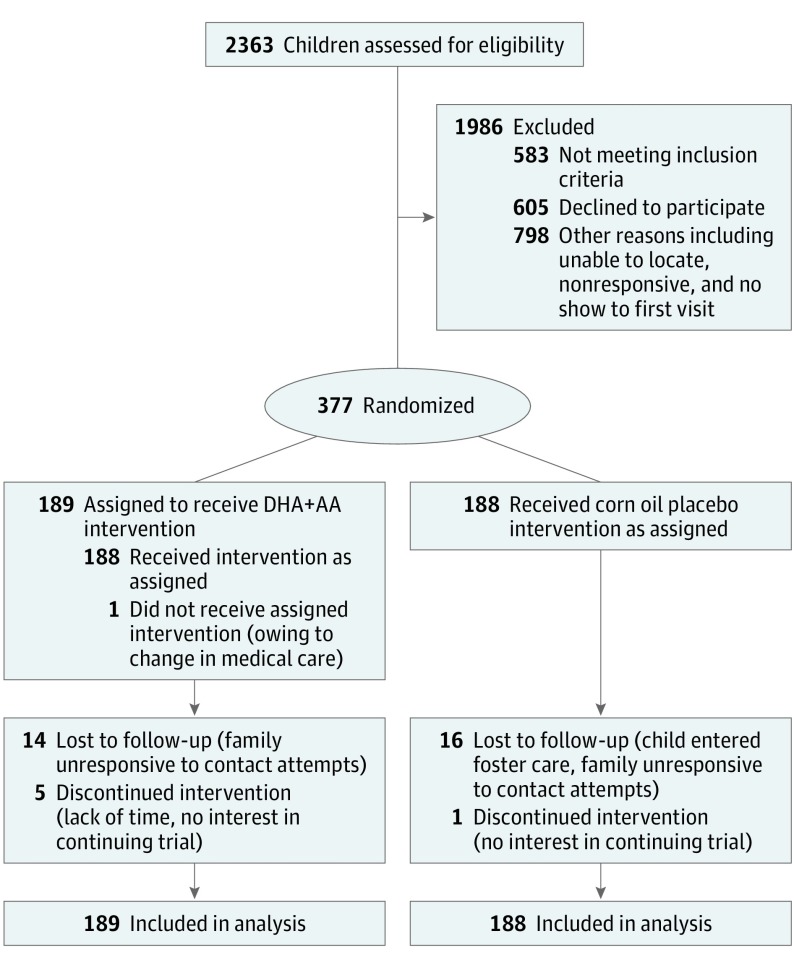
A randomized, fully masked, placebo-controlled trial was conducted from April 26, 2012, to March 24, 2017, at a large US pediatric academic center with 9 neonatal intensive care units. Children born at less than 35 weeks’ gestation who were 10 to 16 months corrected age underwent 6 months of intervention. Of 2363 children assessed, 982 were eligible, 605 declined, and 377 enrolled and were randomized. Analyses were according to intent to treat.
Interventions
One-to-one allocation to receive daily microencapsulated DHA, 200 mg, and arachidonic acid (AA), 200 mg (DHA+AA), or microencapsulated corn oil (placebo).
Main Outcomes and Measures
The primary outcome specified a priori was Bayley Scales of Infant and Toddler Development, third edition (Bayley-III), cognitive composite score at 16 to 22 months corrected age. Secondary outcomes were Bayley-III language and motor composite scores and Infant Behavior Questionnaire–Revised and Early Childhood Behavior Questionnaire effortful control and activity level scores. Subgroup analyses defined a priori were by income, sex, and birth weight.
Results
Among 377 children randomized and included in the analysis (182 girls and 195 boys; median corrected age, 15.7 months), 338 children (89.7%) had complete data on the primary outcome. Bayley-III cognitive scores did not differ between the DHA+AA and placebo groups (difference in change, 0.5 [95% CI, –1.8 to 2.8]; effect size, 0.05; P = .66). Assignment to the DHA+AA group had a small to medium negative effect on Bayley-III language scores among children with lower birth weights (eg, a child with a birth weight of 1000 g assigned to receive DHA+AA experienced a 4.1-point relative decrease, while a child assigned to placebo did not; P = .03 for interaction). Supplementation had a similar negative effect on effortful control scores among children with annual household incomes greater than $35 000 (difference in change, –0.3 [95% CI, –0.4 to –0.1]; effect size, –0.37; P = .01). Bayley-III motor scores and activity level scores were unaffected.
Conclusions and Relevance
Daily supplementation with 200 mg of DHA and 200 mg of AA for 6 months resulted in no improvement in cognitive development and early measures of executive function vs placebo, and may have resulted in negative effects on language development and effortful control in certain subgroups of children. These findings do not support DHA supplementation in the second year of life for children who are born preterm.
Trial Registration
ClinicalTrials.gov Identifier: NCT01576783
This randomized clinical trial examines whether 6 months of daily docosahexaenoic acid supplementation during the second year of life improves developmental outcomes of children who were born preterm.
Key Points
Question
What is the effect of dietary supplementation with docosahexaenoic acid during the second year of life on the development of children who were born preterm?
Findings
In a randomized clinical trial of 377 US children aged 1 year who were born preterm, 6 months of daily docosahexaenoic acid supplementation resulted in no overall effect on Bayley Scales of Infant and Toddler Development, third edition cognitive composite scores compared with placebo.
Meaning
In toddlers born prematurely, daily supplementation with docosahexaenoic acid did not result in improvements in global cognitive development.
Introduction
Cognitive deficits are common, burdensome long-term consequences of prematurity.1 Around age 1 year, many preterm children enter early intervention programs, which can improve long-term outcomes. However, these programs are costly, require substantial family involvement, and do not eliminate the developmental gap.2,3,4 Children with mild deficits may receive no intervention but are most of those born preterm and remain at risk for poor outcomes.5 Disparities in outcomes also exist by socioeconomic status.6
Docosahexaenoic acid (DHA), an ω-3 long-chain polyunsaturated fatty acid, is the brain’s dominant structural fatty acid.7 Docosahexaenoic acid is crucial for neurotransmitter function, signal transduction, gene expression, neurogenesis, and anti-inflammation.8,9,10,11,12 Preterm infants are born DHA deficient.13 In some trials, dietary DHA supplementation initiated in the neonatal period has improved global developmental outcomes in preterm infants.14,15 Certain sex, birth weight, or socioeconomic groups may experience differential effects of DHA supplementation, but results have been inconsistent.16,17
The second year of life is also a period of DHA deficiency because dietary intake of DHA decreases from approximately 50 mg/d in midinfancy to 20 mg/d after the child weans from breast milk or formula.18,19 Nevertheless, the brain continues incorporating DHA rapidly to at least age 2 years.7 Despite no rigorous trial evidence supporting DHA supplementation during the second year of life for children born preterm, numerous DHA-supplemented foods and formula products are marketed for toddlers. This trial tested the efficacy of DHA supplementation in improving developmental outcomes among toddlers born across a spectrum of premature gestational ages.
Methods
Trial Design and Setting
Omega Tots was a randomized, fully masked, placebo-controlled trial that began with a seed grant–funded phase to enroll 112 children; enrollment expanded with subsequent funding (trial protocol in Supplement 1). The study physician reviewed adverse events. The study was approved by the Nationwide Children’s Hospital (NCH) Institutional Review Board. Children’s caregivers provided written informed consent.
Participants and Trial Procedures
A census identified all children aged 10 to 16 months (adjusted for prematurity) born at less than 35 weeks’ gestation who were former patients in 1 of 9 NCH-affiliated neonatal intensive care unit or referred to the neonatology clinic for clinical follow-up. Electronic medical records provided initial eligibility and neonatal data. Eligible children weighed between the 5th and 95th percentiles for corrected age and sex per World Health Organization standards,20 had discontinued consumption of human milk and formula, and had English as their primary language. Children were excluded for consuming fatty acid supplements, fatty fish, or nutritional support beverages with DHA more than twice weekly; having allergies to fish, corn, or soy; planning to relocate; or having a major malformation or feeding, metabolic, or digestive disorder precluding participation or absorption. Recruitment occurred from April 26, 2012, to September 2, 2016; all children completed the study by March 24, 2017.
Caregivers were contacted by mail and telephone to invite participation. Three study visits occurred: baseline (day 0), interim (day 60 ± 14), and final (day 180 ± 20, at 16-22 months’ corrected age). Baseline questionnaires included demographics, infant feeding, health care, and a brief food frequency questionnaire previously validated against blood fatty acid values for intake of DHA and eicosapentaenoic acid (EPA, a precursor to DHA).21 Caregivers could select 1 or more race and ethnicity category for their child to enable comparison with the US preterm population. The Bayley Scales of Infant and Toddler Development, third edition (Bayley-III) was administered by a trained research assistant.22 Bayley-III was selected to offer comparability with prior infant trials and because it is used clinically to evaluate preterm children.15,17,23,24 It provides subscale composites for cognitive, language (receptive and expressive), and motor (fine and gross) skills (standardized mean, 100; SD, 15). Caregivers completed the Infant Behavior Questionnaire–Revised (IBQ-R; very short form) to assess effortful control (12 items) and activity level (3 items).25 The IBQ-R measures early temperament-based inhibitory control, a key component of executive function as children mature. Scores range from 1 to 7, with higher scores indicating greater frequency of behaviors in the past week. Effortful control and activity level were selected as secondary outcomes because children born preterm often exhibit impairments in executive functions, such as working memory and effortful control, and dysregulated behaviors, such as hyperactivity and inattention, and these impairments are associated with poor academic outcomes.26,27 Hyperactivity and inattention have been shown to be reduced with ω-3 supplementation.28
The final visit repeated the Bayley-III and food frequency questionnaire. Caregivers completed the Early Childhood Behavior Questionnaire very short form, the companion to the IBQ-R very short form but for older children, with demonstrated longitudinal continuity and identical score interpretations.29,30 Caregivers were asked to guess their child’s randomization assignment. Families with difficulty keeping appointments were rescheduled even if outside the target window.
Randomization, Masking, and Intervention
Children were allocated to 1 of 4 masked color codes—2 for the treatment group, which received DHA and arachidonic acid (AA), and 2 for placebo—using a randomization scheme with varying block size of multiples of 4 and 8 and with 1:1 allocation to the DHA+AA group or placebo group (Figure). A statistician (A.N.T.) with no contact with participants prepared the scheme; assigned ID numbers; prepared opaque, sealed, tamper-resistant envelopes; and conducted the statistical analysis with another statistician (J.R.). Twins and triplets were randomized as sets to prevent crossover. Each sequentially numbered envelope was prelabeled with the next ID number and was opened by a research assistant to assign children to the DHA+AA group or placebo group upon caregiver’s provision of written informed consent. All caregivers, investigators, and staff remained masked throughout. One of the statisticians (J.R.) was unmasked after data collection ended. The principal investigator (S.A.K.) was unmasked after analysis.
Figure. Participant CONSORT Flow Diagram, Omega Tots Trial, 2012-2017.
DHA+AA indicates daily microencapsulated docosahexaenoic acid, 200 mg, and arachidonic acid, 200 mg.
The DHA+AA group was assigned to 180 days of daily oral supplementation with dissolvable, microencapsulated DHA, 200 mg (from Schizochytrium species algal oil), and AA, 200-mg (from fungal Mortierella alpina oil) powder (DSM Nutritional Products). The dose was selected to approximate the peak daily DHA consumed per body weight during infancy (ie, for a typical 4-month-old infant, 20 mg/kg/d). The 180-day period was selected to ensure significant incorporation of DHA into neuronal cell membranes while maximizing enrollment and retention. This product is a dietary supplement under US Food and Drug Administration regulations, and an Investigational New Drug application was filed (No. 112885). The placebo group was assigned to receive 400 mg of daily microencapsulated corn oil powder. Both products came in identical foil packets and were dissoluble in milk. The investigational drug service dispensed the masked products directly to families.
Outcomes
The Bayley-III cognitive composite score was chosen a priori as the primary outcome measure because prior trials of preterm neonates have most consistently reported effects on global cognitive development measured with the Bayley Scales of Infant Development–2 Mental Development Index.15,17,24,31 The Bayley-III language and motor composite scores and the IBQ-R and Early Childhood Behavior Questionnaire effortful control and activity level scores were specified a priori as secondary outcomes.
Fatty Acid Biomarkers
Blood was collected via venipuncture to examine erythrocyte fatty acid levels at baseline and the final visit. One funder of the study prohibited collection of biospecimens, so children enrolled after May 2015 (1 month after the funding started and procedures were put in place to manage this issue) were randomly allocated before recruitment to undergo blood collection or not. Red blood cells were separated from whole blood by centrifugation at collection and stored at –80°C. Esterified fatty acids were isolated using organic extraction, then hydrolyzed and methylated using standard techniques.32,33 Resultant fatty acid methyl esters were separated and quantified using gas chromatography with flame ionization detection. The fatty acid contents were quantified using experimentally derived standard curves.
Adherence and Follow-up
Adherence was calculated as the packets consumed out of those dispensed based on diaries. Families were contacted regularly to address adherence and participation barriers.
Statistical Analysis
A sample size of 448 was planned to achieve greater than 80% power to detect a 4.2-point difference (0.27 SD) in Bayley-III cognitive composite scores with 10% loss to follow-up. The product manufacturer discontinued production per preexisting business plans once 377 participants were enrolled. This discontinuation had a negligible effect on power; a sample size of 377 provided 80% power to detect a 0.29-SD difference in Bayley-III scores. This effect size is similar to those reported for several subgroups in the largest DHA trial involving preterm neonates and would be meaningful at the population level.15
Except when noted otherwise, analyses were conducted according to intent to treat (all randomized children were included). No interim analyses were conducted. No correction for multiple comparisons was made because only 1 primary outcome was analyzed. The Bayley-III plus IBQ-R and Early Childhood Behavior Questionnaire continuous scores, calculated per the manuals and age-corrected for prematurity when prescribed, served as the outcome measures.22 To address statistical nonindependence because twins and triplets were included, all analyses included a random effect for family within a mixed model.
Analyses compared the change between baseline and the final visit in outcome scores between groups, controlling for baseline scores, using a mixed-effects regression approach (analogous to analysis of covariance) that leverages maximum likelihood to include children with missing outcome data.34 Treatment-by-time interaction terms were included as fixed effects and served as estimates of treatment effect. No demographic or clinical characteristics were included as covariates. Group mean differences divided by the SD of the residuals from an analysis of covariance model were calculated as standardized effect sizes (ie, Cohen d) for each outcome based on the model already mentioned except that the random within-family effect was excluded to enable estimation of effect size using a conventional approach. A post hoc sensitivity analysis repeated the primary analysis, with outcome data treated as missing for children whose last visit occurred more than 200 days after randomization.
Prespecified subgroup analyses were conducted to explore effects by household income (≤$35 000 vs >$35 000), child sex, and birth weight (<1250 g vs ≥1250 g); the latter 2 variables mirror the trial by Makrides et al.15 Interactions were tested by 3-way interaction terms including time, treatment, and the moderator. Post hoc analyses explored alternative specifications of categorical moderators (income, ≤$20 000 vs >$20 000 and birth weight as a continuous variable) when the prespecified analyses revealed interactions with P < .20.
The Bayley-III analysis was repeated with post hoc dichotomization at a score of 85 (mild or moderate delay) and analyzed using generalized estimating equations and logistic regression, adjusted for baseline score. The number of adverse events per child, the change in erythrocyte fatty acids, and the change in dietary DHA and EPA (aside from the supplement) were examined by treatment group using mixed-effects regression, with a random effect for within-family dependence. Analyses used SAS, version 9.3 (SAS Institute Inc). All P values were from 2-sided tests, and results were deemed statistically significant at P < .05.
Results
The children screened for eligibility, enrolled, and followed up through the trial are displayed in the Figure. All but 1 of 377 children received their allocated intervention. None of the children were randomized out of sequence. Primary outcome data were available for 338 children (89.7%; same proportion in each group). Characteristics were similar across treatment groups (Table 1). Gestational age ranged from 23 to 34 completed weeks, and 64 children (17.0%) were small for gestational age.
Table 1. Participant Demographic and Clinical Characteristics at Baseline.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| DHA+AA (n = 189) | Placebo (n = 188) | All (N = 377) | |
| Maternal educational levela | |||
| High school or GED or less | 46/187 (24.6) | 56/184 (30.4) | 102/371 (27.5) |
| Some college or associate’s degree | 73/187 (39.0) | 59/184 (32.1) | 132/371 (35.6) |
| Bachelor’s degree or higher | 68/187 (36.4) | 69/184 (37.5) | 137/371 (36.9) |
| Spouse or partner educational level | |||
| High school or GED or less | 36/126 (28.6) | 25/113 (22.1) | 61/239 (25.5) |
| Some college or associate’s degree | 48/126 (38.1) | 45/113 (39.8) | 93/239 (38.9) |
| Bachelor’s degree or higher | 42/126 (33.3) | 43/113 (38.1) | 85/239 (35.6) |
| Maternal age, mean (SD), y | 30.6 (6.1) | 30.7 (7.1) | 30.7 (6.6) |
| Maternal marital status | |||
| Married or living with partner | 126/185 (68.1) | 115/175 (65.7) | 241/360 (66.9) |
| Separated or divorced | 7/185 (3.8) | 8/175 (4.6) | 15/360 (4.2) |
| Single: never married or not living with partner | 52/185 (28.1) | 52/175 (29.7) | 104/360 (28.9) |
| Household size, median (IQR) | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) | 4.0 (3.0-5.0) |
| Public or no health insurance | 96/188 (51.1) | 91/184 (49.5) | 187/372 (50.3) |
| Annual household income, $ | |||
| <20 000 | 43/188 (22.9) | 42/184 (22.8) | 85/372 (22.8) |
| 20 000 to <35 000 | 42/188 (22.3) | 47/184 (25.5) | 89/372 (23.9) |
| 35 000 to <45 000 | 38/188 (20.2) | 37/184 (20.1) | 75/372 (20.2) |
| ≥45 000 | 65/188 (34.6) | 58/184 (31.5) | 123/372 (33.1) |
| Have a medical home | 58/105 (55.2) | 49/100 (49.0) | 107/205 (52.2) |
| Sets of twins or triplets | 29 | 26 | 55 |
| Female sex | 84 (44.4) | 98 (52.1) | 182 (48.3) |
| Child’s race | |||
| White | 114 (60.3) | 122 (64.9) | 236 (62.6) |
| Black or African American | 52 (27.5) | 53 (28.2) | 105 (27.9) |
| Asian or Pacific Islander | 2 (1.1) | 1 (0.5) | 3 (0.8) |
| Other or multiple | 21 (11.1) | 12 (6.4) | 33 (8.8) |
| Child’s ethnicity, Hispanic | 7 (3.7) | 10 (5.3) | 17 (4.5) |
| Gestational age at delivery, median (IQR), completed wk | 32.0 (30.0-34.0) | 32.0 (30.0-34.0) | 32.0 (30.0-34.0) |
| Birth weight, mean (SD), gb | 1705 (534) | 1749 (570) | 1727 (552) |
| Birth weight <1250 g | 45/188 (23.9) | 36 (19.1) | 81/376 (21.5) |
| Small for gestational age | 32/188 (17.0) | 32 (17.0) | 64/376 (17.0) |
| Child weight at baseline, mean (SD), g | 10 164 (1041) | 10 144 (1139) | 10 154 (1090) |
| Attended neonatology follow-up clinic | 102 (54.0) | 107 (56.9) | 209 (55.4) |
| Received community-based early intervention services | 66 (34.9) | 79 (42.0) | 145 (38.5) |
| Received breast milk | 168 (88.9) | 163 (86.7) | 331 (87.8) |
| Breast milk feeding duration, median (IQR), d | 117 (44-269) | 101 (41-209) | 113 (44-239) |
| Dietary DHA+EPA intake before randomization, median (IQR), mg/d | 44.3 (29.8-83.5) | 55.3 (26.0-95.0) | 48.0 (26.8-90.8) |
| Child age at baseline (corrected for prematurity), median (IQR), mo | 15.7 (13.8-16.5) | 15.6 (13.1-16.5) | 15.7 (13.6-16.5) |
| Child age at baseline (unadjusted for prematurity), median (IQR), mo | 17.3 (15.7-18.3) | 17.4 (15.3-18.4) | 17.3 (15.6-18.4) |
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GED, General Education Development certificate; IQR, interquartile range.
Children with missing data include 6 for maternal education, 2 for partner’s education (of the 241 with a partner), 5 for maternal age, 17 for maternal marital status, 2 for household size, 5 for health insurance, 5 for income, 0 for medical home (only the 205 last-enrolled families were surveyed), 1 for birth weight and small for gestational age, 4 for breast milk feeding duration (among those who were fed breast milk), and 1 for dietary DHA+EPA intake.
Birth weight ranged from 595 to 3545 g among those who received any breast milk (n = 167 in the DHA+AA group and n = 160 in the placebo group).
Adherence, Adverse Events, and Masking
Children consumed a mean of 80.6% of the packets (of children with adherence data, mean of 80.6% in the DHA+AA group and mean of 80.7% in the placebo group). No complaints about products’ sensory characteristics (eg, taste or smell) were reported. A total of 256 children experienced at least 1 adverse event, totaling 683 events (1.7 events per child in the DHA+AA group and 2.0 events per child in the placebo group; group difference, –0.37; 95% CI, –0.07 to 0.80; P = .10), mainly minor gastrointestinal illness and respiratory tract infections. No adverse events were judged to be serious and related to the intervention. At the last visit, 69 of 126 caregivers with children in the DHA+AA group (54.8%) and 73 of 122 caregivers with children in the placebo group (59.8%) guessed that their child was assigned to the DHA+AA group (P = .42). No participant or staff was unmasked.
Erythrocyte Fatty Acids and Diet
Of 206 children assigned to undergo blood collection, 205 had analyzable samples at the baseline visit and 171 had analyzable samples at the final visit. Baseline DHA levels were low, and the DHA+AA group experienced statistically significantly greater mean (SD) increases in erythrocyte DHA and AA compared with the placebo group (reported as percentage of total fatty acid: DHA, 1.1% [1.0%] vs –0.1% [0.2%]; and AA, 2.0% [2.6%] vs –0.2% [1.1%]) (Table 2). Dietary DHA and EPA intake unrelated to the intervention increased similarly in both groups (median DHA+EPA intake at the end of the trial for DHA+AA group, 67.0 mg/d; placebo group, 60.0 mg/d; group difference in change, –0.70 mg; 95% CI, –20.9 to 19.5; P = .95).
Table 2. Changes in Erythrocyte Fatty Acid Levelsa.
| Erythrocyte Fatty Acidb | Median (IQR), mol% | Change, Mean (SD), mol% | Difference in Change (95% CI), mol% | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End of Trial | ||||||
| DHA+AA (n = 99) | Placebo (n = 106) | DHA+AA (n = 85) | Placebo (n = 88) | DHA+AA | Placebo | ||
| Linoleic acid (18:2n-6) | 30.2 (27.6 to 32.6) | 29.3 (26.6 to 31.9) | 28.5 (24.9 to 31.2) | 29.9 (27.2 to 32.6) | −2.1 (5.2) | 0.6 (4.4) | −2.2 (−3.4 to −0.9) |
| Arachidonic acid (20:4n-6) | 5.7 (4.9 to 6.6) | 5.6 (4.8 to 6.4) | 7.7 (6.0 to 9.4) | 5.4 (4.5 to 6.2) | 2.0 (2.6) | −0.2 (1.1) | 2.2 (1.7 to 2.8) |
| Total n-6 | 37.3 (34.1 to 39.4) | 36.0 (33.7 to 38.9) | 37.5 (33.8 to 40.2) | 37.1 (33.6 to 39.7) | 0.2 (6.0) | 0.4 (5.0) | 0.4 (−1.0 to 1.7) |
| α-Linolenic acid (18:3n-3) | 0.7 (0.5 to 0.9) | 0.7 (0.5 to 0.9) | 0.6 (0.5 to 0.8) | 0.6 (0.5 to 0.7) | −0.1 (0.4) | 0.0 (0.3) | 0.0 (−0.1 to 0.1) |
| Eicosapentaenoic acid (20:5n-3) | 0.4 (0.3 to 0.4) | 0.4 (0.3 to 0.4) | 0.3 (0.3 to 0.4) | 0.3 (0.3 to 0.4) | 0.0 (0.1) | −0.1 (0.2) | 0.0 (0.0 to 0.1) |
| Docosapentaenoic acid (22:5n-3) | 0.4 (0.4 to 0.5) | 0.4 (0.4 to 0.5) | 0.3 (0.3 to 0.4) | 0.4 (0.4 to 0.5) | −0.1 (0.1) | 0.0 (0.1) | −0.1 (−0.1 to 0.0) |
| Docosahexaenoic acid (22:6n-3) | 1.0 (0.8 to 1.1) | 0.9 (0.8 to 1.1) | 2.3 (1.1 to 3.0) | 0.8 (0.7 to 1.1) | 1.1 (1.0) | −0.1 (0.2) | 1.2 (1.0 to 1.5) |
| Total n-3 | 2.5 (2.2 to 2.8) | 2.5 (2.3 to 2.8) | 3.6 (2.6 to 4.3) | 2.3 (2.1 to 2.6) | 0.9 (1.1) | −0.2 (0.5) | 1.2 (0.9 to 1.4) |
| n-6:n-3 ratio | 14.9 (13.0 to 16.4) | 14.4 (12.7 to 15.6) | 10.6 (8.4 to 12.8) | 15.9 (14.2 to 17.8) | −3.2 (3.9) | 1.6 (2.8) | −4.6 (−5.5 to −3.7) |
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; IQR, interquartile range; mol%, percentage of total quantified fatty acids.
A total of 205 children had analyzable samples at the baseline visit.
The DHA+AA supplement contained only DHA and AA. Because units are determined as a percentage of total fatty acids, other fatty acids may show a decrease because DHA and AA increased.
Primary Outcome
The change in primary outcome did not differ between the DHA+AA group and placebo group (0.5-point difference in change; both groups declined, but DHA+AA experienced less decline; effect size = 0.05; P = .66) (Table 3). Mean (SD) baseline Bayley-III cognitive composite scores (based on corrected age, 100.5 [12.7]; 36 of 376 [9.6%] with a score <85) were similar to norms. No statistically significant interactions were observed between treatment assignment and income (≤$35 000 vs >$35 000), sex, or birth weight (<1250 g vs ≥1250 g). Supplementation had no effect on the odds of mild or moderate developmental delay (Table 4). The sensitivity analysis, in which the primary outcome was set to missing for 34 children whose final visit was late (median, 17 days), yielded results similar to the main analysis (–0.07-point difference in change; 95% CI, –2.50 to 2.36; P = .95).
Table 3. Change From Baseline to Trial Completion in Developmental Outcomes.
| Outcome | DHA+AA/Placebo, No. at Baseline | Baseline Score, Mean (SD) | DHA+AA/Placebo, No. at End of Trial | Score at End of Trial, Mean (SD) | Change, Mean (SD) | Difference in Change (95% CI)a | ANCOVA Effect Size | P Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA+AA | Placebo | DHA+AA | Placebo | DHA+AA | Placebo | ||||||
| Cognitive composite | 188/188b | 99.7 (13.9) | 101.3 (11.4) | 169/169 | 97.7 (11.8) | 97.7 (11.5) | −2.6 (13.3) | −3.7 (12.2) | 0.5 (−1.8 to 2.8) | 0.05 | .66 |
| Language composite | 187/188 | 92.5 (12.1) | 93.2 (12.1) | 169/169 | 92.5 (12.3) | 93.2 (13.2) | −0.5 (12.0) | 0.2 (12.6) | −0.7 (−3.1 to 1.6) | −0.07 | .55 |
| Motor composite | 187/188 | 96.1 (13.8) | 96.3 (11.1) | 168/169 | 96.3 (10.9) | 96.0 (12.5) | −0.6 (12.5) | −0.4 (12.7) | 0.2 (−2.1 to 2.4) | 0.02 | .88 |
| Effortful control | 188/185 | 5.3 (0.7) | 5.2 (0.8) | 160/163 | 4.6 (0.7) | 4.7 (0.8) | −0.7 (0.8) | −0.5 (0.8) | −0.1 (−0.3 to 0.0) | −0.16 | .13 |
| Activity level | 188/186 | 4.8 (1.2) | 4.9 (1.2) | 159/163 | 5.4 (1.1) | 5.4 (1.1) | 0.6 (1.5) | 0.5 (1.5) | 0.0 (−0.2 to 0.3) | 0.01 | .76 |
Abbreviations: AA, arachidonic acid; ANCOVA, analysis of covariance; DHA, docosahexaenoic acid.
Difference in Change column is based on a mixed-effects model (analogous to ANCOVA) using maximum likelihood to account for missing data (39 children did not participate in the last trial visit) and thus may differ from the results of the raw summary statistics presented for the within-group mean change and SD. Positive numbers indicate the effect tended to favor DHA even if not statistically significant.
One child’s baseline Bayley-III assessment was deemed invalid.
Table 4. Odds of Mild or Moderate Developmental Delay.
| Outcome | DHA+AA/Placebo, No. at Baseline | Baseline Score <85, No. (%) | DHA+AA/Placebo, No. at End of Trial | Trial End Score <85, No. (%) | Odds Ratio (95% CI)a | ||
|---|---|---|---|---|---|---|---|
| DHA+AA | Placebo | DHA+AA | Placebo | ||||
| Cognitive composite | 188/188 | 21 (11.1) | 15 (8.0) | 169/169 | 14 (8.3) | 11 (6.5) | 1.26 (0.55-2.91) |
| Language composite | 187/188 | 43 (23.0) | 39 (20.7) | 169/169 | 41 (24.3) | 38 (22.5) | 1.03 (0.59-1.78) |
| Motor composite | 187/188 | 25 (13.4) | 26 (13.8) | 168/169 | 14 (8.3) | 20 (11.8) | 0.81 (0.36-1.81) |
Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid.
All models adjusted for baseline score (<85 vs ≥85).
Secondary Outcomes
No difference was detected between the DHA+AA and placebo groups in the change in Bayley-III language or motor composite scores. Mean (SD) language scores (92.9 [12.1]; 82 of 375 [21.9%] with a score <85) and motor scores (96.2 [12.5]; 51 of 375 [13.6%] with a score <85) at baseline were lower than norms. A significant interaction was observed with birth weight (continuous) in post hoc analysis for the language outcome, with a moderate negative effect of DHA+AA supplementation observed among children with lower birth weights (eg, a child with a birth weight of 1000 g assigned to receive DHA+AA experienced a relative 4.1-point decrease in language scores compared with a child assigned to receive placebo based on the model estimate; regression slope of treatment effect for 1-g increase in birth weight, 0.0046; 95% CI, 0.0005-0.0088; P = .03 for interaction) (eTable in Supplement 2).
There was no overall treatment effect on the secondary outcomes of effortful control and activity level, but in prespecified subgroup analyses, children from households with income greater than $35 000 exhibited a small to medium decline in effortful control scores when assigned to the DHA+AA group compared with those assigned to the placebo group (difference in change, –0.3; effect size, –0.37; P = .01; P = .17 for interaction) (eTable in Supplement 2). Post hoc analysis revealed that the effect applied to all groups with income greater than $20 000, while children from families with income of $20 000 or less experienced no negative effect (difference between treatment and placebo groups in change comparing ≤$20 000 and >$20 000 groups, –0.44; 95% CI, –0.81 to –0.06; P = .02).
Two other interactions with .05 < P < .20 were observed: income and motor composite scores as well as sex and activity level. In post hoc analyses, alternative specification of the income variable did not reveal a statistically significant result for the test for interaction.
Discussion
In this randomized, masked trial of 1-year-old children born preterm, 6 months’ daily DHA+AA supplementation did not improve cognitive development compared with placebo. Motor development and activity level ratings were also unaffected. Subgroup analyses by income, sex, and birth weight with prespecified categories (≤$35 000 vs >$35 000 and <1250 g vs ≥1250 g) suggested treatment effects in certain birth weight groups on the secondary outcome of language and treatment effects in certain income groups on the secondary outcome of effortful control. Specifically, DHA supplementation resulted in moderate negative effects on early language ability for the children with the lowest birth weight and moderate declines in effortful control among children from all but the poorest households.
To our knowledge, this is the first trial to test the effect of supplementation on developmental outcomes among children born preterm just after weaning to cow’s milk and foods low in long-chain ω-3 fatty acids. Despite the developmental differences between infants and toddlers, comparison of our findings with prior trials of preterm neonates is informative. The findings for our primary outcome are compatible with the largest trial of Australian preterm neonates that reported no overall short-term cognitive benefit from high-dose DHA based on the Bayley Mental Developmental Index at age 18 months.15 However, girls in that study assigned to receive high-dose vs low-dose DHA exhibited a 4.5-point advantage on the Bayley Mental Developmental Index. Other trials in infants have reported no cognitive benefit at 12 to 18 months or reported benefits specific to boys only.17,35 We observed no sex-specific differences in our study.
Makrides et al15 also observed a 43% reduced risk of mild mental delay from high-dose supplementation in children with birth weights less than 1250 g. Our result for poorer language outcomes among children with the lowest birth weight who received DHA contrasts that finding, although the Bayley-II, used in the study by Makrides et al,15 did not separately assess language. For the smallest children who are at particular risk for language delay, this possible negative effect is concerning.
To our knowledge, this is the first trial involving preterm children to consider socioeconomic status as a potential treatment effect modifier. The moderate negative effect of DHA on effortful control among all but the very lowest income groups may have been a chance finding and should be interpreted with caution but is concerning given the importance of self-regulation for long-term academic and social outcomes.36,37 The previously mentioned Australian trial15 and another trial that studied DHA supplementation in pregnant women reported poorer parent-reported executive function and more dysregulated behavior in their intervention groups when followed up to 4 or 7 years of age.38,39,40 Although the Omega Tots toddlers in our study were too immature to evaluate fine-grained aspects of executive function, ours is the earliest report of negative effects in this domain to our knowledge. Effortful control was reported by caregivers, but caregivers were poor at guessing their child’s group assignment and so were unlikely to give biased reports.
Effects may have been isolated to the children with the lowest birth weight and higher income groups perhaps because these children were not truly DHA deficient, and supplementation upset a previously healthy balance of fatty acids in the brain, resulting in poorer neurotransmitter function and consequent changes in behavior in children in the higher income groups. The ω-6 to ω-3 ratio has previously been shown to be associated with executive function in children.41 However, baseline DHA status in this sample appeared to be lower than reported for several other samples of young children, although measurement units vary across studies.42,43,44
Strengths and Limitations
Strengths of this study include its large, socioeconomically diverse sample, broad inclusion criteria to improve generalizability to the preterm population, masking, and multi-informant assessment of outcomes. The sample was smaller than planned, but this factor had a negligible effect. The study was powered for the primary outcome, not to detect subgroup differences. Some children were lost to follow-up, but our statistical methods leveraged their baseline data in estimating effects. As a tradeoff for our broad inclusion criteria, the median gestational age of children in this study was higher than in some infant trials. This increased heterogeneity may have hampered the detection of treatment effects specific to children with the lowest birth weights. Finally, the children were too young for robust evaluation of executive function and cognitive ability, which limited estimation of long-term outcomes, but follow-up would help clarify whether supplementation during toddlerhood has detectable effects later.
Conclusions
Among 1-year-old children born at less than 35 weeks’ gestation, DHA+AA supplementation had no developmental benefit vs placebo. Small- to medium-magnitude negative effects were observed for language ability among children of lower birth weight and for effortful control for children from all but the very lowest income strata. Ongoing follow-up is needed, particularly given the ubiquity of DHA-supplemented formulas and foods for children and the cognitive deficits associated with prematurity.
Trial Protocol
eTable. Change from Baseline to Trial Completion in Developmental Outcomes, Subgroup Results, Omega Tots Trial (n = 377), 2012-2017
References
- 1.Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 2.Ramey SL, Ramey CT Early experience and early intervention for children at risk for developmental delay and mental retardation. In: Ramey SL, Ramey CT, Friedlander MJ, eds. Mental Retardation and Developmental Disabilities Research Reviews, No. 5. New York, NY: Wiley & Sons; 1999. [Google Scholar]
- 3.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900-1902. doi: 10.1126/science.1128898 [DOI] [PubMed] [Google Scholar]
- 4.Duncan AF, Watterberg KL, Nolen TL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Effect of ethnicity and race on cognitive and language testing at age 18-22 months in extremely preterm infants. J Pediatr. 2012;160(6):966-971. doi: 10.1016/j.jpeds.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44(5):276-280. doi: 10.1111/j.1440-1754.2007.01251.x [DOI] [PubMed] [Google Scholar]
- 6.Potijk MR, Kerstjens JM, Bos AF, Reijneveld SA, de Winter AF. Developmental delay in moderately preterm–born children with low socioeconomic status: risks multiply. J Pediatr. 2013;163(5):1289-1295. doi: 10.1016/j.jpeds.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 7.Martínez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998;71(6):2528-2533. doi: 10.1046/j.1471-4159.1998.71062528.x [DOI] [PubMed] [Google Scholar]
- 8.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4-5):259-269. doi: 10.1016/j.plefa.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263-271. doi: 10.1016/j.tins.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Kitajka K, Puskás LG, Zvara A, et al. The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc Natl Acad Sci U S A. 2002;99(5):2619-2624. doi: 10.1073/pnas.042698699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coti Bertrand P, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136(6):1570-1575. doi: 10.1093/jn/136.6.1570 [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495-505. doi: 10.1080/07315724.2002.10719248 [DOI] [PubMed] [Google Scholar]
- 13.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(suppl A):S70-S75. doi: 10.1016/j.placenta.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 14.Moon K, Rao SC, Schulzke SM, Patole SK, Simmer K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst Rev. 2016;12:CD000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makrides M, Gibson RA, McPhee AJ, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009;301(2):175-182. doi: 10.1001/jama.2008.945 [DOI] [PubMed] [Google Scholar]
- 16.Makrides M. DHA supplementation during the perinatal period and neurodevelopment: do some babies benefit more than others? Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):87-90. doi: 10.1016/j.plefa.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Fewtrell MS, Abbott RA, Kennedy K, et al. Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr. 2004;144(4):471-479. doi: 10.1016/j.jpeds.2004.01.034 [DOI] [PubMed] [Google Scholar]
- 18.Keim SA, Branum AM. Dietary intake of polyunsaturated fatty acids and fish among US children 12-60 months of age. Matern Child Nutr. 2015;11(4):987-998. doi: 10.1111/mcn.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grote V, Verduci E, Scaglioni S, et al. ; European Childhood Obesity Project . Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur J Clin Nutr. 2016;70(2):250-256. doi: 10.1038/ejcn.2015.162 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization; WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 21.Kuratko C. Food-frequency questionnaire for assessing long-chain ω-3 fatty-acid intake: re: assessing long-chain ω-3 polyunsaturated fatty acids: a tailored food-frequency questionnaire is better. Nutrition. 2013;29(5):807-808. doi: 10.1016/j.nut.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 22.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 23.Vohr BR, Stephens BE, Higgins RD, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Are outcomes of extremely preterm infants improving? impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222-228.e3. doi: 10.1016/j.jpeds.2012.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clandinin MT, Van Aerde JE, Merkel KL, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146(4):461-468. doi: 10.1016/j.jpeds.2004.11.030 [DOI] [PubMed] [Google Scholar]
- 25.Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of short and very short forms of the Infant Behavior Questionnaire–Revised. J Pers Assess. 2014;96(4):445-458. doi: 10.1080/00223891.2013.841171 [DOI] [PubMed] [Google Scholar]
- 26.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717-728. doi: 10.1542/peds.2008-2816 [DOI] [PubMed] [Google Scholar]
- 27.Poehlmann J, Schwichtenberg AJ, Shah PE, Shlafer RJ, Hahn E, Maleck S. The development of effortful control in children born preterm. J Clin Child Adolesc Psychol. 2010;39(4):522-536. doi: 10.1080/15374416.2010.486319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri BK, Martins JG. Which polyunsaturated fatty acids are active in children with attention-deficit hyperactivity disorder receiving PUFA supplementation? a fatty acid validated meta-regression analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2014;90(5):179-189. doi: 10.1016/j.plefa.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 29.Putnam SP, Jacobs J, Gartstein MA, Rothbart MK Development and assessment of short and very short forms of the Early Childhood Behavior Questionnaire. Presented at: International Conference on Infant Studies; March 10, 2010; Baltimore, MD. [Google Scholar]
- 30.Putnam SP, Rothbart MK, Gartstein MA. Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant Child Dev. 2008;17:387-405. doi: 10.1002/icd.582 [DOI] [Google Scholar]
- 31.Bayley N. The Bayley Scales of Infant Development. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 32.Eder K. Gas chromatographic analysis of fatty acid methyl esters. J Chromatogr B Biomed Appl. 1995;671(1-2):113-131. doi: 10.1016/0378-4347(95)00142-6 [DOI] [PubMed] [Google Scholar]
- 33.Valentine CJ, Morrow G, Fernandez S, et al. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J Pediatr. 2010;157(6):906-910. doi: 10.1016/j.jpeds.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 34.Winkens B, van Breukelen GJ, Schouten HJ, Berger MP. Randomized clinical trials with a pre- and a post-treatment measurement: repeated measures versus ANCOVA models. Contemp Clin Trials. 2007;28(6):713-719. doi: 10.1016/j.cct.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 35.O’Connor DL, Hall R, Adamkin D, et al. ; Ross Preterm Lipid Study . Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108(2):359-371. doi: 10.1542/peds.108.2.359 [DOI] [PubMed] [Google Scholar]
- 36.Meinert CL. Clinical Trials: Design, Conduct, and Analysis. New York: Oxford University Press; 1986. doi: 10.1093/acprof:oso/9780195035681.001.0001 [DOI] [Google Scholar]
- 37.Friedman LM, Furberg CD, DeMets D, Reboussin DM, Granger CB. Fundamentals of Clinical Trials. New York, NY: Springer International Publishing; 2015. doi: 10.1007/978-3-319-18539-2 [DOI] [Google Scholar]
- 38.Makrides M, Gould JF, Gawlik NR, et al. Four-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA. 2014;311(17):1802-1804. doi: 10.1001/jama.2014.2194 [DOI] [PubMed] [Google Scholar]
- 39.Gould JF, Treyvaud K, Yelland LN, et al. Seven-year follow-up of children born to women in a randomized trial of prenatal DHA supplementation. JAMA. 2017;317(11):1173-1175. doi: 10.1001/jama.2016.21303 [DOI] [PubMed] [Google Scholar]
- 40.Collins CT, Gibson RA, Anderson PJ, et al. Neurodevelopmental outcomes at 7 years’ corrected age in preterm infants who were fed high-dose docosahexaenoic acid to term equivalent: a follow-up of a randomised controlled trial. BMJ Open. 2015;5(3):e007314. doi: 10.1136/bmjopen-2014-007314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheppard KW, Cheatham CL. Executive functions and the ω-6-to-ω-3 fatty acid ratio: a cross-sectional study. Am J Clin Nutr. 2017;105(1):32-41. doi: 10.3945/ajcn.116.141390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo J, Jill Shaddy D, Kerling EH, Gustafson KM, Carlson SE. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot Essent Fatty Acids. 2017;121:52-56. doi: 10.1016/j.plefa.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parks CA, Brett NR, Agellon S, et al. DHA and EPA in red blood cell membranes are associated with dietary intakes of omega-3-rich fish in healthy children. Prostaglandins Leukot Essent Fatty Acids. 2017;124:11-16. doi: 10.1016/j.plefa.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 44.Devlin AM, Chau CMY, Dyer R, et al. Developmental outcomes at 24 months of age in toddlers supplemented with arachidonic acid and docosahexaenoic acid: results of a double blind randomized, controlled trial. Nutrients. 2017;9(9):E975. doi: 10.3390/nu9090975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Change from Baseline to Trial Completion in Developmental Outcomes, Subgroup Results, Omega Tots Trial (n = 377), 2012-2017