Key Points
Question
Is the risk of bleeding requiring a return to the operating room when ibuprofen is used for postoperative analgesia after pediatric tonsillectomy with or without adenoidectomy no worse than the risk of bleeding when acetaminophen is used for postoperative pain management?
Findings
In this randomized, multicenter, double-blind, noninferiority clinical trial that included 741 children, the rate of bleeding requiring operative intervention was 1.2% in the acetaminophen group and 2.9% in the ibuprofen group. The criteria to conclude that ibuprofen is noninferior to acetaminophen were not met.
Meaning
Ibuprofen may increase the risk of severe bleeding after tonsillectomy with or without adenoidectomy; further work is needed to understand if shorter duration or combination therapy reduce this risk.
Abstract
Importance
Ibuprofen is an effective analgesic after tonsillectomy alone or tonsillectomy with adenoidectomy, but concerns remain about whether it increases postoperative hemorrhage.
Objective
To investigate the effect of ibuprofen compared with acetaminophen on posttonsillectomy bleeding (PTB) requiring surgical intervention in children.
Design, Setting, and Participants
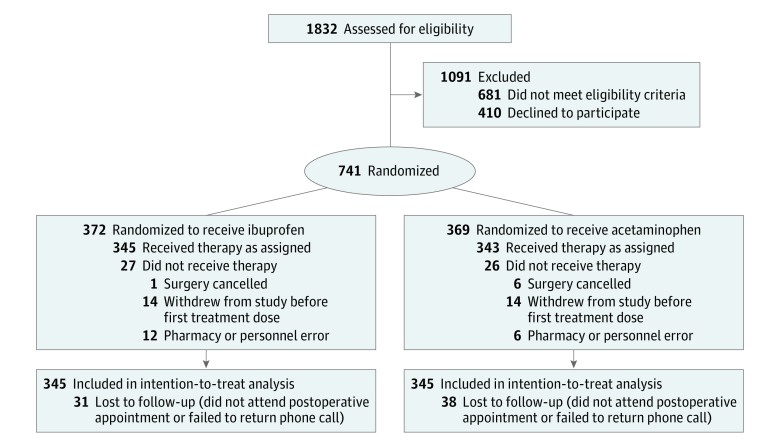
A multicenter, randomized, double-blind noninferiority trial was conducted at 4 tertiary medical centers (Massachusetts Eye and Ear Infirmary, Boston; Naval Medical Center, San Diego, California; Naval Medical Center, Portsmouth, Virginia; Madigan Army Medical Center, Tacoma, Washington). A total of 1832 children were assessed for eligibility (presence of sleep-disordered breathing or obstructive sleep apnea, adenotonsillar hypertrophy, or infectious tonsillitis undergoing extracapsular tonsillectomy by electrocautery). Of these, 1091 were excluded because they did not meet eligibility criteria (n = 681) or refused to participate (n = 410); thus, 741 children aged 2 to 18 years undergoing tonsillectomy alone or tonsillectomy with adenoidectomy were enrolled between May 3, 2012, and January 20, 2017.
Interventions
Participants were randomized to receive ibuprofen, 10 mg/kg (n = 372), or acetaminophen, 15 mg/kg (n = 369), every 6 hours for the first 9 postoperative days.
Main Outcomes and Measures
Rate and severity of posttonsillectomy bleeding were recorded using a postoperative bleeding severity scale: type 1 (bleeds that were observed at home or evaluated in the emergency department without further intervention), type 2 (bleeds that required readmission for observation), and type 3 (bleeds that required a return to the operating room for control of hemorrhage). Type 3 bleeding was the main outcome measure. The noninferiority margin was set at 3%, and modified intention-to-treat analysis was used.
Results
Of the 741 children enrolled, 688 children (92.8%) (median [interquartile range] age, 5 [4] years; 366 boys [53.2%]) received the study medication and were included in a modified intention-to-treat analysis. The rate of bleeding requiring operative intervention was 1.2% in the acetaminophen group and 2.9% in the ibuprofen group (difference, 1.7%; 97.5% CI upper limit, 3.8%; P = .12 for noninferiority). There were no significant adverse events or deaths.
Conclusions and Relevance
This study could not exclude a higher rate of severe bleeding in children receiving ibuprofen after tonsillectomy alone or tonsillectomy with adenoidectomy. This finding should be considered when selecting a postoperative analgesic regimen. Further studies are needed to understand if bleeding risk is affected when ibuprofen is used for a shorter duration or in combination with acetaminophen for postoperative analgesia.
Trial Registration
ClinicalTrials.gov identifier: NCT01605903
This noninferiority randomized clinical trial evaluates the risk of severe bleeding with ibuprofen vs acetaminophen use in children undergoing tonsillectomy with or without adenoidectomy.
Introduction
Tonsillectomy alone or tonsillectomy with adenoidectomy is a common surgical procedure performed in approximately 530 000 children in the United States annually.1,2 Postoperatively, children are at risk for developing pain and associated dehydration, as well as potentially life-threatening hemorrhage.3 Posttonsillectomy bleeding (PTB) can be categorized by its severity, with high-volume or brisk bleeding requiring a return to the operating room, or by the time at which events occur. Overall, PTB rates range from 3.3% to 20%, with a mean bleeding rate of 4.5%.4
Nonsteroidal anti-inflammatory drugs (NSAIDs) block prostaglandin-induced inflammation.5,6 Unlike narcotics, NSAIDs do not cause respiratory and central nervous system depression, making them a useful therapeutic option in young children and patients with sleep-disordered breathing. However, NSAIDs may interfere with platelet aggregation, raising concerns about their safety owing to the risk of PTB. Aspirin, an irreversible inhibitor, affects coagulation for up to 10 days and is associated with increased hemorrhage.7 Reversible inhibitors, such as ibuprofen, do not have the same prolonged effects on coagulation.8 Several meta-analyses of reversible NSAIDs have not shown increased PTB.9,10 However, when study is restricted to NSAID use during the postoperative period, rates of PTB may be significantly higher.10 Retrospective medical record reviews of postoperative ibuprofen have not demonstrated elevated rates of PTB.11,12 There are few randomized trials of ibuprofen use after tonsillectomy, however, and these studies were powered to assess analgesic effect rather than bleeding.5,6 This multicenter, randomized, double-blind trial was performed to address concerns about an association between postoperative ibuprofen administration and severe PTB.
Methods
Study Design and Conduct
This study was a randomized, double-blind noninferiority trial and was approved by the institutional review boards of participating institutions (Massachusetts Eye and Ear Infirmary, Boston; Naval Medical Center San Diego, San Diego, California; Naval Medical Center Portsmouth, Portsmouth, Virginia; and Madigan Army Medical Center, Tacoma, Washington). All patients or their guardians provided written informed consent or assent for older children. There was no financial compensation. The trial protocol is available in Supplement 1.
Study Participants
Children between the ages of 2 and 18 years with sleep-disordered breathing or obstructive sleep apnea, adenotonsillar hypertrophy, or infectious tonsillitis undergoing extracapsular tonsillectomy by electrocautery were eligible. Patients were excluded for the following reasons: known personal or family history of any bleeding disorder, asthma requiring ongoing treatment, and red or blue dye allergy. Children with guardians unable to understand study protocol, read instructions, or communicate needs without assistance were excluded owing to safety concerns.
Randomization
Random number assignments were generated using a random number generator and block randomization was performed. Patients were assigned to a treatment group as they were enrolled, in chronological order. Only each site’s pharmacist had access to the assignments. Participants, their families, and clinicians remained blinded unless circumstances required unblinding for safety; otherwise, unblinding occurred only after all data were collected.
Intervention
Participants received a preoperative dose of acetaminophen, 15 mg/kg (maximum dose, 650 mg), by mouth and an intraoperative dose of parenteral dexamethasone, 0.5 mg/kg (maximum dose, 8 mg) or methylprednisolone, 2.5 mg/kg (maximum dose, 40 mg). Extracapsular tonsillectomy was performed using electrocautery at standardized settings (excision using monopolar with spatula tip on 12W), hemostasis using suction cautery at 12W to 18W, and adenoidectomy using suction cautery at 30W.
Site pharmacies prepared and delivered a bottle containing 36 doses of either grape-flavored acetaminophen, 15 mg/kg (maximum dose, 650 mg), or grape-flavored ibuprofen, 10 mg/kg (maximum dose, 600 mg), in identical packaging to the postoperative recovery area. Ibuprofen volume per kilogram was matched to that of acetaminophen using a clear, tasteless suspension (Ora-Blend; Perrigo) to create equal volumes per dose of each treatment.
The first treatment dose was administered orally 4 hours after the preoperative acetaminophen dose. Participants were instructed to continue the study medication every 6 hours around the clock for the first 9 postoperative days, for a total of 36 doses (4 doses per day) and avoid supplemental acetaminophen or ibuprofen. Oxycodone, 0.05 to 0.1 mg/kg, could be prescribed at the surgeon’s discretion. All participants had discharge instructions to come to the medical center emergency department and discontinue study medication in the event of bleeding. All participants were asked to complete and return postoperative study medication dosage questionnaires, 1 for each of the 9 study days.
Outcomes
At the postoperative clinic visit or phone call, a questionnaire about bleeding during the 14-day postoperative period was completed and dosage questionnaires were collected. Results were supplemented with a medical record review to assess timing and severity of any PTB during the first 14 postoperative days. Postoperative bleeding was stratified by 3 levels of severity: type 1 referred to bleeds that were observed at home or evaluated in the emergency department without further intervention, type 2 referred to bleeds that required readmission for observation, and type 3 referred to bleeds that required a return to the operating room for control of hemorrhage. Type 3 bleeding was the primary outcome measure. Less severe forms of bleeding were also recorded. Bleeding events were also categorized by the time at which they occurred, with primary bleeding developing within 24 hours of surgery and secondary bleeding occurring after 24 hours.
Statistical Analysis
This was designed as a noninferiority trial in which the null hypothesis states that the rate of type 3 bleeding differed between treatment groups; the alternative hypothesis is that the rate of type 3 bleeding between the 2 groups was not different by more than the noninferiority margin. A noninferiority margin of 3% was determined by several methods. Site investigators were polled about an acceptable difference in bleed rates between treatment groups. At Massachusetts Eye and Ear Infirmary, the type 3 bleed rate was 1.8% in 2011. In 2012, Gallagher et al13 published data indicating a type 3 bleed rate of approximately 2%. The national benchmark used by the Massachusetts Eye and Ear Infirmary for type 3 bleeding is between 1.25% and 3.25%. A 3% margin was selected because it is approximately twice the difference between the upper margin of the US benchmark and expected bleed rate of 2%; a bleed rate above this percentage would be considered excessive.
Sample size was calculated to have a power of 80% to detect the difference of 3% between treatment groups using a 1-sided test and significance level of .025, factoring in interim analyses at 14% and 50% enrollment. Sample size calculations assumed an O’Brien-Fleming spending function and incorporated a 5% dropout rate. The noninferiority hypothesis required that the ibuprofen group have no more than a 3% absolute increase in the rate of type 3 bleeding compared with the acetaminophen group. The expected bleed rate in the acetaminophen group was set at 2% based on prior studies of children receiving intraoperative dexamethasone and postoperative acetaminophen.13 A sample size of 722 patients (361 per group) was calculated to achieve the 686 participants needed for intention-to-treat (ITT) analysis. With approval of the institutional review and data and safety monitoring boards, the sample size was increased after enrollment of the first 722 participants when fewer than 686 children were eligible for modified ITT (mITT) analysis.
An mITT analysis was performed, including all participants who were enrolled, underwent surgery, and received at least 1 dose of the study drug; participants were analyzed according to their original randomized group. Patients lost to follow-up without postoperative bleeding information were included and presumed not to have bled. Interim analyses were performed by constructing 1-sided 97.5% CIs around the difference in type 3 bleeding between treatment groups. Using EAST software, it was determined one would reject the alternative hypothesis of equivalent rates of bleeding if the P values were ≤.000001 at 14% enrollment and ≤.001525 at 50% enrollment. A data and safety monitoring board reviewed interim analyses after 14% and 50% enrollment; the data did not meet criteria for premature trial termination, which were defined as a type 3 bleed rate of greater than 6% in either arm or a greater than 3% increased risk of type 3 bleeding in the ibuprofen arm.
Baseline characteristics were summarized. A multivariable logistic regression model adjusting for site, age, sex, and surgical indication was performed.
Type 3 bleeding rates were compared using a 1-sided CI approach. One-sided testing was used owing to the noninferiority trial design, with a null hypothesis that children receiving ibuprofen would have more type 3 bleeding than those receiving acetaminophen. Two-sided testing, which is applied in equivalence trials, was not performed because it was not hypothesized that ibuprofen would protect against type 3 bleeding. Noninferiority for bleeding in the ibuprofen group could not be demonstrated if the upper bound of a 97.5% CI constructed around the difference in bleeding rate between the 2 groups was greater than 3%. Noninferiority analysis was not conducted on less-severe bleeding categories.
A per protocol noninferiority analysis was also performed, which only included subjects who were confirmed to have received their assigned study medication through postoperative day 8 or until a bleeding event occurred, based on collected dosage questionnaires, and to not have received additional acetaminophen or ibuprofen during the study period. A noninferiority analysis of secondary type 3 PTB was also performed.
Data were analyzed using SAS, version 9.4 (SAS Institute Inc). The significance level was set to .025 for noninferiority analyses and .05 for other analyses.
Results
A total of 1832 children were assessed for eligibility; of these, 1091 were excluded (681 did not meet eligibility criteria; 410 refused to participate). Thus, 741 patients were enrolled between May 3, 2012, and January 20, 2017 (Figure 1). Fifty-three patients (7.2%) were excluded from analysis, either because they dropped out prior to the first dose of study medication, the procedure was cancelled, or pharmacy or study personnel error occurred. A total of 688 patients (92.8%) (median [interquartile range] age, 5 [4] years; 366 boys [53.2%]) received at least 1 dose of study medication and were eligible for mITT analysis. Of these, 69 patients (10.0%) were lost to follow-up; rates did not differ significantly between treatment groups (31 in the ibuprofen arm [9.0%] and 38 in the acetaminophen arm [11.1%]). Of patients lost to follow-up, 3 had documented bleeding events. The remaining 66 patients were presumed not to have had postoperative bleeding. Premature unblinding was performed for 4 patients because of pharmacy error (n = 1), medical necessity due to allergy or prescriber error (n = 2), and parent request (n = 1); all 4 of these children were included in these analyses. There were no significant adverse events or deaths.
Figure 1. Participant Flow.
Patients were enrolled between May 3, 2012, and January 20, 2017.
A total of 343 children (49.9%) were randomized to the acetaminophen group and 345 children (50.1%) were randomized to the ibuprofen group (Table 1). Sixty-five children (9.4%) developed at least 1 PTB event, including 27 children in the acetaminophen group (7.9%) and 38 in the ibuprofen group (11.0%). Among children in the acetaminophen group who bled, 2 children (7.4%) experienced 2 bleeding events. Among children who bled in the ibuprofen group, 4 children (10.5%) experienced 2 bleeding events, 1 child (2.6%) experienced 3 bleeding events, and 1 child (2.6%) experienced 4 bleeding events; thus 15.8% of children who bled in the ibuprofen group experienced multiple bleeding events.
Table 1. Characteristics of Study Patients.
| Characteristic | Ibuprofen (n = 345) | Acetaminophen (n = 343) |
|---|---|---|
| Age, median (IQR), y | 5 (4-8) | 5 (3-7) |
| Boys | 177 (51.3) | 189 (55.1) |
| Reason for surgery | ||
| OSA and/or ATH | 274 (79.4) | 264 (77.0) |
| Tonsillitis only | 39 (11.3) | 45 (13.1) |
| Tonsillitis and OSA and/or ATH | 29 (8.4) | 34 (9.9) |
| Other only | 3 (0.9) | 0 |
| Surgery type | ||
| Tonsillectomy alone | 23 (6.7) | 22 (6.4) |
| Tonsillectomy with adenoidectomy | 321 (93.0) | 321 (93.6) |
| Unknown | 1 (0.3) | 0 |
| Study location | ||
| MAMC | 62 (18.0) | 63 (18.4) |
| MEEI | 155 (44.9) | 152 (44.3) |
| NMCP | 55 (15.9) | 57 (16.6) |
| NMCSD | 73 (21.2) | 71 (20.7) |
Abbreviations: ATH, adenotonsillar hypertrophy; IQR, interquartile range; MAMC, Madigan Army Medical Center; MEEI, Massachusetts Eye and Ear Infirmary; NMCP, Naval Medical Center Portsmouth; NMCSD, Naval Medical Center San Diego; OSA, obstructive sleep apnea.
When the most severe form of bleeding was analyzed, of the 27 children who bled in the acetaminophen group, 14 children (51.9%) experienced type 1, 9 children (33.3%) experienced type 2, and 4 children (14.8%) experienced type 3 bleeding events. In the ibuprofen group, of the 38 children who bled, 16 children (42.1%) experienced type 1, 12 children (31.6%) experienced type 2, and 10 children (26.3%) experienced type 3 bleeding events (Table 2).
Table 2. Bleeding Event Rate by Noninferiority mITT Analysisa.
| Bleeding Event | No. (%) of Patients | % Difference (1-Sided 97.5% CI)b | Noninferiority P Valuec | |
|---|---|---|---|---|
| Ibuprofen (n = 345) | Acetaminophen (n = 343) | |||
| Most Severe Bleeding Eventd | ||||
| Type 1 | 16 (4.6) | 14 (4.1) | NA | NA |
| Type 2 | 12 (3.5) | 9 (2.6) | NA | NA |
| Type 3e | 10 (2.9) | 4 (1.2) | 1.7 (3.8) | .12 |
| Total children with bleeding | 38 (11.0) | 27 (7.9) | NA | NA |
| Most Severe Secondary Bleeding Event (mITT Analysis)d | ||||
| Type 1 | 16 (4.6) | 12 (3.5) | NA | NA |
| Type 2 | 10 (2.9) | 5 (1.5) | NA | NA |
| Type 3e | 8 (2.3) | 4 (1.2) | 1.17 (3.1) | .03 |
| Total children with bleeding | 34 (10.0) | 21 (6.2) | NA | NA |
| Most Severe Bleeding Event Rate (per Protocol Analysis)d,f | ||||
| Type 1 | 5 (3.4) | 1 (0.8) | NA | NA |
| Type 2 | 4 (2.8) | 3 (2.5) | NA | NA |
| Type 3e | 2 (1.4) | 2 (1.6) | 0.26 (2.69) | .02 |
| Total children with bleeding | 11 (7.6) | 6 (4.9) | NA | NA |
Abbreviations: mITT, modified intention-to-treat; NA, not applicable.
Noninferiority mITT analysis was performed only for type 3 bleeding.
Noninferiority is not achieved if the 1-sided 97.5% CI surpasses the noninferiority margin of 3%.
P values correspond to 1-sided CIs with noninferiority margin of 3.0%. P values <.025 are considered statistically significant.
The most severe type of bleeding event was included for children with multiple bleeding events.
Primary outcome measure.
Populations for these outcomes were 145 for ibuprofen and 122 for acetaminophen.
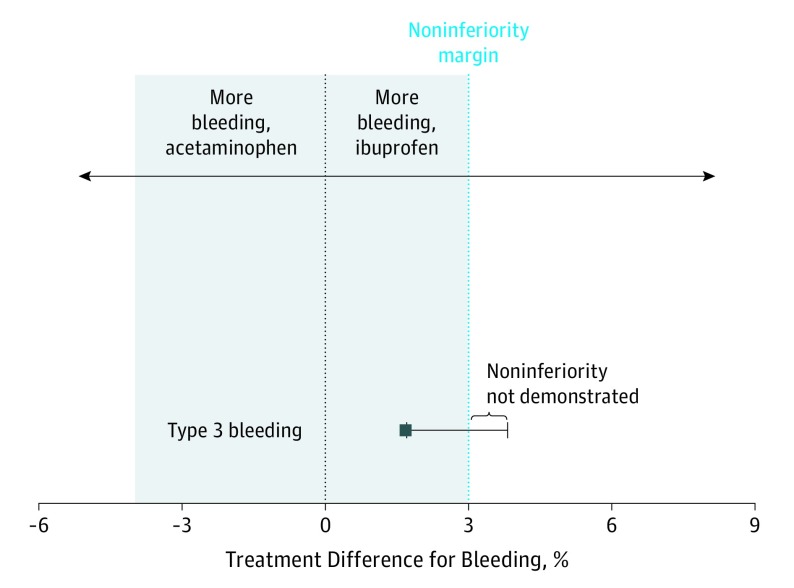
The type 3 bleed rate was 1.2% in the acetaminophen group and 2.9% in the ibuprofen group; for type 3 bleeding, ibuprofen did not meet criteria for noninferiority (difference, 1.7%; 97.5% CI upper limit, 3.8%; P = .12 for noninferiority) (Table 2, Figure 2). Modified ITT analysis of secondary bleeding events was also performed by excluding participants with bleeding within the first 24 hours after surgery. For type 3 secondary bleeding, the ibuprofen group failed to demonstrate noninferiority because the upper limit of the 97.5% CI surpassed 3% (difference, 1.17%; 97.5% CI upper limit, 3.1%; P = .03 for noninferiority) (Table 2).
Figure 2. Type 3 Bleeding Event Rate by Noninferiority Modified Intention-to-Treat Analysis.
Treatment difference determined as ibuprofen treatment minus acetaminophen treatment.
Of the 741 patients randomized, 385 children (52.0%) returned their postoperative dosage questionnaires. A total of 303 of the 688 patients eligible for mITT analysis (44.0%) were missing postoperative dosage data, and these data were incomplete for 116 patients (30.1%) who returned the questionnaires. There was no significant difference in the rate of early withdrawal from the study between treatment groups (72 patients in the acetaminophen group [21.0%] and 57 patients in the ibuprofen group [16.5%]). Per protocol analysis was performed for only 267 participants (38.8% of the mITT population) owing to missing or incomplete dosage data. In this analysis, ibuprofen met the criteria for noninferiority for type 3 bleeding (difference, 0.26%; 97.5% CI upper limit, 2.69%; P = .02 for noninferiority) (Table 2).
In the multivariable model predicting the probability of a type 3 bleeding event adjusted for site, age, sex, and surgical indication, none of the variables of interest was significantly associated with type 3 bleeding (Table 3).
Table 3. Adjusted Logistic Regression Model.
| Variable | Type 3 Bleeding, OR (95% CI)a |
|---|---|
| Study arm (ibuprofen vs acetaminophen) | 2.56 (0.78-8.42) |
| Sex (girl vs boy) | 2.58 (0.79-8.39) |
| Age, y | 1.05 (0.91-1.22) |
| Study site (MEEI vs other sites) | 2.96 (0.89-9.85) |
| Surgical indication | |
| Tonsillitis and OSA and/or ATH vs OSA and/or ATH only | 1.55 (0.30-8.17) |
| Tonsillitis only vs OSA and/or ATH | 2.91 (0.69-12.29) |
Abbreviations: ATH, adenotonsillar hypertrophy; MEEI, Massachusetts Eye and Ear Infirmary; OR, odds ratio; OSA, obstructive sleep apnea.
Primary outcome measure.
Discussion
This randomized, double-blind, multicenter clinical trial was designed to evaluate whether postoperative ibuprofen causes more bleeding requiring surgical intervention after tonsillectomy. Noninferiority of ibuprofen could not be concluded for type 3 bleeding in mITT analysis. Therefore, the possibility that ibuprofen causes more severe hemorrhage than acetaminophen when used for analgesia after tonsillectomy in children could not be excluded.
Ibuprofen use after tonsillectomy is becoming increasingly popular, in part owing to recognition of the dangers of narcotic administration, particularly in young children and patients with sleep-disordered breathing. A subset of patients rapidly metabolize codeine, causing elevated, potentially fatal blood opioid levels; a black box warning has been issued against codeine use after tonsillectomy.14 Even in children who are not hypermetabolizers, postoperative narcotics are associated with persistent obstructive sleep apnea and oxygen desaturation in the immediate postoperative period.15 In 2011, the American Academy of Otolaryngology incorporated ibuprofen use into its clinical practice guidelines for tonsillectomy, indicating that postoperative administration of ibuprofen is safe.16 There are persistent concerns, however, about increased bleeding complications when ibuprofen is used postoperatively.
In 2013, a meta-analysis demonstrated that, in 2961 patients from 18 studies, the odds of bleeding were 2 times higher when NSAIDs were administered in the postoperative period after tonsillectomy (odds ratio [OR], 2.02; 95% CI, 1.25-3.27). This difference was not observed for patients receiving preoperative (OR, 1.24; 95% CI, 0.43-3.55) and perioperative (OR, 0.77; 95% CI, 0.37-1.62) doses of NSAIDs.10 Other retrospective studies have failed to demonstrate an increased risk of PTB with postoperative ibuprofen.11,12 However, a recent retrospective cohort study suggested that, when it occurs, hemorrhage is more severe, with a nearly 3-fold increase in the odds that transfusion will be needed in children receiving ibuprofen.12
Smaller randomized clinical trials have evaluated ibuprofen use after pediatric tonsillectomy, but these studies were powered to investigate analgesic effect rather than postoperative hemorrhage. In 1997, St Charles et al6 reported a bleed rate of 7.3% among 55 children who underwent tonsillectomy and received ibuprofen for postoperative pain vs a bleed rate of 9.1% for 55 children who received acetaminophen with codeine (difference, 1.8%; 95% CI, −9.4% to 13.2%). There are several limitations of this trial that undermine conclusions that can be drawn about the association between postoperative ibuprofen use and postoperative hemorrhage. These limitations include a failure to blind both research participants and research staff, inclusion of a variety of surgical techniques, and a small sample size, which contribute to an imprecise estimate (ie, large width of the CI) of the true difference in bleed rates between ibuprofen and acetaminophen.
In 1998, Harley et al5 published a randomized, double-blind study of 27 patients who received either postoperative acetaminophen with codeine or ibuprofen. The rate of hemorrhage requiring surgical intervention was 12.5% in the ibuprofen arm (2 of 16 patients) and 0% in the acetaminophen with codeine arm (0 of 11 patients) (OR, 3.97; 95% CI, 0.17-91.09). The small sample size and imprecise estimates prevent a definitive conclusion about the effect of ibuprofen on postoperative hemorrhage despite a large OR estimate of increased bleeding risk with ibuprofen use.
For the present study, type 3 bleeding was chosen as the main outcome measure because it is the most objective measure of PTB, exhibiting less regional variation, and is a clinically important indicator of severe bleeding.13,17 Many patients had self-limited bleeding; this type 1 bleeding was identified only at follow-up, and high loss to follow-up may have underestimated these events. Although type 2 bleeding is more objective, requiring physician documentation and treatment, it may still be subject to practice pattern differences.18 More bleeding was observed in the ibuprofen arm across all bleeding severity types in this study. Noninferiority analysis was restricted to type 3 bleeding; therefore, it is not known whether the observed differences in rates of less-severe bleeding are precise or clinically meaningful.
Our analysis distinguished between primary and secondary bleeding events owing to proposed differences in their causative factors, such as an association between early bleeding and technical error.17 Ibuprofen affects platelet aggregation within 2 hours after a single dose.19,20 Therefore, exclusion of primary events may have led to underestimation of the number of bleeds attributable to ibuprofen. Even when these events were excluded, noninferiority of ibuprofen could not be demonstrated for secondary type 3 bleeding.
Limitations
This study has several limitations. First, conclusions are reliant on a carefully selected noninferiority margin, which was set at 3% based on available literature and quality and outcomes data on severe bleeding. Lack of PTB literature that distinguishes between bleeding severity levels restricted noninferiority analysis to type 3 bleeding, and noninferiority analysis could not be performed for type 1 and type 2 bleeding.
Second, loss to follow-up was high but equivalent between treatment groups. Children lost to follow-up without postoperative information were presumed not to have bled. More marked differences in bleeding may have been seen between treatment groups had more data been available.
Third, mITT analysis did not distinguish between children who took the study medication as prescribed and those who took the medication less frequently or stopped use early. Per protocol analysis, performed for children confirmed to have taken the study medication throughout the trial, showed noninferiority of ibuprofen. These results should be interpreted with caution, however, because of the small sample size secondary to the limited number of dosage questionnaires that were returned. It is not known whether differences in timing, dose, or cumulative amount of ibuprofen affect bleeding risk. A study using variable dosing of ibuprofen would have required a larger sample size and been infeasible.
Conclusions
This study could not support the conclusion that there is no difference in severe bleeding risk when ibuprofen is used for postoperative analgesia compared with acetaminophen after pediatric tonsillectomy. The possibility that ibuprofen causes more severe postoperative bleeding requiring a return to the operating room could not be excluded. This finding should be taken into consideration when selecting a postoperative analgesic regimen. More research is needed to determine whether ibuprofen results in increased bleeding when it is used for a shorter duration, less frequently, or as part of a multidrug postoperative analgesic regimen.
Trial Protocol
Data Sharing Statement
References
- 1.Erickson BK, Larson DR, St Sauver JL, Meverden RA, Orvidas LJ. Changes in incidence and indications of tonsillectomy and adenotonsillectomy, 1970-2005. Otolaryngol Head Neck Surg. 2009;140(6):894-901. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone CD. Current indications for tonsillectomy and adenoidectomy. Ann Otol Rhinol Laryngol Suppl. 1992;155:58-64. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz RG, Zalzal GH. Tonsillectomy in children under 3 years of age. Arch Otolaryngol Head Neck Surg. 1990;116(6):685-686. [DOI] [PubMed] [Google Scholar]
- 4.Blakley BW. Post-tonsillectomy bleeding: how much is too much? Otolaryngol Head Neck Surg. 2009;140(3):288-290. doi: 10.1016/j.otohns.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Harley EH, Dattolo RA. Ibuprofen for tonsillectomy pain in children: efficacy and complications. Otolaryngol Head Neck Surg. 1998;119(5):492-496. doi: 10.1016/S0194-5998(98)70107-X [DOI] [PubMed] [Google Scholar]
- 6.St Charles CS, Matt BH, Hamilton MM, Katz BP. A comparison of ibuprofen versus acetaminophen with codeine in the young tonsillectomy patient. Otolaryngol Head Neck Surg. 1997;117(1):76-82. doi: 10.1016/S0194-5998(97)70211-0 [DOI] [PubMed] [Google Scholar]
- 7.Reuter SH, Montgomery WW. Aspirin vs acetaminophen after tonsillectomy. Arch Otolaryngol. 1964;80:214-217. doi: 10.1001/archotol.1964.00750040220021 [DOI] [PubMed] [Google Scholar]
- 8.Schafer AI. Effects of nonsteroidal antiinflammatory drugs on platelet function and systemic hemostasis. J Clin Pharmacol. 1995;35(3):209-219. doi: 10.1002/j.1552-4604.1995.tb04050.x [DOI] [PubMed] [Google Scholar]
- 9.Cardwell M, Siviter G, Smith A. Non-steroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev. 2005;(2):CD003591. [DOI] [PubMed] [Google Scholar]
- 10.Riggin L, Ramikrishna J, Sommer DD, Koren GA. A 2013 updated systematic review & meta-analysis of 36 randomized controlled trials; no apparent effects of non steroidal anti-inflammatory agents on the risk of bleeding after tonsillectomy. Clin Otolaryngol. 2013;38:115-129. doi: 10.1111/coa.12106 [DOI] [PubMed] [Google Scholar]
- 11.Pfaff JA, Hsu K, Chennupati SK. The use of ibuprofen in posttonsillectomy analgesia and its effect on posttonsillectomy hemorrhage rate. Otolaryngol Head Neck Surg. 2016;155(3):508-513. doi: 10.1177/0194599816646363 [DOI] [PubMed] [Google Scholar]
- 12.Mudd PA, Thottathil P, Giordano T, et al. . Association between ibuprofen and the severity of surgically managed posttonsillectomy hemorrhage. JAMA Otolaryngol Head Neck Surg. 2017;143(7):712-717. doi: 10.1001/jamaoto.2016.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher TQ, Hill C, Ojha S, et al. . Perioperative dexamethasone administration and risk of bleeding following tonsillectomy in children: a randomized controlled trial. JAMA. 2012;308(12):1221-1226. doi: 10.1001/2012.jama.11575 [DOI] [PubMed] [Google Scholar]
- 14.Kelly LE, Rieder M, van den Anker J, et al. . More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129(5):e1343-e1347. doi: 10.1542/peds.2011-2538 [DOI] [PubMed] [Google Scholar]
- 15.Kelly LE, Sommer DD, Ramakrishna J, et al. . Morphine or ibuprofen for post-tonsillectomy analgesia: a randomized trial. Pediatrics. 2015;135(2):307-313. doi: 10.1542/peds.2014-1906 [DOI] [PubMed] [Google Scholar]
- 16.Baugh RF, Archer SM, Mitchell RB, et al. ; American Academy of Otolaryngology-Head and Neck Surgery Foundation . Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1)(suppl):S1-S30. doi: 10.1177/0194599810389949 [DOI] [PubMed] [Google Scholar]
- 17.Conley SF, Ellison MD. Avoidance of primary post-tonsillectomy hemorrhage in a teaching program. Arch Otolaryngol Head Neck Surg. 1999;125(3):330-333. doi: 10.1001/archotol.125.3.330 [DOI] [PubMed] [Google Scholar]
- 18.Evans AS, Khan AM, Young D, Adamson R. Assessment of secondary haemorrhage rates following adult tonsillectomy: a telephone survey and literature review. Clin Otolaryngol Allied Sci. 2003;28(6):489-491. doi: 10.1046/j.1365-2273.2003.00763.x [DOI] [PubMed] [Google Scholar]
- 19.Cronberg S, Wallmark E, Söderberg I. Effect on platelet aggregation of oral administration of 10 non-steroidal analgesics to humans. Scand J Haematol. 1984;33(2):155-159. doi: 10.1111/j.1600-0609.1984.tb02390.x [DOI] [PubMed] [Google Scholar]
- 20.McIntyre BA, Philp RB, Inwood MJ. Effect of ibuprofen on platelet function in normal subjects and hemophiliac patients. Clin Pharmacol Ther. 1978;24(5):616-621. doi: 10.1002/cpt1978245616 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement