Abstract
This study investigates the susceptibilities of CoNS-causing endophthalmitis to fluoroquinolones over a 22-year time period at the Bascom Palmer Eye Institute, Miami, Florida, are reported.
Coagulase-negative Staphylococcus (CoNS) is the most common cause of acute-onset post–cataract surgery endophthalmitis.1 Fluoroquinolones are frequently used prophylactically (either topical or intracameral) in an attempt to reduce the rates of postoperative endophthalmitis. In this study, the susceptibilities of CoNS-causing endophthalmitis to fluoroquinolones over a 22-year period at the Bascom Palmer Eye Institute, Miami, Florida, are reported.
Methods
This study was a retrospective, consecutive case series of microbiology isolates, with no identifying patient information. Microbiology studies were used to obtain sensitivities for second-generation (ciprofloxacin), third-generation (levofloxacin), and fourth-generation (moxifloxacin) fluoroquinolones derived from E-test and VITEK testing of vitreous CoNS isolates causing endophthalmitis between January 1, 1995, and December 31, 2016, at Bascom Palmer Eye Institute, Miami, Florida. In the earlier period, all isolates were not tested for levofloxacin and moxifloxacin. This study did not require informed consent and the University of Miami Miller School of Medicine institutional review board waived approval because samples were taken as part of routine medical care unrelated to this study and no patient-identifying information was collected. For the same reasons, the Health Insurance Portability and Accountability Act compliance did not apply to this study. We used a 2-sided P value of .05 at 95% confidence interval significance level.
Results
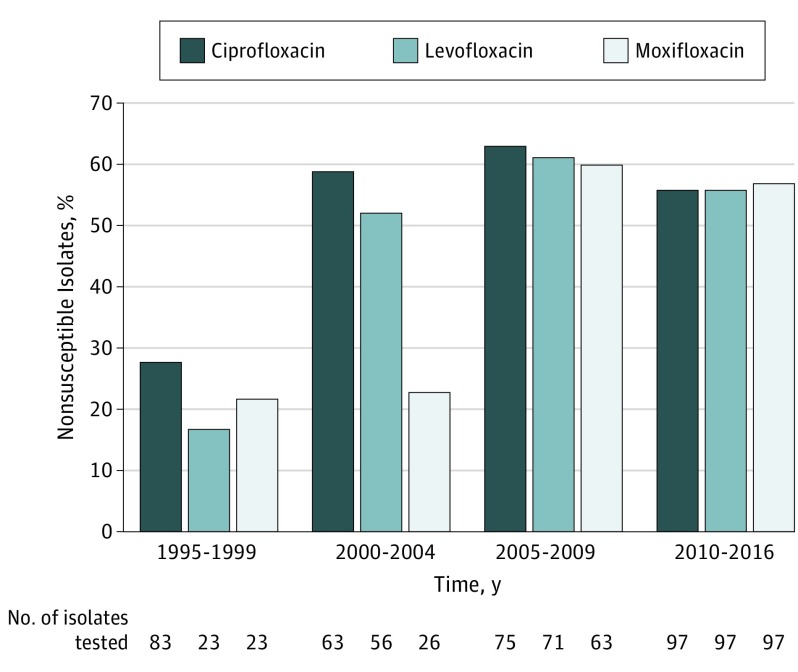
In this study of coagulase-negative Staphylococcus from patients with endophthalmitis, susceptibility rates among fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin) were reviewed between January 1, 1995, and December 31, 2016 (Figure).
Figure. Trends in Fluoroquinolone Nonsusceptibility Among Coagulase-Negative Staphylococcus Isolates.
Trends in fluoroquinolone nonsusceptibility among coagulase-negative Staphylococcus isolates causing endophthalmitis at the Bascom Palmer Eye Institute, Miami, Florida, from January 1, 1995, to December 31, 2016. Data are divided by each fluoroquinolone antibiotic (ciprofloxacin, levofloxacin, and moxifloxacin). Not all isolates were tested for antimicrobial susceptibility. The total number of isolates in each time frame was 83 (1995-1999); 63 (2000-2004); 75 (2005-2009); and 97 (2010-2016), respectively.
Ciprofloxacin
The nonsusceptibility rates for ciprofloxacin were 28% (n = 83) from 1995 to 1999, 59% (n = 63) from 2000 to 2004, 63% (n = 75) from 2005 to 2009, and 56% (n = 97) from 2010 to 2016. The nonsusceptibility to ciprofloxacin increased from 28% between 1995 and 1999 to 56% between 2010 and 2016 (95% CI, 12.88-41.71; P < .001).
Levofloxacin
The nonsusceptibility rates for levofloxacin were 17% (n = 23) from 1995 to 1999, 52% (n = 56) from 2000 to 2004, 61% (n = 71) from 2005 to 2009, and 56% (n = 97) from 2010 to 2016. The nonsusceptibility to levofloxacin increased from 17% between 1995 and 1999 to 56% between 2010 and 2016 (95% CI, 15.25-54.86, P < .001).
Moxifloxacin
The nonsusceptibility rates for moxifloxacin were 22% (n = 23) from 1995 to 1999, 23% (n = 26) from 2000 to 2004, 60% (n = 63) from 2005 to 2009, and 57% (n = 97) from 2010 to 2016. The nonsusceptibility to moxifloxacin increased from 22% between 1995 and 1999 to 57% between 2010 and 2016 (95% CI, 10.65-52.52, P = .003).
Discussion
In this study, the nonsusceptibility of CoNS to all 3 generations of fluoroquinolones increased during the time period of 22 years. In another study, nonsusceptibility to these antibiotics was shown to rapidly occur with repeated exposure of conjunctival Staphylococcus epidermidis to fluoroquinolones or azithromycin.2 Increase in the resistance among all of conjunctival flora with repeated exposure to topical antibiotics (ofloxacin, 0.3%; azithromycin, 1%; gatifloxacin, 0.3%; or moxifloxacin hydrochloride, 0.5%) has been reported.2,3 Similarly, in a prospective study, CoNS resistant to fluoroquinolones were also reported to have resistance to multiple other antibiotics. Endophthalmitis caused by CoNS nonsusceptible to fluoroquinolones has been reported to be associated with a poorer visual prognosis.4
Various studies have advocated the prophylactic use of moxifloxacin (topical or intracameral) to reduce postcataract surgery endophthalmitis rates.5 Although endophthalmitis prophylaxis with intracameral antibiotics is commonly used in Europe, this practice is less consistently used in the United States. However, these studies do not constitute level I evidence, and thus, there is no worldwide consensus regarding endophthalmitis prophylaxis with cataract surgery.6
Limitations
This study is limited by its retrospective nature and the possibility of selection bias. The mechanism of this increasing nonsusceptibility to fluoroquinolones is not fully understood, but it may be associated with widespread use of fluoroquinolones, use of antibiotics outside of the health care sector, and emergence of intrinsic genetic factors promoting resistance. Although this study involves only in vitro testing, the clinical implications of this laboratory data requires further investigations.
References
- 1.Schimel AM, Miller D, Flynn HW. Evolving fluoroquinolone resistance among coagulase-negative Staphylococcus isolates causing endophthalmitis. Arch Ophthalmol. 2012;130(12):1617-1618. [DOI] [PubMed] [Google Scholar]
- 2.Dave SB, Toma HS, Kim SJ. Ophthalmic antibiotic use and multidrug-resistant staphylococcus epidermidis: a controlled, longitudinal study. Ophthalmology. 2011;118(10):2035-2040. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Toma HS. Antimicrobial resistance and ophthalmic antibiotics: 1-year results of a longitudinal controlled study of patients undergoing intravitreal injections. Arch Ophthalmol. 2011;129(9):1180-1188. [DOI] [PubMed] [Google Scholar]
- 4.Chiquet C, Maurin M, Altayrac J, et al. Correlation between clinical data and antibiotic resistance in coagulase-negative Staphylococcus species isolated from 68 patients with acute post-cataract endophthalmitis. Clin Microbiol Infect. 2015;21(6):592.e1-592.e8. [DOI] [PubMed] [Google Scholar]
- 5.Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: analysis of 600 000 surgeries. Ophthalmology. 2017;S0161-6420(16)30790-4. [DOI] [PubMed] [Google Scholar]
- 6.Grzybowski A, Schwartz SG, Matsuura K, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns around the world. Curr Pharm Des. 2017;23(4):565-573. [DOI] [PubMed] [Google Scholar]