Key Points
Question
Which genes and gene sets harbor rare variants that affect the response to short-term antipsychotic medication treatment for patients with schizophrenia?
Findings
In this randomized clinical trial of 3023 patients with schizophrenia, nonresponders to antipsychotic drugs had a greater burden of rare damaging variants in 2 gene sets (reduced N-methyl-D-aspartate–mediated synaptic currents and reduced AMPA [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid]–mediated synaptic currents). This finding was replicated in the reduced N-methyl-D-aspartate–mediated synaptic currents gene set by targeting gene sequencing in an independent sample.
Meaning
The trial provides evidence that gene sets in the glutamatergic system may affect the efficacy of short-term antipsychotic medications.
Abstract
Importance
The underlying mechanism for individual differences in patient response to antipsychotic medication remains unknown.
Objective
To discover genes and gene sets harboring rare variants associated with short-term antipsychotic medication efficacy.
Design, Setting, and Participants
In this multicenter, open-label, randomized clinical trial conducted between July 6, 2010, and December 31, 2011, 3023 patients recruited in China of Chinese Han descent with schizophrenia with total Positive and Negative Syndrome Scale (PANSS) score ≥ 60 received a 6-week treatment of antipsychotic medications randomly chosen from 5 atypical and 2 typical antipsychotic medications. Whole-exome sequencing (WES) was performed in 316 participants (grouped into those with the best response [n=156] and those who had no response [n=160] to the antipsychotic medication prescribed), according to the total PANSS score reduction rate after 6 weeks of treatment. Validation was performed using targeted sequencing in an independent sample of 1920 patients. Data analyses was performed between March 15, 2016, and March 1, 2017.
Main Outcomes and Measures
Drug efficacy at week 6 was assessed according to the change in PANSS scores from baseline. Extremely good and extremely poor responders were selected for an initial WES association study, from which a subset of genes showing putative association was selected for independent replication with a targeted sequencing approach.
Results
Of the 3023 patients (1549 [51.24%] female and 1474 [48.8%] male; mean [SD] age, 31.2 [7.9] years), 2336 (77.3%) were eligible for genetic analysis. After quality-control exclusions, 316 patients (10.5%) were included for WES and 1920 (63.5%) were included for replication. In the WES discovery stage, 2 gene sets (reduced NMDA [N-methyl-D-aspartate]–mediated synaptic currents and reduced AMPA [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid]–mediated synaptic currents) were found to be enriched with rare damaging variants in the nonresponder group, suggesting the involvement of these gene sets in antipsychotic medication efficacy. Reduced NMDA–mediated synaptic currents gene set was further replicated in an independent sample using targeting sequencing. No statistically significant differences in antipsychotic drug response were found among the patients who received different antipsychotic drugs.
Conclusions and Relevance
Genetic variation in glutamatergic or NMDA neurotransmission is implicated in short-term antipsychotic medication efficacy; WES may have utility in the study of rare genetic variation in pharmacogenetics.
Trial Registration
Chinese Clinical Trials Registry Identifier: ChiCTR-TRC-10000934
This randomized clinical trial examines genes and gene sets using exome sequencing technology to determine the genetic mechanism behind the responses to antipsychotic drugs prescribed to patients with schizophrenia.
Introduction
Schizophrenia is a chronic mental disorder that affects approximately 1 in 100 people worldwide. It is characterized by hallucinations, delusions, disorganized speech and behavior, and negative symptoms that cause social dysfunction.1 Its typical age at onset is in late adolescence or early adulthood, and it imposes a considerable health care burden on society.2 Antipsychotic medications are the mainstay of the clinical management of schizophrenia, but individually these drugs have much variability in efficacy and adverse effects. Better understanding of the factors that influence antipsychotic drug response will facilitate optimal clinical care tailored to individual patients.
The heritability of antipsychotic drug response is not easy to quantify because the phenotype can be assessed only in individuals who have schizophrenia and who received antipsychotic medications, and gathering a large number of families or twin pairs with 2 or more such individuals is difficult.3 Nevertheless, case reports of monozygotic twins show similar responses to olanzapine4 and similar adverse effects from clozapine (particularly antipsychotic medication–induced weight gain),5 suggesting a genetic contribution to individual variation in efficacy and adverse effects of antipsychotic medication. However, systematic search for common variants influencing antipsychotic drug response by genome-wide association study so far has been unsuccessful.6,7
The role of rare functional variants has been recognized in several neuropsychiatric disorders, including schizophrenia,8,9,10,11,12 intellectual disability,13,14,15 and autism spectrum disorder,16,17,18 and in serotonin reuptake inhibitor treatment response in major depression.19 Because most drugs exert their effects through protein binding, whole-exome sequencing (WES) offers a cost-effective strategy for investigating rare variants in drug-response studies.20 In addition, rare variants in specific genes or gene sets may define a disease subtype that is relatively nonresponsive to the standard drug treatment for a disorder.
This randomized clinical trial of antipsychotic drug response in schizophrenia used WES and targeted sequencing. Patients with schizophrenia who received 6-week (short-term) antipsychotic monotherapy in the trial were evaluated for antipsychotic drug response. Extremely good and extremely poor responders were selected for an initial WES association study, from which a subset of genes showing putative association was selected for independent replication using a targeted sequencing approach.
Methods
This randomized clinical trial was registered for the Chinese Antipsychotics Pharmacogenomics Consortium (Chinese Clinical Trials Registry Identifier: ChiCTR-TRC-10000934) and was executed according to the principles of the Declaration of Helsinki.21 The study protocols were approved by the institutional review board at each trial site throughout China. Written informed consent was obtained from all participating patients or their legal guardians. Data analyses were performed between March 15, 2016, and March 1, 2017.
Participants
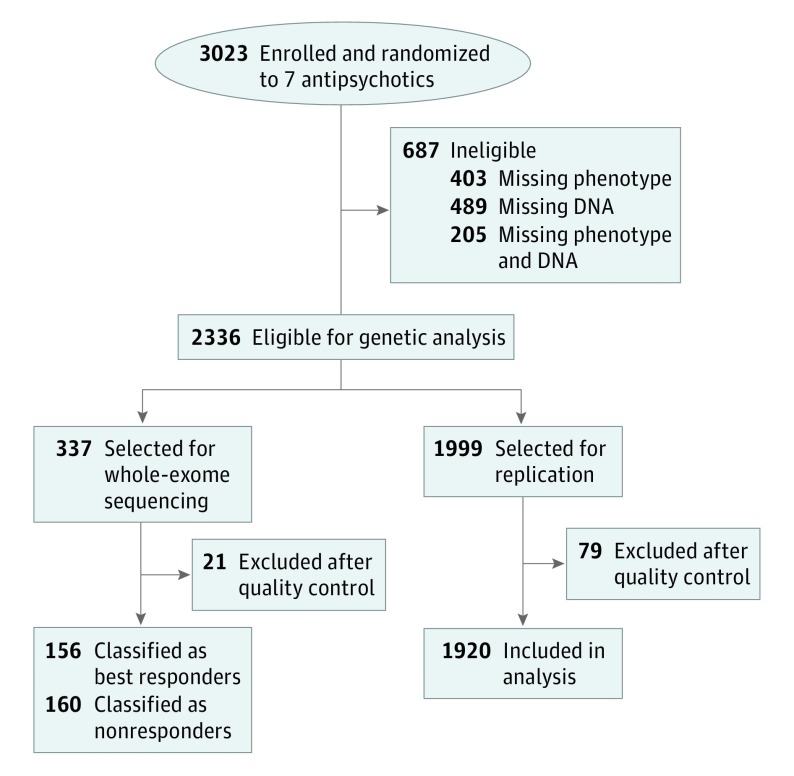
A clinical cohort of 3023 patients with schizophrenia was recruited between July 6, 2010, and November 30, 2011, for a multicenter, randomized and open-label clinical trial in China (Figure 1) conducted from July 6, 2010, to December 31, 2011; the trial protocol is found in Supplement 1. The inclusion criteria for participation were as follows: Chinese Han descent, diagnosis of first onset or chronic schizophrenia, total Positive and Negative Syndrome Scale (PANSS) score of more than 60, and written informed consent. The exclusion criteria were pregnancy or breastfeeding, contraindications of the recommended drugs, severe or unstable physical disease, and existence of certain heart diseases.
Figure 1. Trial Profile.
All patients received a 6-week treatment of 1 antipsychotic drug, randomly chosen from 5 atypical antipsychotic medications (risperidone, olanzapine, quetiapine fumarate, aripiprazole, and ziprasidone hydrochloride) and 2 typical antipsychotic medications (perphenazine and haloperidol lactate) (eTable 1 in Supplement 2) using a table of random computer-generated numbers. Clinical symptom assessment was based on the PANSS22 (score range: 60-90, with the highest score indicating the most severe symptoms). During the 6-week antipsychotic medication treatment, drug efficacy was assessed 4 times (at baseline and the end of week 2, week 4, and week 6 after the initial prescription of the drug) according to the change in PANSS scores from baseline. For each patient, at week 6, the PANSS reduction rate was defined as 100 × (PANSS score at baseline – PANSS score at week 6)/(PANSS score at baseline – 30). The reduction rates of all patients were ranked in decreasing order; the highest-ranking 300 patients and the lowest-ranking 300 patients were chosen as the 2 extreme groups (best responders and nonresponders). Of these patients, 340 individuals (170 in each group) with a baseline PANSS total score of 60 or higher (patients with the severity of moderately ill or greater) were selected for WES. Three patients were excluded because of poor DNA quality, leaving 337 patients with extreme trait values for inclusion in the WES discovery stage. The 1999 patients in the remaining study sample were included in the validation stage (although 79 patients were excluded after quality control, leaving 1920 patients for inclusion in targeted sequencing). A detailed description of the participants can be found in the eMethods in Supplement 2.
Differences in PANSS score reduction among the 6 groups that received different drugs (5 groups received atypical antipsychotic medications and 1 group received 1 of 2 typical antipsychotic drugs) were examined using χ2 tests. In the absence of statistically significant differences, and given that all antipsychotic drugs are likely to act on common pathways,23 we combined the 6 groups in subsequent analyses to improve statistical power, adjusting for the effect of age, sex, the first 2 principal components, and antipsychotic medication types when the statistical test allowed. The full methods for library construction and preparation, exome capture, alignment, preprocessing, variant calling, and quality control are described in the eMethods in Supplement 2.
Variant-Based and Gene-Based Association Analysis
For common-variant testing, we performed a single-nucleotide variant (SNV) association analysis (eTable 3 in Supplement 2).
For rare-variant testing, we used a gene-based approach to analyze the combined effects of nonsynonymous variants, including stop-gain, stop-loss, and missense (which are predicted by genetics platform KGGSeq24 to be damaging), with a minor allele frequency (MAF) lower than 0.01 (ie, variants that are likely to have functional effect). The MAF was calculated in our sample. Only genes with at least 2 rare damaging variants were included in the association test. A 1-sided burden test8 was carried out to evaluate whether nonresponders had a greater burden of rare variants in a gene than did best responders, given an assumption that rare functional variants were enriched in nonresponders. Phenotype permutation was then performed to obtain an empirical P value. In addition, variable threshold25 tests were done to assess the association between drug response and rare-variant burden, allowing for various MAF thresholds. Finally, the optimized sequence kernel association test26 was performed to achieve greater power for genes with bidirectional effects (ie, both risk-increasing and protective rare variants) while allowing sex, age, antipsychotic medication type (typical or atypical), and the first 2 principal components as covariates. Bonferroni correction was used to correct for multiple tests, with an overall type I error of 5%. All analyses were conducted in PLINK/SEQ (open source).27
Candidate genes for drug effects were considered separately. We selected genes shown to be associated with antipsychotic drug response from pharmacodynamic candidate gene studies and genome-wide association studies. eTable 2 in Supplement 2 lists the most promising candidate genes, as reviewed by Changasi et al,28 and the genes shown to be associated with drug response from the 2 published genome-wide association studies.6,7
Gene Set Association Analysis
Schizophrenia has been hypothesized to be caused by alterations in the neurotransmitter, signal transduction, and synaptic activity. Genes that encode protein involving the gene pathways mentioned earlier have long been an area of intensive investigation in schizophrenia and therefore are potentially relevant to antipsychotic medication efficacy. Recently, the involvement of synaptic activity in the action of antipsychotic medications has been supported by independent studies, including proteomic data in cultured mouse neurons and mouse brains after treatment.29 Using this knowledge, we retrieved gene sets relevant to synaptic activity from multiple knowledge databases (Kyoto Encyclopedia of Genes and Genomes [KEGG], Reactome, and Mouse Genome Informatics [MGI]) and performed the set-based analysis separately. To reduce the burden of multiple testing and avoid testing overly narrow or broad functional gene sets or pathways, we selected gene sets or pathways that contained at least 10 and at most 200 genes. On the basis of these selection criteria, we extracted 10 KEGG pathways under the nervous system and 30 Reactome pathways under the neuronal system. Because of the detailed classification of phenotype terms in MGI, it contains a large number of gene sets (1365 gene sets under the nervous system phenotype), compared with KEGG and Reactome. Therefore, we narrowed our candidates of interest on the basis of the following selection procedure.
Under nervous system phenotype, we selected (from the classification of phenotype terms in MGI) abnormal nervous system physiology over abnormal nervous system morphology. Under abnormal nervous system physiology, we chose 2 phenotype terms associated with synaptic activity, on which our hypothesis was based: abnormal synaptic plasticity and abnormal synaptic transmission. We checked for the gene overlap between the 2 phenotype terms and found that 88.7% of the genes in abnormal synaptic plasticity are present in abnormal synaptic transmission. To avoid testing for redundant gene sets, we retained abnormal synaptic transmission for our selection of candidate. From MGI, we chose 39 gene sets under abnormal synaptic transmission. In total, 79 gene sets were considered across the KEGG, Reactome, and MGI knowledge databases.
In the gene set association analysis, we took a gene set or pathway as a basic unit to find biological sets or pathways associated with antipsychotic drug response. Nonsynonymous variants predicted to be damaging with a MAF lower than 0.01 were included in the analysis. To determine whether the association with rare nonsynonymous variants is confounded by population stratification, we evaluated the burden of rare synonymous variants. For statistical testing, 1-sided burden test was used to assess the burden of all variants in the gene set. Because of the highly overlapped genes within the gene sets tested, Bonferroni correction, which assumes that an independent test is overconservative, was used. As a result, the false discovery rate using the Benjamin-Hochberg30 and histogram31 methods was used to control for family-wise error rate.
Validation Analysis in an Independent Sample Set
Genes and gene sets from the WES discovery stage were further verified using targeted sequencing in an independent cohort of 1920 patients. Details about the library preparation, gene capture, sequencing, and quality controls are presented in the eMethods in Supplement 2. Treatment response was treated as a quantitative trait in the Combined Multivariate and Collapsing burden test, with sex, age, and antipsychotic medication type (typical or atypical) as covariates. This validation analysis was conducted in RvTest, version 1.0.32
Statistical Analysis
We used χ2 test to compare the frequency difference in qualitative characteristics (ie, sex) between the best responders and nonresponders, a 2-tailed t test to compare the difference in the mean value of quantitative characteristics (ie, age and duration of untreated psychosis) that met the assumption of normal distribution, and Mann-Whitney test for the non-normally distributed characteristics. All analyses were conducted in R, version 3.4.0 (R Foundation for Statistical Computing), with a 2-sided P < .05 defined as statistically significant after multiple corrections.
Results
Demographic and Clinical Characteristics of Participants
Among 3023 patients recruited at baseline, 2336 (77.3%) were eligible for genetic analysis. After quality-control exclusions, 316 patients (10.5%) were included for WES and 1920 (63.5%) were included for replication (Figure 1). After sex, age, and drug used were matched, a total of 337 patients (169 best responders and 168 nonresponders) were selected for WES in the discovery stage of the trial. The remaining participants were included for targeted sequencing in the second stage of the trial.
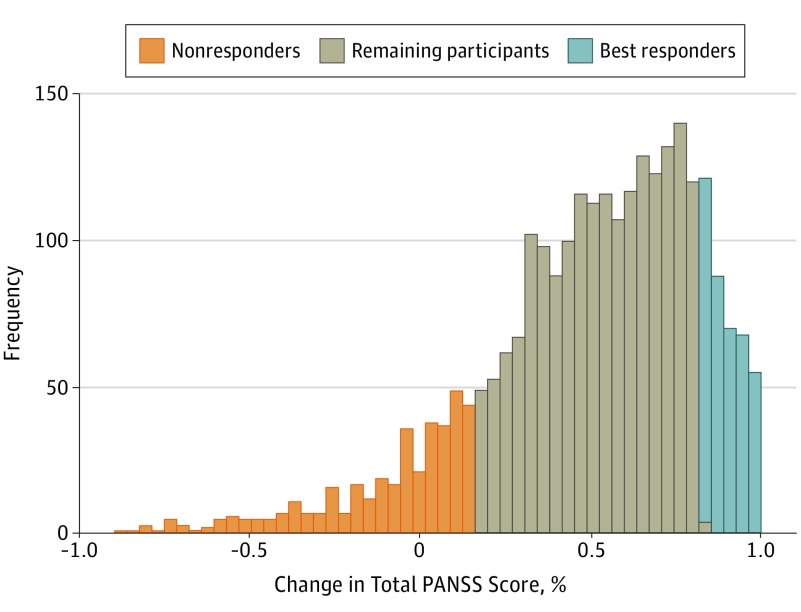
At recruitment, 1549 (51.24%) of the 3023 patients were female, 1474 (48.8%) were male, with a mean (SD) age of 31.2 (7.9) years. The mean (SD) age at illness onset was 24.3 (6.8) years, and the mean (SD) years of educational attainment were 10.4 (3.2) years. No substantial differences were observed in age, sex ratio, educational attainment, or the ratio of first illness onset among the 6 groups that received different antipsychotic drugs. The distribution of the reduction in total PANSS score is shown in Figure 2. Details on the demographic characteristics of all patients are presented in eTable 1 in Supplement 2.
Figure 2. Histogram of the Change in Positive and Negative Syndrome Scale (PANSS) Score Reduction Rate Distribution .
The proportional change in total PANSS score was calculated between baseline and week 6 to evaluate the efficacy of the prescribed antipsychotic drugs. Patients at the extreme ends of the change in total PANSS distribution (15% at both ends) were selected and classified as nonresponders or best responders. Gray bars indicate the distributions of remaining participants after selecting extreme response participants.
Table 1 lists the clinical characteristics of the best responders and nonresponders, including information about the prescribed antipsychotic drugs. The final data set comprised 316 patients (166 [52.5%] were female, 150 [47.5%] were male, with a mean [SD] age of 25.8 [8.0] years), of whom 156 (49.4%) were best responders and 160 (50.6%) were nonresponders to antipsychotic medications. Whole-exome sequencing was performed on 337 best responders and nonresponders, but 21 patients were excluded after quality control (18 patients were excluded because of a discordance between reported and genotyped sex, 1 because of sample contamination, and 1 because of genetic relatedness checking [ϕ (kinship coefficient), ≥0.125]). No statistically significant differences in age and sex were observed between the best responder and nonresponder groups (Table 1).
Table 1. Demographic and Clinical Characteristics of Patients Who Underwent Whole-Exome Sequencing .
| Variable | Best Responder Group | Nonresponder Group |
|---|---|---|
| No. | 156 | 160 |
| Male, No. (%) | 68 (43.6) | 82 (51.2) |
| Female, No. (%) | 88 (56.4) | 78 (48.8) |
| Age, mean (SD), y | 25.7 (7.7) | 25.9 (7.3) |
| Antipsychotic drugs | ||
| Olanzapine, 5-20 mg | 24 (15.4) | 33 (20.6) |
| Quetiapine fumarate, 400-750 mg | 25 (16.0) | 31 (19.4) |
| Ziprasidone hydrochloride, 80-160 mg | 29 (18.6) | 21 (13.1) |
| Aripiprazole, 10-30 mg | 20 (12.8) | 26 (16.3) |
| Risperidone, 2-6 mg | 25 (16.0) | 21 (13.1) |
| Perphenazine, 20-60 mg | 19 (12.2) | 19 (11.9) |
| Haloperidol lactate, 6-20 mg | 14 (8.9) | 9 (5.6) |
| PANSS total score, mean (SD), % | ||
| Baseline | 87.4 (6.8) | 86.0 (6.9) |
| 6 wk | 35.8 (3.0) | 87.8 (12.1) |
| PANSS positive symptom score, mean (SD), % | ||
| Baseline | 24.9 (3.6) | 25.5 (3.5) |
| 6 wk | 8.1 (1.3) | 22.0 (5.3) |
| PANSS negative symptom score, mean (SD), % | ||
| Baseline | 21.4 (5.1) | 21.1 (4.8) |
| 6 wk | 8.7 (1.3) | 23.8 (5.2) |
| PANSS general psychopathologic score, mean (SD), % | ||
| Baseline | 41.1 (5.0) | 40.4 (4.8) |
| 6 wk | 19.1 (2.0) | 42.0 (7.1) |
| Percentage reduction in PANSS total scorea | 90 (5) | −4 (20) |
Abbreviation: PANSS, Positive and Negative Syndrome Scale (score range: 60-90, with the highest score indicating the most severe symptoms).
Calculated as 100 × (baseline – 6 weeks) / (baseline – 30).
We observed no statistically significant differences in the effect of antipsychotic drugs, including response status (χ2 = 6.09; P = .30), percentage change in PANSS positive symptom score (F = 1.09; P = .37), percentage change in PANSS negative symptom score (F = 0.58; P = .71), and percentage change in PANSS general symptom score (F = 1.05; P = .39), among the 6 groups who received different drugs (5 groups received 1 of an atypical medication, and 1 group received 1 of 2 typical antipsychotic drugs). In addition, we observed no statistically significant differences in the effect of antipsychotic drugs between the 2 groups who received atypical or typical antipsychotic drugs (response status: χ2 = 0.44 [P = .51]; percentage change in PANSS positive symptom score: t = 0.59 [P = .56]; percentage change in PANSS negative symptom score: t = 1.02 [P = .31]; percentage change in PANSS general symptom score: t = 0.27 [P = .79]).
Whole-Exome Sequencing
The WES data generated on the 337 patients had a mean per-target depth of coverage of ×55.7, with 97.5% of all targeted bases covered at ×10 or greater (93.7% at ≥20×), which is more than the minimum 8× required for heterozygous variant calling. After taking a series of quality control steps, we identified 154 332 SNV in the protein-coding regions. Of these, 103 (0.1%) were stop-loss variants, 1534 (1.0%) were stop-gain variants, 669 (0.43%) were splicing-site variants, 89 442 (58.0%) were missense variants, and 62 584 (40.6%) were synonymous variants. The mean transition to transversion ratio (which was close to the expected ratio) for the known variants was 3.38 (expected ratio, 3.5) and for the novel variants was 2.95 (expected ratio, 3.0) in the exonic region.33
Common-Variant Association Analyses
We examined associations between the response status and the variants with a MAF of 5% or higher by performing logistic regression in PLINK,34 with age, sex, antipsychotic medication types, and the first 2 principal components as covariates. No population stratification in nonresponders and best responders was found from the results of the principal component analysis (eFigure 1 in Supplement 2). eFigure 2 in Supplement 2 depicts the quantile-quantile plots of the SNV-based association analysis of all exonic variants, nonsynonymous variants, and damaging nonsynonymous variants. No single-nucleotide polymorphism achieved genome-wide significance (P < 5 × 10−8) (eTable 3 in Supplement 2).
Gene-Based Association Analyses
In total, we identified 9900 genes that carry at least 2 rare damaging nonsynonymous variants. We defined a statistically significant threshold of P < 5.05 × 10−6 to reflect Bonferroni correction on the basis of total number of tests (P < .05 for 9900 tests) performed. No single gene was significantly associated with response status (eTable 4 in Supplement 2). In addition, we examined the gene-based association of our predefined candidate genes. Six candidate genes (GRID1 [OMIM 610659], ZNF804A [OMIM 612282], NRXN1 [OMIM 600565], CYP2J2 [OMIM 601258], GABRA6 [OMIM 137143], and GRM7 [OMIM 604101]) were nominally associated (P < .05) with the response status (eTable 5 in Supplement 2).
Gene-Set Association Analyses
To identify the underlying pathways or gene modules associated with response to antipsychotic medications, we performed gene set–based analyses using a list of predefined candidate pathways or gene sets. A total of 79 neuronal- or synaptic-associated gene sets extracted from MGI, Reatome, and KEGG were tested, using the 1-sided burden test, for enrichment of rare nonsynonymous variants predicted to be damaging. Two candidate gene sets achieved statistical significance (q<0.05) by having more rare alleles present in nonresponders than in best responders. These gene sets were the reduced N-methyl-D-aspartate (NMDA)–mediated synaptic currents (total number of genes = 13; number of rare alleles = 42 in nonresponders vs 19 in best responders) and the reduced AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid)–mediated synaptic currents (total number of genes = 17; number of rare alleles = 36 in nonresponders vs 14 in best responders = 14) (Table 2; see eTable 6 in Supplement 2 for the full results). Interestingly, most of the top gene sets were associated with biological processes of the glutamate receptors signaling pathway. For example, the top 3 gene sets (reduced NMDA-mediated synaptic currents, reduced AMPA-mediated synaptic currents, and abnormal AMPA-mediated synaptic currents) were under the same parent category (abnormal glutamate-mediated currents) in the MGI phenotype ontologies. Details about the variants, their functional and gene annotations, MAF in the sample and external databases, and deleteriousness prediction by multiple bioinformatics tools of the top 3 gene sets are provided in eTables 7, 8, and 9 in Supplement 2. In addition, we showed that enrichment was specific to rare damaging nonsynonymous variants and was absent for rare synonymous variants in all tested gene sets (eTable 10 in Supplement 2), suggesting that the original enrichment of rare functional variants was unlikely caused by population substructure.
Table 2. Set-Based Association Results on Gene Sets in the Whole-Exome Sequencing Discovery and Follow-up Stagesa.
| Gene Set (No. of Genes) | Discovery | Follow-up | |||||
|---|---|---|---|---|---|---|---|
| No. of Variants | P Value | Q Value | Allele Count, NR; BR | No. of Variants | P Value | ||
| B-H Method | Histogram Method | ||||||
| MGI: reduced NMDA-mediated synaptic currents (13) | 49 | .001 | 0.0495b | 0.0463 | 42; 19b | 261 | .009 |
| MGI: reduced AMPA-mediated synaptic currents (17) | 43 | .001 | 0.0495b | 0.0463 | 36; 14b | 231 | .11 |
| MGI: abnormal AMPA-mediated synaptic currents (12) | 20 | .012 | 0.2544b | 0.2375 | 17; 6b | 78 | .67 |
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; B-H, Benjamin-Hochberg; BR, best responder; MGI, Mouse Genome Informatics; NMDA, N-methyl-D-aspartate; NR, nonresponder.
Rare nonsynonymous variants predicted to be damaging with a minor allele frequency lower than 1% were included in this analysis.
P < .05 indicates statistically significant associations.
To examine whether the importance of gene sets was driven by a single highly critical gene, we conducted the burden test again and excluded the gene with the lowest gene-based P value in each set. After removal of the most important gene in each set, only the top 2 gene sets remained nominally associated (1-sided burden test for both sets: P < .05) with response status: reduced NMDA-mediated synaptic currents with a P = .01 (number of rare alleles: 30 in nonresponders vs 15 in best responders [1-sided burden test]), and reduced AMPA-mediated synaptic currents with a P = .009 (number of rare alleles: 30 in nonresponders vs 14 in best responders [1-sided burden test]).
Validation in Independent Sample
Targeted gene sequencing of the 78 candidate genes, including GRID1, GRM7, and ZNF804A; 34 unique genes involved in the top 3 gene sets; and 41 genes selected according to our interest (eTable 11 in Supplement 2), was applied in a sample of 1999 patients (980 [49.0%] were female and 1019 [51.0%] were male, with a mean [SD] age of 31.2 [7.97] years).
After quality controls were performed, 1920 patients and 5009 variants were retained for analysis. Consistent with our previous analyses, we tested selected genes and gene sets for enrichment of rare (MAF, <0.01) damaging nonsynonymous variants. The total PANSS score reduction rate was treated as a quantitative trait in the Combined Multivariate and Collapsing burden test, with sex, age, and antipsychotic medication type as covariates. Bonferroni correction was used for multiple testing adjustments.
No single gene was statistically significantly associated (adjusted P > .05) with total PANSS score reduction rate (eTable 12 in Supplement 2). Among the gene sets tested, reduced NMDA-mediated synaptic currents achieved a significance of P = .009 (Table 2). Details about the identified variants, their functional and gene annotation, MAF, and deleteriousness prediction by multiple bioinformatics tools of the involved genes are provided in eTable 13 in Supplement 2. In addition, the enrichment of rare variants was not found in synonymous variants (eTable 14 in Supplement 2), suggesting that the enrichment of nonsynonymous variants in this gene set was unlikely influenced by population substructure. Given that the burden test did not provide the effect sizes, we dichotomized the sample into 2 groups by median splitting and counted the number of rare alleles in each group as a simplistic way to assess the direction of effect. All of the 3 gene sets showed the same direction as the WES discovery study, with a higher frequency of rare alleles in patients with a lower total PANSS reduction rate (ie, less responsive to antipsychotic treatments) (eTable 15 in Supplement 2).
Discussion
To our knowledge, to date, this is the largest sequencing study on antipsychotic drug response. In the discovery stage, we used WES to discover genes and gene sets harboring rare variants associated with antipsychotic medication efficacy. We observed a greater burden of rare damaging variants in the reduced NMDA-mediated synaptic currents and reduced AMPA-mediated synaptic currents gene sets curated by MGI in patients with poorer response to antipsychotic medications. We were able to replicate the finding in the gene set of reduced NMDA-mediated synaptic currents by targeting gene sequencing in an independent sample set.
The reduced NMDA-mediated synaptic currents gene set caused a reduction in NMDA-mediated synaptic currents in glutamatergic neurotransmission when mutated or knocked out in a mouse model. The enrichment of rare damaging variants in the reduced NMDA-mediated synaptic currents suggests the potential role of reduction of synaptic transmission of glutamatergic neurons in antipsychotic medication efficacy. It is hypothesized that genes that code for any subtle molecular abnormalities linked to NMDA function could potentially create inefficient processing at glutamate synapses that mediate the symptoms of schizophrenia.35 Therefore, modulation of NMDA receptor function has been suggested as a novel treatment to reverse the hypothesized abnormality of glutamatergic transmission in schizophrenia. Several clinical studies found substantial improvement in negative symptoms, along with improvement in positive and cognitive symptoms when NMDA agonist, such as glycine and D-serine, were used in combination with either typical or atypical antipsychotic drugs.36,37,38,39
Another example is clozapine, which is often referred to as the criterion standard for schizophrenia with poorer response or resistance to treatment and is known to be effective in attenuating negative symptoms. The superior efficacy of clozapine may be due to its intrinsic agonist action at the D1 or NMDA receptor and at a glycine site of the NMDA receptor.40,41,42 Together, these findings suggest that modulation of the glutamatergic system, in addition to the dopaminergic system, might be essential to improving clinical manifestations.
The present study provides evidence of the importance of gene sets involved in the glutamatergic system to the response to antipsychotic drugs. Because the involved genes potentially have an effect on glutamate transmission, drugs that target glutamate transmission may be of benefit to patients who do not respond to traditional antipsychotic drugs. In fact, glutamatergic drugs have been estimated to be useful to 20% to 30% of patients with schizophrenia who do not respond to dopaminergic agents, particularly in the early stage of illness when the medications may be disease-modifying.42
Furthermore, the reduced AMPA-mediated synaptic currents gene set was found to be associated with poorer antipsychotic drug response in our WES study. No significant association was found in our validation study using targeted sequencing analysis, but the same direction of effect has been observed, where an increased burden of rare damaging variants was observed in patients with poorer response to antipsychotic drugs. Similar to the NMDA receptors, the AMPA receptors are iontropic glutamate receptors that mediate synaptic transmission at many postsynaptic members. These NMDA and AMPA receptors often coexist at the same synapse, where the AMPA receptor might facilitate the NMDA receptor activity. At rest, the ion channels of NMDA receptors are blocked by magnesium ions, but the channels open when a sufficient number of AMPA receptors are activated to depolarize the membrane potential from resting to positive potentials.43 Considering that AMPA works in tandem with NMDA, the effect of AMPA receptor function on antipsychotic drug response may warrant further investigation. This might be due to the different study designs of the WES discovery and the validation studies. In the WES discovery stage, we aimed to maximize the power by selecting patients in the study cohort from the extreme ends of the PANSS score reduction, matched by age, sex and antipsychotic medications. In the validation study, we sent the remaining patients in the cohort to undergo targeted sequencing. Therefore, this difference in study design might contribute to the inadequate power to detect associated genes and gene sets.
Limitations
This trial has a number of limitations. First, rare-variant studies require a large sample size to allow the identification of rare variants that are associated with complex diseases. To enhance the power of our study, we selected patients from the extreme ends of the treatment response distribution for the WES discovery stage, and then we aggregated the effect of rare damaging variants of protein coding regions on genes and a list of candidate gene sets. Yet, this trial still lacked the statistical power to perform gene- and gene set–based associations in a hypothesis-free approach. Second, a common limitation of clinical trials is that the patients tend to be chronically ill and with prior exposure to multiple antipsychotic medications and prolonged treatment histories, which resulted in our inclusion of less responsive and more severe patients. In addition, these chronically ill cohorts have been found to have a longer duration of psychotic symptoms and higher rate of substance abuse and functional disabilities, all of which have a potential effect on the drug response rate and could introduce increased variance into data analysis.44 An alternative, to enhance the power of pharmacogenetic study, is to use first-episode patients; however, a large number of first-episode patients are difficult to gather. Third, environmental factors, such as cigarette smoking, and concomitant diet, which might affect interindividual differences in antipsychotic drug response,45 were not collected and considered in our analyses. Fourth, increasing dose was given to patients who initially did not respond to the assigned antipsychotic drug. Drug dose, which essentially affects drug efficacy, was not accounted for in our analyses. However, the sensitivity threshold of a drug to become effective varies interindividually, making the generalization about dose adjustment challenging.
Conclusions
This trial has identified the genes involved in reduced NMDA-mediated synaptic currents gene set harboring rare damaging variants associated with a poorer response to short-term antipsychotic drug treatment. To our knowledge, this is the first study to demonstrate that rare genetic variants in the glutamate transmission are potentially implicated in the response to antipsychotic drugs among patients with schizophrenia. These findings warrant further replication and investigation.
Trial Protocol
eMethods. Materials and Methods
eFigure 1. The first two principal components of worst-responders (n=160) and best-responders (n=156) from PCA analysis result
eFigure 2. Quantile-quantile plot of genome-wide P values of single-variant (SNPs with MAF > 0.05) associations
eTable 1. Use of antipsychotics in the present study
eTable 2. List of 175 pharmacogenetics associated genes selected as candidates
eTable 3. Single variant based association results of the top 100 SNPs
eTable 4. The gene-based association results of rare variants using VT test, one-sided burden test and SKAT-O, top 100 genes are shown for each test
eTable 5. Nominally significant rare variant association (gene-based) results on candidate genes
eTable 6. Set-based association result of rare (MAF < 1%) damaging non-synymous variant in the 79 candidate gene sets
eTable 7. Information about variants identified in the reduced NMDA-mediated synaptic current in the discovery exome study
eTable 8. Information about variants identified in the reduced AMPA-mediated synaptic current in the discovery exome study
eTable 9. Information about variants identified in the abnormal AMPA-mediated synaptic current in the discovery exome study
eTable 10. Set-based association results on candidate gene-sets in the discovery exome on rare (MAF<0.01) synonymous variants
eTable 11. Full list of genes selected for targeted sequencing
eTable 12. The gene-based association results of rare variants (damaging non-synonymous with MAF < 1%) using CMC burden test in follow-up study
eTable 13. Information about variants identified in the reduced NMDA-mediated synaptic current in the follow-up study
eTable 14. Set-based association results on the 3 candidate gene-sets in the follow-up study on rare (MAF<0.01) synonymous variants in CMC burden test
eTable 15. Burden of rare allele (damaging nonsyn with MAF < 0.01) in follow-up by median splitting, with subjects with lower PANSS were treated as “less responsive”
References
- 1.Jufe GS. Schizophrenia according to DSM-5 [in Spanish]. Vertex. 2014;25(113):36-42. [PubMed] [Google Scholar]
- 2.Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30(2):279-293. doi: 10.1093/oxfordjournals.schbul.a007078 [DOI] [PubMed] [Google Scholar]
- 3.Brennand KJ, Simone A, Tran N, Gage FH. Modeling psychiatric disorders at the cellular and network levels. Mol Psychiatry. 2012;17(12):1239-1253. doi: 10.1038/mp.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mata I, Madoz V, Arranz MJ, Sham P, Murray RM. Olanzapine: concordant response in monozygotic twins with schizophrenia. Br J Psychiatry. 2001;178(1):86. doi: 10.1192/bjp.178.1.86 [DOI] [PubMed] [Google Scholar]
- 5.Wehmeier PM, Gebhardt S, Schmidtke J, Remschmidt H, Hebebrand J, Theisen FM. Clozapine: weight gain in a pair of monozygotic twins concordant for schizophrenia and mild mental retardation. Psychiatry Res. 2005;133(2-3):273-276. doi: 10.1016/j.psychres.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 6.McClay JL, Adkins DE, Aberg K, et al. Genome-wide pharmacogenomic analysis of response to treatment with antipsychotics. Mol Psychiatry. 2011;16(1):76-85. doi: 10.1038/mp.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavedan C, Licamele L, Volpi S, et al. Association of the NPAS3 gene and five other loci with response to the antipsychotic iloperidone identified in a whole genome association study. Mol Psychiatry. 2009;14(8):804-819. doi: 10.1038/mp.2008.56 [DOI] [PubMed] [Google Scholar]
- 8.Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185-190. doi: 10.1038/nature12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179-184. doi: 10.1038/nature12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulsuner S, Walsh T, Watts AC, et al. ; Consortium on the Genetics of Schizophrenia (COGS); PAARTNERS Study Group . Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154(3):518-529. doi: 10.1016/j.cell.2013.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Li M, Yang Z, et al. Increased co-expression of genes harboring the damaging de novo mutations in Chinese schizophrenic patients during prenatal development. Sci Rep. 2015;5:18209-18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genovese G, Fromer M, Stahl EA, et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci. 2016;19(11):1433-1441. doi: 10.1038/nn.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauch A, Wieczorek D, Graf E, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380(9854):1674-1682. doi: 10.1016/S0140-6736(12)61480-9 [DOI] [PubMed] [Google Scholar]
- 14.Gilissen C, Hehir-Kwa JY, Thung DT, et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511(7509):344-347. doi: 10.1038/nature13394 [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Haas SA, Chelly J, et al. X-exome sequencing of 405 unresolved families identifies seven novel intellectual disability genes. Mol Psychiatry. 2016;21(1):133-148. doi: 10.1038/mp.2014.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74(2):285-299. doi: 10.1016/j.neuron.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242-245. doi: 10.1038/nature11011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237-241. doi: 10.1038/nature10945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tammiste A, Jiang T, Fischer K, et al. Whole-exome sequencing identifies a polymorphism in the BMP5 gene associated with SSRI treatment response in major depression. J Psychopharmacol. 2013;27(10):915-920. doi: 10.1177/0269881113499829 [DOI] [PubMed] [Google Scholar]
- 20.Holsboer F. How can we realize the promise of personalized antidepressant medicines? Nat Rev Neurosci. 2008;9(8):638-646. doi: 10.1038/nrn2453 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 23.Jones HM, Pilowsky LS. Dopamine and antipsychotic drug action revisited. Br J Psychiatry. 2002;181:271-275. doi: 10.1192/bjp.181.4.271 [DOI] [PubMed] [Google Scholar]
- 24.Li MX, Gui HS, Kwan JS, Bao SY, Sham PC. A comprehensive framework for prioritizing variants in exome sequencing studies of Mendelian diseases. Nucleic Acids Res. 2012;40(7):e53. doi: 10.1093/nar/gkr1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price AL, Kryukov GV, de Bakker PI, et al. Pooled association tests for rare variants in exon-resequencing studies. Am J Hum Genet. 2010;86(6):832-838. doi: 10.1016/j.ajhg.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Emond MJ, Bamshad MJ, et al. ; NHLBI GO Exome Sequencing Project—ESP Lung Project Team . Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am J Hum Genet. 2012;91(2):224-237. doi: 10.1016/j.ajhg.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li MX, Gui HS, Kwan JS, Sham PC. GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet. 2011;88(3):283-293. doi: 10.1016/j.ajhg.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Changasi AH, Shams TA, Pouget JG, Müller DJ. Genetics of antipsychotic drug outcome and implications for the clinician: into the limelight. Transl Dev Psychiatry. 2014;2(1):246-263. doi: 10.3402/tdp.v2.24663 [DOI] [Google Scholar]
- 29.Dzyubenko E, Juckel G, Faissner A. The antipsychotic drugs olanzapine and haloperidol modify network connectivity and spontaneous activity of neural networks in vitro. Sci Rep. 2017;7(1):11609-11622. doi: 10.1038/s41598-017-11944-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsueh HM, Chen JJ, Kodell RL. Comparison of methods for estimating the number of true null hypotheses in multiplicity testing. J Biopharm Stat. 2003;13(4):675-689. doi: 10.1081/BIP-120024202 [DOI] [PubMed] [Google Scholar]
- 31.Nettleton D, Hwang JTG, Caldo RA, Wise RP. Estimating the number of true null hypotheses from a histogram of P values. J Agric Biol Environ Stat. 2006;11(3):337-356. doi: 10.1198/108571106X129135 [DOI] [Google Scholar]
- 32.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics. 2016;32(9):1423-1426. doi: 10.1093/bioinformatics/btw079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Guo Y, Li J, Long J, Zhang B, Shyr Y. Steps to ensure accuracy in genotype and SNP calling from Illumina sequencing data. BMC Genomics. 2012;13(suppl 8):S8-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7 (31):1-12. doi: 10.3389/fncel.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55(2):165-171. doi: 10.1016/S0006-3223(03)00707-8 [DOI] [PubMed] [Google Scholar]
- 37.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56(1):29-36. doi: 10.1001/archpsyc.56.1.29 [DOI] [PubMed] [Google Scholar]
- 38.Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44(11):1081-1089. doi: 10.1016/S0006-3223(98)00279-0 [DOI] [PubMed] [Google Scholar]
- 39.Stip E, Trudeau LE. Glycine and D-serine improve the negative symptoms of schizophrenia. Evid Based Ment Health. 2005;8(3):82. doi: 10.1136/ebmh.8.3.82 [DOI] [PubMed] [Google Scholar]
- 40.de Bartolomeis A, Fiore G, Iasevoli F. Dopamine-glutamate interaction and antipsychotics mechanism of action: implication for new pharmacological strategies in psychosis. Curr Pharm Des. 2005;11(27):3561-3594. doi: 10.2174/138161205774414538 [DOI] [PubMed] [Google Scholar]
- 41.Yang CR, Chen L. Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11(5):452-470. doi: 10.1177/1073858405279692 [DOI] [PubMed] [Google Scholar]
- 42.Stone JM. Glutamatergic antipsychotic drugs: a new dawn in the treatment of schizophrenia? Ther Adv Psychopharmacol. 2011;1(1):5-18. doi: 10.1177/2045125311400779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming JJ, England PM. AMPA receptors and synaptic plasticity: a chemist’s perspective. Nat Chem Biol. 2010;6(2):89-97. doi: 10.1038/nchembio.298 [DOI] [PubMed] [Google Scholar]
- 44.Zhang JP, Malhotra AK. Pharmacogenetics of antipsychotics: recent progress and methodological issues. Expert Opin Drug Metab Toxicol. 2013;9(2):183-191. doi: 10.1517/17425255.2013.736964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandl EJ, Kennedy JL, Müller DJ. Pharmacogenetics of antipsychotics. Can J Psychiatry. 2014;59(2):76-88. doi: 10.1177/070674371405900203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Materials and Methods
eFigure 1. The first two principal components of worst-responders (n=160) and best-responders (n=156) from PCA analysis result
eFigure 2. Quantile-quantile plot of genome-wide P values of single-variant (SNPs with MAF > 0.05) associations
eTable 1. Use of antipsychotics in the present study
eTable 2. List of 175 pharmacogenetics associated genes selected as candidates
eTable 3. Single variant based association results of the top 100 SNPs
eTable 4. The gene-based association results of rare variants using VT test, one-sided burden test and SKAT-O, top 100 genes are shown for each test
eTable 5. Nominally significant rare variant association (gene-based) results on candidate genes
eTable 6. Set-based association result of rare (MAF < 1%) damaging non-synymous variant in the 79 candidate gene sets
eTable 7. Information about variants identified in the reduced NMDA-mediated synaptic current in the discovery exome study
eTable 8. Information about variants identified in the reduced AMPA-mediated synaptic current in the discovery exome study
eTable 9. Information about variants identified in the abnormal AMPA-mediated synaptic current in the discovery exome study
eTable 10. Set-based association results on candidate gene-sets in the discovery exome on rare (MAF<0.01) synonymous variants
eTable 11. Full list of genes selected for targeted sequencing
eTable 12. The gene-based association results of rare variants (damaging non-synonymous with MAF < 1%) using CMC burden test in follow-up study
eTable 13. Information about variants identified in the reduced NMDA-mediated synaptic current in the follow-up study
eTable 14. Set-based association results on the 3 candidate gene-sets in the follow-up study on rare (MAF<0.01) synonymous variants in CMC burden test
eTable 15. Burden of rare allele (damaging nonsyn with MAF < 0.01) in follow-up by median splitting, with subjects with lower PANSS were treated as “less responsive”