Key Points
Question
What is the latent structure of negative symptoms in schizophrenia?
Findings
Three cross-sectional studies were conducted on 860 outpatients with schizophrenia who underwent rating with the 3 most conceptually contemporary measures. Confirmatory factor analysis indicated that the 1- and 2-factor models provided a poor fit for the data; however, 5-factor and hierarchical models provided an excellent fit.
Meaning
These findings suggest that a change is warranted regarding diagnostic criteria for schizophrenia, how pathophysiological mechanisms are explored, and how to search for targeted treatments for negative symptoms.
This cross-sectional study used confirmatory factor analysis to evaluate the fit of 4 models regarding the latent structure of negative symptoms using confirmatory factor analysis in a population of outpatients with schizophrenia.
Abstract
Importance
Negative symptoms are associated with a range of poor clinical outcomes, and currently available treatments generally do not produce a clinically meaningful response. Limited treatment progress may be owing in part to poor clarity regarding latent structure. Prior studies have inferred latent structure using exploratory factor analysis, which has led to the conclusion that there are 2 dimensions reflecting motivation and pleasure (MAP) and diminished expressivity (EXP) factors. However, whether these conclusions are statistically justified remains unclear because exploratory factor analysis does not test latent structure. Confirmatory factor analysis (CFA) is needed to test competing models regarding the latent structure of a construct.
Objective
To evaluate the fit of 4 models of the latent structure of negative symptoms in schizophrenia using CFA.
Design, Setting, and Participants
Three cross-sectional studies were conducted on outpatients with schizophrenia who were rated on the 3 most conceptually contemporary measures: Scale for the Assessment of Negative Symptoms (SANS), Brief Negative Symptom Scale (BNSS), and Clinical Assessment Interview for Negative Symptoms (CAINS). Confirmatory factor analysis evaluated the following 4 models: (1) a 1-factor model; (2) a 2-factor model with EXP and MAP factors; (3) a 5-factor model with separate factors for the 5 domains of the National Institute of Mental Health consensus development conference (blunted affect, alogia, anhedonia, avolition, and asociality); and (4) a hierarchical model with 2 second-order factors reflecting EXP and MAP and 5 first-order factors reflecting the 5 consensus domains.
Main Outcomes and Measures
Outcomes included CFA model fit statistics derived from symptom severity scores on the SANS, BNSS, and CAINS.
Results
The study population included 860 outpatients with schizophrenia (68.0% male; mean [SD] age, 43.0 [11.4] years). Confirmatory factor analysis was conducted on each scale, including 268 patients for the SANS, 192 for the BNSS, and 400 for the CAINS. The 1- and 2-factor models provided poor fit for the SANS, BNSS, and CAINS as indicated by comparative fit indexes (CFIs) and Tucker Lewis indexes (TLIs) less than 0.950, RMSEAs that exceeded the 0.080 threshold, and WRMRs greater than 1.00. The 5-factor and hierarchical models provided excellent fit, with the 5-factor model being more parsimonious. The CFIs and TLIs met the 0.95 threshold and the 1.00 threshold for both factor models with all 3 measures. Interestingly, the RMSEAs for the 5-factor model and the hierarchical model fell under the 0.08 threshold for the BNSS and the CAINS but not the SANS.
Conclusions and Relevance
These findings suggest that the recent trend toward conceptualizing the latent structure of negative symptoms as 2 distinct dimensions does not adequately capture the complexity of the construct. The latent structure of negative symptoms is best conceptualized in relation to the 5 consensus domains. Implications for identifying pathophysiological mechanisms and targeted treatments are discussed.
Introduction
Negative symptoms have long been considered a core symptom of schizophrenia.1,2 Factor analytic studies support these early clinical impressions, indicating that negative symptoms are distinct from positive and disorganized symptoms.3,4,5 However, studies examining the factor structure of items within negative symptom scales alone suggest that the construct may not be unidimensional.6 Consistent evidence suggests that there are 2 distinct factors reflecting diminished motivation and pleasure (MAP, including anhedonia, avolition, and asociality) and diminished expressivity (EXP, including blunted affect and alogia) across a variety of scales.7,8,9,10,11,12,13 These findings have led the field to shift away from a unidimensional conceptualization of negative symptoms in favor of a 2-dimensional conceptualization consisting of MAP and EXP.14
However, whether current views on the latent structure of negative symptoms are theoretically or statistically justified remains unclear. Evidence supporting the 2-dimensional structure comes primarily from studies using exploratory factor analysis (EFA). Exploratory factor analysis is a data reduction technique that infers the presence of latent factors responsible for shared variance among a set of items. It does not specify an underlying structure, but rather assumes that each item could be related to each latent factor. Exploratory factor analysis is an important first step in generating hypotheses regarding the latent structure of a construct, but it does not actually test competing models regarding the number of dimensions that exist or that evaluate which items are part of those dimensions. Confirmatory factor analysis (CFA) is needed to achieve these aims and make definitive conclusions regarding the latent structure of a construct, because CFA allows for direct comparison of competing theoretical models. Prior CFA studies15,16 have been restricted to the Scale for the Assessment of Negative Symptoms (SANS) and have included items that are no longer considered part of the negative symptom construct (eg, inappropriate affect, inattention), limiting conclusions regarding the latent structure of negative symptoms.
The present study used CFA to evaluate competing hypotheses regarding the latent structure of negative symptoms. Four models were examined across 3 studies using data on outpatients with schizophrenia who underwent rating using the SANS,17 the Brief Negative Symptom Scale (BNSS),18 or the Clinical Assessment Interview for Negative Symptoms (CAINS).12 The first model was unidimensional, which considered whether all items best reflect a single latent construct. A unidimensional model is important to test because original conceptualizations of the construct posited a single dimension.19 In addition, most negative symptom scales are still evaluated using a single total score, which may or may not be justified. The second model evaluated the 2 dimensions identified in prior EFA studies,7,11,12,13 which indicate separate EXP and MAP factors. The EFA studies supporting the 2 factors have been influential, informing how researchers search for pathophysiological mechanisms of negative symptoms20,21,22 and how pharmaceutical companies have recently been approaching targeted treatment development.23 However, these decisions may not be empirically supported, and a more fine-grained approach may be warranted. The most contemporary conceptualization resulted from the 2005 National Institute of Mental Health (NIMH) consensus development conference, which proposed the existence of the following 5 core domains: blunted affect, alogia, avolition, anhedonia, and asociality.24 Although EFAs indicate that these 5 domains load onto EXP and MAP factors, examination of more complex theoretical models is required to understand the underlying structure of negative symptoms. Confirmatory factor analysis can be used for this purpose. If more complex models are superior, the current focus on the 2 factors may preclude identification of pathophysiological mechanisms or treatment effects that are specific to the 5 domains. As such, more complex models were also examined. The third model was a 5-factor model that specified 1 factor for each of the 5 consensus domains. The fourth model was a hierarchical model with 2 second-order factors reflecting EXP and MAP and 5 first-order factors reflecting the 5 consensus domains. In the hierarchical model, first-order factors representing anhedonia, avolition, and asociality were specified to load on the MAP second-order factor, whereas the first-order factors blunted affect and alogia were specified to load on the EXP second-order factor. Collectively, evaluating the fits of 1-, 2-, 5-, and hierarchical-model solutions provides a comprehensive test of the latent structure of negative symptoms that is informed by theory.
Methods
Participants
Data were examined for 3 studies that used different negative symptom scales to evaluate samples of predominantly outpatients with chronic schizophrenia. This study was approved by the local institutional review boards, and all participants provided written informed consent.
Study 1 included data from 268 outpatients with schizophrenia who underwent rating using the SANS.25 Participants were recruited from the outpatient research clinics at the Maryland Psychiatric Research Center, Catonsville, and community mental health clinics in the Baltimore, Maryland, metropolitan area. Study 2 included 192 outpatients with schizophrenia who underwent rating using the BNSS.18 Participants were recruited from the following 3 sites: (1) the outpatient research clinics at the Maryland Psychiatric Research Center and community mental health clinics in the Baltimore metropolitan area (n = 65); (2) the State University of New York at Binghamton, including community outpatient mental health clinics in upstate New York (n = 60); and (3) the University of Nevada, Las Vegas, including community outpatient mental health clinics in Las Vegas (n = 67). Study 3 included data from 400 outpatients with schizophrenia who underwent rating using the CAINS.12 These patients were recruited from the Maryland Psychiatric Research Center and community mental health clinics in the Baltimore metropolitan area (n = 117) and the University of California, San Diego, Department of Psychiatry via Assertive Community Treatment teams in the San Diego metropolitan area (n = 283).
All participants met DSM-IV-TR26 criteria for schizophrenia or schizoaffective disorder as determined by the Structured Clinical Interview for DSM-IV interview27 (see Table 1 for demographic characteristics). Most participants were prescribed a second-generation antipsychotic, and all were clinically stable as indicated by no change in the type or the dose of antipsychotic for 4 weeks before evaluation. Data from each study had not been used in prior EFAs.
Table 1. Demographic Characteristics.
| Variable | Substudy | ||
|---|---|---|---|
| SANS (n = 268) | BNSS (n = 192) | CAINS (n = 400) | |
| Age, mean (SD), y | 40.6 (11.9) | 40.3 (11.7) | 45.8 (10.6) |
| Male, No. (%) | 188 (70.1) | 124 (64.6) | 273 (68.3) |
| Race/ethnicity, No. (%) | |||
| Non-Hispanic white | 143 (53.4) | 114 (59.4) | 169 (42.3) |
| African American | 102 (38.1) | 48 (25.0) | 139 (34.8) |
| Hispanic | 0 | 9 (4.7) | 52 (13.0) |
| Asian | 8 (3.0) | 6 (3.1) | 14 (3.5) |
| Native American or Alaskan | 2 (0.7) | 3 (1.6) | 14 (3.5) |
| Biracial | 10 (3.7) | 9 (4.7) | 6 (1.5) |
| Other | 3 (1.1) | 3 (1.6) | 6 (1.5) |
Abbreviations: BNSS, Brief Negative Symptom Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; SANS, Scale for the Assessment of Negative Symptoms.
Procedures
At each site, the SANS, the BNSS, or the CAINS was administered as part of larger protocols examining cognition, reward, or the efficacy of cognitive behavioral social skills training (baseline data are reported from that study). Raters at each site were trained to minimum reliability standards (interrater agreement >0.80 with criterion-standard training tapes) before performing study procedures. Rater training consisted of an in-depth review of the manual of and procedures for rating each instrument. Raters watched and rated a series of initial videos that were developed by the BNSS and CAINS authors or internally by the research team for the SANS. Ratings were then discussed as a group using criterion standard rationales, and interviewers were instructed in interview technique. Interviewers subsequently received ongoing supervision and participated in regular (approximately monthly) criterion-standard reliability meetings to maintain quality assurance. All raters had earned a bachelor’s degree or higher and had 1 or more years of clinical experience.
Data Analysis
Mplus software (version 5.0; Muthén and Muthén)28 was used to conduct the CFAs. The CFAs compared 4 models that differed in their number of factors and item-loading patterns (models are described in eTables 1-3 in the Supplement). Owing to violation of multivariate normality for the BNSS and SANS as indicated by a Mardia coefficient greater than 3,29 robust estimation procedures were used. These procedures included the weighted least-squares estimator with SEs and mean- and variance-adjusted χ2 test that used a full-weight matrix and the maximum likelihood with robust SEs. A numerical integration algorithm was used in maximum likelihood robust SE model estimation. Numerical integration becomes computationally demanding in the estimation of models as the number of factors increases. Therefore, a Monte Carlo method of designating integration points was used. The number of selected integration points ranged from 5000 to 10 000 in the estimation of tested models.
For the BNSS, the lack of a normal distress item was not included in the CFA models because the distress item was not part of the agreed NIMH consensus conference domains, and prior EFA studies reported low communalities for this item.13 For the SANS, the anhedonia item was specified to load by itself on the anhedonia factor, because no other items on the SANS assessed anhedonia. Similarly, for the CAINS, the quantity of speech item was specified to load by itself on the alogia factor because it is the only CAINS item that assesses alogia (eTables 1-3 in the Supplement).30
Model fit was evaluated using indices of absolute fit, including the model χ2 test, the comparative fit index (CFI), the Tucker Lewis index (TLI), the root mean square error of approximation (RMSEA), the standardized root mean square residual (SRMR), and the weighted root mean square residual (WRMR). Information criteria including the Akaike information criterion, Bayesian information criteria, and the sample size–adjusted Bayesian information criteria evaluated the relative fit of alternate models. The model χ2 reflects the degree to which the data agree with the hypothesized model.31 The CFI and TLI are incremental fit indices that compare the independence model with the hypothesized model.32 The SRMR and the WRMR are residual-based indices of the difference between sample and hypothesized variance-covariance matrices. Whereas the SRMR is obtained in EFA estimation, WRMR is obtained in the estimation of CFA models. The RMSEA is a parsimony index that evaluates the fit between the hypothesized model and the population covariance matrix.33 The information criteria are relative fit indices of model parsimony that take into account model complexity based on degrees of freedom.34 Evidence of model fit was determined according to standard interpretations of the fit indices, including a χ2 value that is not statistically significant,35 CFI and TLI values of at least 0.950, and an RMSEA no greater than 0.080.31 The SRMR values range from 0 to 1, with values of 0.080 or lower indicative of good-fitting models. The WRMR values of 1.00 and lower are considered strong fits. The information criteria allow for comparisons between nonnested models, with lower values indicating better model fit,34 so the lowest value was used to determine optimal model fit. Of note, the χ2 test tends to falsely reject adequate statistical model fit with large sample sizes,36 and, thus, the descriptive fit indices are preferred for interpretation of model fit.1
Results
A total of 860 outpatients were included in the study (585 men [68.0%] and 275 women [32.0%]; mean [SD] age, 43.0 [11.4] years). Results of the CFAs of evaluated models are presented in Table 2. The 1- and 2-factor models provided poor fit for the SANS, BNSS, and CAINS as indicated by CFIs and TLIs less than 0.950, RMSEAs that exceeded the 0.080 threshold, and WRMRs greater than 1.00.
Table 2. Model Fit Results From CFA of Negative Symptom Measures.
| Model by Substudya | Log likelihood | Free Parameters, No. | Confirmatory Factor Analysisb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AIC | BIC | aBIC | χ2 Value (df) | CFI | TLI | RMSEA | WRMR | |||
| SANSc | ||||||||||
| 1-Factor | −5729.04 | 99 | 11 656.08 | 12 010.48 | 11 696.59 | 539.97 (45)d | 0.782 | 0.879 | 0.204 | 1.98 |
| 2-Factor | −5607.89 | 100 | 11 415.79 | 11 773.76 | 11 456.71 | 237.75 (47)d | 0.916 | 0.955 | 0.124 | 1.30 |
| 5-Factor | −5556.59 | 109 | 11 331.08 | 11 721.27 | 11 375.68 | 161.51 (46)d | 0.949 | 0.972 | 0.097 | 1.00 |
| Hierarchical | −5564.74 | 101 | 11 331.48 | 11 693.03 | 11 372.81 | 154.92 (46)d | 0.952 | 0.974 | 0.095 | 0.99 |
| BNSSe | ||||||||||
| 1-Factor | −2939.06 | 84 | 6046.13 | 6319.76 | 6053.67 | 225.95 (11)d | 0.934 | 0.952 | 0.319 | 2.13 |
| 2-Factor | −2748.99 | 85 | 5668.00 | 544.88 | 5675.63 | 104.17 (16)d | 0.973 | 0.986 | 0.169 | 1.08 |
| 5-Factor | −2606.95 | 94 | 5401.90 | 5708.10 | 5410.34 | 39.87 (19)f | 0.994 | 0.997 | 0.076 | 0.43 |
| Hierarchical | −2630.18 | 84 | 5428.36 | 5701.99 | 5435.90 | 21.77 (16)g | 0.998 | 0.999 | 0.043 | 0.47 |
| CAINSh | ||||||||||
| 1-Factor | −6769.83 | 65 | 13 669.66 | 13 929.11 | 13 722.86 | 982.64 (19)d | 0.770 | 0.794 | 0.356 | 4.25 |
| 2-Factor | −6433.37 | 66 | 12 998.74 | 13 262.18 | 13 052.75 | 481.65 (20)d | 0.890 | 0.906 | 0.240 | 2.80 |
| 5-Factor | −6418.02 | 75 | 12 986.03 | 13 285.39 | 13 047.41 | 76.52 (19)d | 0.986 | 0.988 | 0.077 | 0.89 |
| Hierarchical | −6436.61 | 66 | 13 005.21 | 13 268.65 | 13 059.23 | 56.44 (21)d | 0.992 | 0.993 | 0.065 | 0.79 |
Abbreviations: AIC, Akaike information criterion; aBIC, sample size–adjusted Bayesian information criterion (BIC); BNSS, Brief Negative Symptom Scale; CAINS, Clinical Assessment Interview for Negative Symptoms; CFA, confirmatory factor analysis; CFI, confirmatory fit index; RMSEA, root mean square error of approximation; SANS, Scale for the Assessment of Negative Symptoms; TLI, Tucker Lewis index; WRMR, weighted root mean square residual.
Models included the unidimensional 1-factor model; the motivation and pleasure (MAP) and diminished expression (EXP) 2-factor model; the 5-factor (anhedonia, asociality, avolition, blunted affect, and alogia) consensus model; and the hierarchical model with the 5 first-order consensus factors and 2 second-order MAP and EXP factors.
Weighted least squares and maximum likelihood with robust SE (MLR) estimators were used in the analyses. Monte Carlo–based numerical integration was used in the MLR estimation of models to ease computation time. The number of Monte Carlo–generated integration points ranged from 5000 to 6000.
χ225 = 2293.82 (P < .001) on the baseline model.
P < .001.
χ28 = 3247.08 (P < .001) on the baseline model.
P < .01.
P = .15
χ217 = 4199.74 (P < .001) on the baseline model.
In contrast, the 5-factor model and the hierarchical model provided strong fit for the SANS, BNSS, and CAINS (Table 2, Figure 1, Figure 2, and Figure 3). The CFIs and TLIs met the 0.95 threshold and the 1.00 threshold for both factor models with all 3 measures. Interestingly, the RMSEAs for the 5-factor model and the hierarchical model fell under the 0.08 threshold for the BNSS and the CAINS but not the SANS.
Figure 1. Five-Factor and Hierarchical Models of the Scale for the Assessment of Negative Symptoms (SANS).
The 5 factors of anhedonia, asociality, avolition, blunted affect, and alogia were included in the consensus model. The hierarchical model consists of the 5 factors and the second-order factors of motivation and pleasure (MAP) and diminished expressivity (EXP). Solid lines represent factor loadings and curved lines represent the correlation among factors (A) and second-order factors (B). Numbers indicate item numbers on each scale.
Figure 2. Five-Factor and Hierarchical Models of the Brief Negative Symptom Scale (BNSS).
The 5 factors of anhedonia, asociality, avolition, blunted affect, and alogia were included in the consensus model. The hierarchical model consists of the 5 factors and the second-order factors of motivation and pleasure (MAP) and diminished expressivity (EXP). Solid lines represent factor loadings and curved lines represent the correlation among factors (A) and second-order factors (B). Numbers indicate item numbers on each scale.
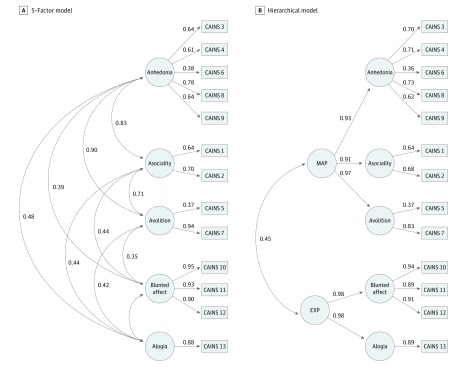
Figure 3. Five-Factor and Hierarchical Models of the Clinical Assessment Interview for Negative Symptoms (CAINS).
The 5 factors of anhedonia, asociality, avolition, blunted affect, and alogia were included in the consensus model. The hierarchical model consists of the 5 factors and the second-order factors of motivation and pleasure (MAP) and diminished expressivity (EXP). Solid lines represent factor loadings and curved lines represent the correlation among factors (A) and second-order factors (B). Numbers indicate item numbers on each scale.
The information criteria indices favored the 5-factor model and the hierarchical model compared with the 1- and the 2-factor models for all 3 measures. Although the 5-factor and hierarchical models are supported, the information criteria by majority demonstrated a preference for the 5-factor model compared with the hierarchical model on the BNSS and the CAINS. In contrast, information criteria slightly favored the hierarchical model on the SANS.
Discussion
We used CFA to evaluate the latent structure of negative symptoms using data from 3 contemporary negative symptom scales (SANS, BNSS, and CAINS). Results were highly consistent across all 3 studies, indicating that the 1- and 2-factor models provided relatively poor fit for the data. The 2 more complex models provided excellent fit for the data. Indices of relative fit favored the 5-factor model over the hierarchical model in the BNSS and CAINS data, and the hierarchical model had a slight edge in the SANS data. When interpreting these results, we should clarify that good fit for the hierarchical model is not simply further support for the 2-dimensional conceptualization, nor does it negate the importance of the 5 domains, because MAP and EXP are secondary dimensions in these hierarchical models and the 5 factors are primary. Because primary dimensions are the ones directly influencing ratings of all negative symptoms in these hierarchical models, this factor suggests that the 5 domains, not the MAP and EXP dimensions, are the most fundamental aspect of negative symptoms that best account for latent structure. The consistency of findings across the 3 scales suggests that nothing about the organization of the scale, manual, or worksheet, for example, arbitrarily produces the 5-domain structure, because these elements are very different across measures.
These findings have several important clinical implications. First, the DSM-5 describes negative symptoms in association with the 2 factors (MAP and EXP). This decision was driven by published EFA studies. Our results suggest that a revision to DSM-5 descriptions of negative symptoms is in order. Specifically, the 5 consensus domains should be defined and considered individually. Second, several important implications apply for clinical trials. Should studies of neurocognitive function, animal models, and biological correlates demonstrate differential relationships to the 5 domains, industry and the US Food and Drug Administration would have a compelling reason to develop targeted treatments for individual domains rather than the broader negative symptom construct. Most studies have explored neurobiological correlates of negative symptoms in association with a total score or the broader MAP and EXP dimensions,21,37 precluding observation of domain-specific neural correlates. However, among the few studies that separate out the domains, some evidence suggests distinct neural correlates among domains38 and the need for further investigation. The NIMH Research Domain Criteria initiative offers a promising framework for exploring mechanisms related to each domain. In particular, the Research Domain Criteria positive valence systems and social processes contain constructs that are conceptually associated with the 5 negative symptom domains. Tasks have been developed in the field of basic neuroscience to assess these constructs, which have distinct cellular and circuit-level mechanisms.39 Some of these tasks have already been translated to human populations40,41,42 and are ideal for exploring neurobiology specific to the 5 domains. Psychosocial intervention trials also demonstrate that taking a targeted approach to treating specific psychological mechanisms (eg, defeatist performance beliefs) underlying individual domains of negative symptoms (eg, avolition)43 is a fruitful approach, which could indeed be adopted by pharmacologic trials once relevant biological mechanisms are identified. In addition, clinical trials should consider adjusting procedures for how they determine primary outcome measures. In pharmaceutical trials, total scores are the most common primary outcome measures. This approach has distinct statistical advantages (eg, sample size, power, or reducing type I error); however, it does not adequately capture the complexity of the construct. Once specific mechanistic targets are identified for individual domains, industry should shift toward using single domains as primary outcome measures in trials. Until that time, the 5 domains should be considered for secondary analyses, and the DSM-5 should retain the 2 dimensions. eTables 1 through 3 in the Supplement list which items should be considered within each of the 5 domains for the SANS, BNSS, and CAINS, which will facilitate exploration of these domains.
We also have several scoring recommendations. When calculating domain scores, we recommend calculating the mean of the items within these domains rather than summing because the different domains have differing numbers of items, complicating comparisons across domains. We also generally do not recommend calculating factor scores using factor loadings because these scores are driven by sample-specific differences, and such calculations prevent comparisons across studies. When using the SANS, we recommend omitting global items when calculating domain scores to prevent redundancy, conflation across domains, and undue influence of halo effects. First-generation negative symptom scales17,44 or subscales45,46 do not adequately cover the 5 domains and conflate constructs. Newer scales, such as the CAINS and BNSS, may be more ideal for measuring negative symptoms, and each of these scales has unique advantages for use in experimental psychopathology and clinical trial studies that make them optimal for specific purposes.47
Limitations
Limitations of our study should be considered. First, only patients in the chronic illness phase were studied, and results may not generalize to earlier phases of illness with higher symptom severity; whether the factor structure would differ between patients whose negative symptoms are primary vs secondary (or deficit vs nondeficit) is also unclear. Second, we were unable to evaluate whether the 5 domains have distinct correlates. Future studies should validate the significance of the 5 domains by exploring a range of external correlates (ie, clinical, cognitive, molecular, cellular, structural, functional, and genetic). Third, the mean severity ratings in our samples were relatively low. However, this finding is not expected to have had an effect on results, because prior studies48 indicate little factorial invariance across samples that differ in negative symptom severity. Fourth, debate as to whether use of single-item indicators is problematic or ideal for testing latent structure is ongoing30,49,50,51,52,53; given that structure was comparable across scales with and without single-item indicators, we doubt that this factor greatly influenced results. Fourth, longitudinal data were not available on these scales to determine whether these factors change along independent trajectories. Finally, results are limited by how well the scales used assess the construct; as measures become more objective and precise, the domains may become even more granular. Furthermore, as acknowledged in the NIMH consensus statement, other aspects of negative symptoms may not have been recognized.
Conclusions
Collectively, these findings suggest that negative symptoms should no longer be considered a single unitary construct, as was assumed when negative symptom scales were originally developed. They should not be considered a simple 2-dimensional construct, as has recently been concluded based on EFA results.11,12,13 Rather, the latent structure of negative symptoms is best conceptualized in relation to the 5 domains identified in the 2005 NIMH consensus development conference: anhedonia, avolition, asociality, alogia, and blunted affect. If distinct clinical and pathophysiological correlates of these 5 domains are identified in future research, this approach will warrant a change in how negative symptoms are conceptualized and how targeted treatment development is approached.
eTable 1. Confirmatory Factor Analysis Models for the Scale for the Assessment of Negative Symptoms (SANS)
eTable 2. Confirmatory Factor Analysis Models for the Brief Negative Symptom Scale (BNSS)
eTable 3. Confirmatory Factor Analysis Models for the Clinical Assessment Interview for Negative Symptoms (CAINS)
References
- 1.Bleuler E. Dementia praecox or the group of schizophrenias [in Spanish]. Vertex. 2010;21(93):394-400. [PubMed] [Google Scholar]
- 2.Kraepelin E, Robertson MR, eds. Dementia Praecox and Paraphrenia. New York, NY: Krieger Publishing; 1919. [Google Scholar]
- 3.Andreasen NC, Arndt S, Miller D, Flaum M, Nopoulos P. Correlational studies of the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms: an overview and update. Psychopathology. 1995;28(1):7-17. doi: 10.1159/000284894 [DOI] [PubMed] [Google Scholar]
- 4.Arndt S, Alliger RJ, Andreasen NC. The distinction of positive and negative symptoms: the failure of a two-dimensional model. Br J Psychiatry. 1991;158:317-322. doi: 10.1192/bjp.158.3.317 [DOI] [PubMed] [Google Scholar]
- 5.Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophr Res. 1998;31(2-3):113-120. doi: 10.1016/S0920-9964(98)00011-5 [DOI] [PubMed] [Google Scholar]
- 6.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238-245. doi: 10.1093/schbul/sbj013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am J Psychiatry. 1999;156(3):406-411. [DOI] [PubMed] [Google Scholar]
- 8.Kimhy D, Yale S, Goetz RR, McFarr LM, Malaspina D. The factorial structure of the Schedule for the Deficit Syndrome in Schizophrenia. Schizophr Bull. 2006;32(2):274-278. doi: 10.1093/schbul/sbi064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakaya M, Ohmori K. A two-factor structure for the Schedule for the Deficit Syndrome in schizophrenia. Psychiatry Res. 2008;158(2):256-259. doi: 10.1016/j.psychres.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Strauss GP, Horan WP, Kirkpatrick B, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47(6):783-790. doi: 10.1016/j.jpsychires.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2011;132(2-3):140-145. doi: 10.1016/j.schres.2011.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170(2):165-172. doi: 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss GP, Hong LE, Gold JM, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1-3):96-98. doi: 10.1016/j.schres.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16(1):14-24. doi: 10.1002/wps.20385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayers S, Curran P, Mueser K. Factor structure and construct validity of the Scale for the Assessment of Negative Symptoms. Psychol Assess. 1996;8(3):269-280. doi: 10.1037/1040-3590.8.3.269 [DOI] [Google Scholar]
- 16.Peralta V, Cuesta MJ. Negative symptoms in schizophrenia: a confirmatory factor analysis of competing models. Am J Psychiatry. 1995;152(10):1450-1457. doi: 10.1176/ajp.152.10.1450 [DOI] [PubMed] [Google Scholar]
- 17.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 18.Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305. doi: 10.1093/schbul/sbq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;7(7):49-58. doi: 10.1192/S0007125000291496 [DOI] [PubMed] [Google Scholar]
- 20.Messinger JW, Trémeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31(1):161-168. doi: 10.1016/j.cpr.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43(4):712-719. doi: 10.1093/schbul/sbx066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24(5):725-736. doi: 10.1016/j.euroneuro.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol. 2014;24(5):737-743. doi: 10.1016/j.euroneuro.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214-219. doi: 10.1093/schbul/sbj053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan RW, Javitt DC, Marder SR, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164(10):1593-1602. doi: 10.1176/appi.ajp.2007.06081358 [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 27.First MB, Gibbon M, Spitzer RL, et al. User’s Guide for the Structured Clinical Interview for DSM-IV Research Version. New York, NY: American Psychiatric Publishing; 1996. [Google Scholar]
- 28.Muthén LK, Muthén BO. Mplus User’s Guide. 5th ed Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- 29.Mardia K. Measures of multivariate skewness and kurtosis with applications. Biometrika. 1970;57(3):519-530. doi: 10.1093/biomet/57.3.519 [DOI] [Google Scholar]
- 30.Hayduk LA, Littvay L. Should researchers use single indicators, best indicators, or multiple indicators in structural equation models? BMC Med Res Methodol. 2012;12:159. doi: 10.1186/1471-2288-12-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1-55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 32.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238-246. doi: 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- 33.Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivariate Behav Res. 1990;25(2):173-180. doi: 10.1207/s15327906mbr2502_4 [DOI] [PubMed] [Google Scholar]
- 34.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317-332. doi: 10.1007/BF02294359 [DOI] [Google Scholar]
- 35.Hoyle RH. Confirmatory factor analysis: the question of regressive effects In: Tinsley HEA, Brown SD, eds. Handbook of Applied Multivariate Statistics and Mathematical Modeling. San Diego, CA: Academic Press; 2000:465-497. doi: 10.1016/B978-012691360-6/50017-3 [DOI] [Google Scholar]
- 36.Kapland D. An overview of Markov chain methods for the study of stage-sequential developmental processes. Dev Psychol. 2008;44(2):457-467. doi: 10.1037/0012-1649.44.2.457 [DOI] [PubMed] [Google Scholar]
- 37.Messinger JW, Trémeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31(1):161-168. doi: 10.1016/j.cpr.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaffer JJ, Peterson MJ, McMahon MA, et al. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol Neuropsychiatry. 2015;1(4):191-200. doi: 10.1159/000440979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes SA, Der-Avakian A, Young JW. Preclinical models to investigate mechanisms of negative symptoms in schizophrenia. Schizophr Bull. 2017;43(4):706-711. doi: 10.1093/schbul/sbx065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the “EEfRT”? the Effort Expenditure for Rewards Task as an objective measure of motivation and anhedonia. PLoS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bismark AW, Thomas ML, Tarasenko M, et al. Relationship between effortful motivation and neurocognition in schizophrenia. Schizophr Res. 2018;193:69-76. doi: 10.1016/j.schres.2017.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, Frost KH. Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a progressive ratio task. Schizophr Res. 2016;170(1):198-204. doi: 10.1016/j.schres.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69(2):121-127. doi: 10.1001/archgenpsychiatry.2011.129 [DOI] [PubMed] [Google Scholar]
- 44.Alphs LD, Summerfelt A, Lann H, Muller RJ. The negative symptom assessment: a new instrument to assess negative symptoms of schizophrenia. Psychopharmacol Bull. 1989;25(2):159-163. [PubMed] [Google Scholar]
- 45.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 46.Overall J, Gorham D. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812. doi: 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- 47.Strauss GP, Gold JM. A psychometric comparison of the Clinical Assessment Interview for Negative Symptoms and the Brief Negative Symptom Scale. Schizophr Bull. 2016;42(6):1384-1394. doi: 10.1093/schbul/sbw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed AO, Kirkpatrick B, Galderisi S, et al. Cross-cultural validation of the 5-factor structure of negative symptoms in schizophrenia [published online April 18, 2018]. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayduk LA. LISREL Issues, Debates and Strategies. Baltimore, MD: Johns Hopkins University Press; 1996. [Google Scholar]
- 50.Hayduk LA, Pazderka-Robinson H. Fighting to understand the world causally: three battles connected to the causal implications of structural equation models In: Outhwaite W, Turner S, eds. Sage Handbook of Social Science Methodology. London, UK: Sage Publications; 2007:147-171. doi: 10.4135/9781848607958.n8 [DOI] [Google Scholar]
- 51.Gosling SD, Rentfrow PJ, Swann WB. A very brief measure of the Big-Five personality domains. J Res Pers. 2003;37(6):504-528. doi: 10.1016/S0092-6566(03)00046-1 [DOI] [Google Scholar]
- 52.MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychol Methods. 1999;4(1):84-99. doi: 10.1037/1082-989X.4.1.84 [DOI] [Google Scholar]
- 53.Raubenheimer J. An item selection procedure to maximise scale reliability and validity. J Industrial Psych. 2004;30(4):a168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Confirmatory Factor Analysis Models for the Scale for the Assessment of Negative Symptoms (SANS)
eTable 2. Confirmatory Factor Analysis Models for the Brief Negative Symptom Scale (BNSS)
eTable 3. Confirmatory Factor Analysis Models for the Clinical Assessment Interview for Negative Symptoms (CAINS)