Key Points
Question
What is the incidence and early course of retinopathy of prematurity (ROP) in a large cohort representative of infants undergoing ROP screening?
Findings
In this secondary analysis of 7483 premature infants who underwent ROP examinations at multiple centers and were retrospectively evaluated, 3224 (43.1%) developed ROP, including 459 (6.1%) with type 1 and 472 (6.3%) with type 2 ROP, and 514 (6.9%) underwent treatment in 1 or both eyes.
Meaning
These data suggest that among all premature infants undergoing ROP screening examinations, approximately 12.5% develop severe ROP, occurring almost exclusively among infants of with a birth weight of less than 1251 g.
Abstract
Importance
The current guidelines for retinopathy of prematurity (ROP) detection programs in the United States include a range of birth weights (BWs) and gestational ages and likely require examinations of many premature infants who are at low risk for developing serious retinopathy.
Objective
To determine the incidence, onset, and early course of ROP in what to our knowledge is the largest cohort to date that is representative of infants who are undergoing ROP screening.
Design, Setting, and Participants
This secondary analysis of data from the Postnatal Growth and Retinopathy of Prematurity (G-ROP) retrospective cohort study was conducted in 29 hospitals in the United States and Canada between January 2006 and December 2011 and included 7483 infants who underwent serial ROP examinations.
Main Outcomes and Measures
Most severe ROP in either eye, classified as no ROP, mild ROP, type 2 ROP, or type 1 ROP (per Early Treatment for ROP Study criteria). Onset at postmenstrual age for zone I disease and stage of ROP, plus disease, and treatment.
Results
This study included 7483 infants with a mean (SD) BW of 1099 (259) g and a mean (SD) gestational age of 28 (3) weeks who underwent ROP examinations. Of these, 3224 infants (43.1%) developed ROP, 459 (6.1%) developed type 1 and 472 (6.3%) type 2 ROP, 514 (6.9%) underwent treatment in 1 or both eyes, and 147 (2%) had zone I disease. Additionally, 98.1% of type 1 or 2 ROP cases occurred in infants with a BW of less than 1251 g. Only about half of the eyes (49.4%) were vascularized into zone III by 37 weeks postmenstrual age.
Conclusions and Relevance
These findings add to our knowledge of ROP screening as they include all eligible infants, not just high-risk infants as in previous studies. More than 40% of at-risk premature infants develop some stage of ROP, and most retinopathy regresses without treatment. However, approximately 12.5% develop severe ROP, which occurs almost exclusively among infants with a BW of less than 1251 g.
This secondary analysis of a cohort study provides an overview of the incidence of retinopathy of prematurity in at-risk premature infants in North America who were enrolled in the Postnatal Growth and Retinopathy of Prematurity Study.
Introduction
Retinopathy of prematurity (ROP), especially in its most severe forms, is seen almost exclusively in prematurely born infants, although the incidence varies in different regions of the world.1,2 Overall, fewer than 10% of the infants who undergo ROP screening examinations require treatment.3 However, most large ROP treatment studies have been limited to those infants with a birth weight (BW) of less than 1251 g who are considered at highest risk for developing severe ROP (defined as type 1 or type 2 ROP per Early Treatment for ROP [ETROP] recommendations; type 1 includes “threshold” ROP in the Cryotherapy for ROP study).3,4 Therefore, earlier studies have not addressed the full extent of ROP risk in infants who are undergoing ROP screening examinations. The current guidelines for ROP detection guidelines in the United States5 recommend ROP examinations for infants with a BW of less than 1501 g or a gestational age (GA) of 30 weeks or younger, with the addition of infants with BWs up to 2000 g if a poor neonatal course indicates an examination is needed as determined by the attending neonatologist. These criteria lead to many low-yield examinations in infants who are larger and more mature at birth.
We recently completed a multicenter, retrospective cohort study called the Postnatal Growth and ROP (G-ROP) Study.6,7 The purpose of the G-ROP Study was to evaluate whether the addition to the current BW and GA guidelines of criteria, such as the rate of postnatal weight gain, could decrease the number of infants who require examinations while maintaining high sensitivity for detecting eyes with a high likelihood of needing treatment. However, the large, broad cohort of at-risk infants in the G-ROP data set also provides a detailed medical and ophthalmological profile of infants who are undergoing ROP screening examinations, in contrast to previous large US-based studies that included only infants with BWs of less than 1251 g to maximize the detection of severe ROP while minimizing the number of infants who are enrolled.3,4,8 We sought to use the G-ROP Study data set to describe the incidence, timing of onset, and early course of ROP, and we considered the association of factors, such as BW and GA, with the risk of ROP and severe ROP.
Methods
We performed a secondary analysis of the G-ROP study data.6 In brief, the G-ROP Study was conducted at 29 hospitals in the United States and Canada. Data were collected by abstractors who underwent a rigorous certification process and followed detailed, standardized procedures for interpreting medical records. Numerous data quality safeguards were used.6 The study included infants who were born between January 1, 2006, and December 31, 2011, and underwent routine ROP screening examinations. The need for examinations in these centers was generally based on the recommended guidelines (BW < 1501 g or GA ≤ 30 weeks and/or at the neonatologist’s request). Specific BW and GA cutoff levels were not used for the G-ROP Study to enroll a sample that was representative of infants who are undergoing examinations in these countries. To be enrolled in the study, an infant had to have a known ROP outcome, defined as meeting 1 of 2 conditions: (1) either eye met criteria for ETROP type 1 or type 2 ROP or underwent treatment for ROP or (2) both eyes had mature retinal vasculature, immature vasculature in zone III with no prior ROP, or a regression of ROP of less than type 1 or type 2 ROP.
The primary outcomes of the current analysis were the risks of developing ROP and severe ROP (type 1 or 2 ROP), calculated as the number of infants who developed the outcome of interest among the infants who were examined. The overall risks and risks of infants who were stratified into subgroups by BW and GA were calculated. An additional outcome was the postmenstrual age (PMA) at onset of ROP, which was reported using descriptive statistics and analyzed by ROP stages 1, 2, and 3 and by type 1 ROP and type 2 ROP. Finally, we examined the proportion of eyes that had not developed ROP and had retinal vasculature that had grown anteriorly into zone III at each PMA week to assess the developmental ages at which retinal vascularization extended into zone III. Institutional review board approval of the study was obtained and a waiver of informed consent was obtained at all study centers for the G-ROP Study.
Results
The G-ROP Study included 7483 infants who underwent retinal examinations and had a known ROP outcome (Table 1). The mean (SD) BW was 1100 (363) g (minimum, 310; maximum, 3000) and mean (SD) GA was 28 (3) weeks (minimum, 22; maximum, 35). Of note, 947 infants (12.7%) had a BW of 1500 g or more and 1440 (19.2%) had a GA of older than 30 weeks. In the G-ROP population, almost half of the infants were white and more than 30% were African American. More than 5512 of 7438 infants (70%) had been born in the study hospital and just over a quarter were members of a multiple-birth gestation.
Table 1. Characteristics of 7483 Infants.
| Characteristic | No. (%) |
|---|---|
| Birth weight, ga | |
| ≤500 | 112 (1.5) |
| 500-750 | 1341 (17.9) |
| 751-900 | 1098 (14.7) |
| 901-1000 | 707 (9.4) |
| 1001-1100 | 725 (9.7) |
| 1101-1250 | 1011 (13.5) |
| 1251-1500 | 1542 (20.6) |
| ≥1501 | 947 (12.7) |
| Gestational age, wkb | |
| ≤23 | 234 (3.1) |
| 24 | 563 (7.5) |
| 25 | 691 (9.2) |
| 26 | 801 (10.7) |
| 27 | 884 (11.8) |
| 28 | 962 (12.9) |
| 29 | 879 (11.7) |
| 30 | 1029 (13.8) |
| 31 | 798 (10.7) |
| 32-33 | 533 (7.1) |
| ≥34 | 109 (1.5) |
| Sex | |
| Male | 3908 (52.2) |
| Female | 3575 (47.8) |
| Maternal ethnicity | |
| Hispanic or Latino | 564 (7.5) |
| Not Hispanic or Latino | 5251 (70.2) |
| Unknown | 1668 (22.3) |
| Maternal race | |
| White | 3615 (48.4) |
| Black/African American | 2310 (30.9) |
| Asian/Asian American | 233 (3.1) |
| Native Hawaiian/Other Pacific Islander | 93 (1.2) |
| American Indian/Alaskan Native | 40 (0.5) |
| Otherc | 1192 (15.9) |
| Birth location | |
| Inborn | 5512 (73.7) |
| Outborn | 1971 (26.3) |
| Multiple gestation | |
| Yes | 2077 (27.8) |
| No | 5406 (72.2) |
| ROP statusd | |
| Type 1 ROP | 459 (6.1) |
| Type 2 ROP | 472 (6.3) |
| Not type 1 or 2 ROP | 2293 (30.6) |
| No ROP | 4259 (56.9) |
| AP-ROPd | |
| Yes | 17 (0.2) |
| No | 7466 (99.8) |
| ROP treatedd | |
| Yes | 514 (6.9) |
| No | 6969 (93.1) |
Abbreviations: AP, aggressive posterior; ROP, retinopathy of prematurity.
Mean (SD; range), 1100 (363; 310-3000).
Mean (SD; range), 28 (3; 22-35).
Includes mixed race, other race not listed previously, or unknown race.
ROP by worse outcome for an infant.
In the G-ROP cohort, most (4259 of 7483 [56.9%]) of the infants had no ROP noted in either eye. Type 1 ROP was documented in 1 or both eyes of 459 infants (6.1%), type 2 ROP in 472 (6.3%), and less severe ROP in 2293 (30.6%). Aggressive posterior ROP was documented in only 17 infants (0.2%). The clinicians caring for the children performed treatment for ROP in 514 infants (6.9%), indicating that treatment was given for less than type 1 ROP in 55 infants (10.7%).
The rates for the presence of ROP stratified by BW and GA are presented in Table 2. The incidence of ROP correlated inversely with BW, with the lowest percentage of infants with ROP in the highest BW strata and the highest rates of ROP among the lowest BW strata. For example, of 2551 infants with a BW of 900 g or less, ROP was noted in 2006 (78.6%), while ROP was noted in 301 (12.1%) of the 2489 infants with a BW of more than 1250 g. A similar pattern was seen across the various GA strata, with the lowest percentage of ROP in the highest GA strata and the highest among the lowest GA strata. More than 95% of those infants with a BW of 900 g or less who had GAs of 24 weeks or younger (747 of 781 [95.6%]) had ROP in 1 or both eyes, while ROP was noted in 10 of 15 infants (66.7%) with a BW of 900 g or greater in these low-GA categories. For infants with a BW of less than 1251 g, ROP was noted in 2923 infants (56.8%). In contrast, among the 529 infants who had both a BW and GA greater than the current ROP screening criteria (ie, BW ≥ 1500 g and GA > 30 weeks), only 33 infants (6.3%) developed ROP.
Table 2. Rates of Any ROP and Types 1 or 2 ROP by Birth Weight and Gestational Age.
| Gestational Age, wk | Birth Weight, g, No. (%) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤500 | 501-750 | 751-900 | 901-1000 | 1001-1100 | 1101-1250 | 1251-1500 | ≥1501 | ||
| Rate of Any ROP | |||||||||
| ≤23 | 19/20 (95) | 203/208 (98) | 6/6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 228/234 (97) |
| 24 | 35/36 (97) | 380/400 (95) | 104/112 (93) | 9/11 (81) | 0/2 (0) | 0/1 (0) | 0 (0) | 1/1 (100) | 529/563 (94) |
| 25 | 24/27 (89) | 279/322 (87) | 206/254 (81) | 55/72 (76) | 8/13 (62) | 1/3 (33) | 0 (0) | 0 (0) | 573/691 (83) |
| 26 | 12/18 (67) | 160/188 (85) | 209/280 (75) | 134/190 (71) | 51/94 (54) | 14/27 (52) | 0/3 (0) | 0/1 (0) | 580/801 (72) |
| 27 | 6/6 (100) | 84/119 (71) | 113/186 (61) | 84/181 (46) | 95/196 (48) | 63/149 (42) | 12/42 (29) | 2/5 (40) | 459/884 (52) |
| 28 | 1/3 (33) | 35/52 (67) | 63/132 (48) | 45/115 (39) | 61/171 (36) | 92/278 (33) | 59/195 (30) | 2/16 (13) | 358/962 (37) |
| 29 | 1/1 (100) | 15/29 (52) | 27/59 (46) | 20/64 (31) | 37/100 (37) | 47/206 (23) | 74/326 (23) | 15/94 (16) | 236/879 (27) |
| 30 | 0 (0) | 4/11 (36) | 14/40 (35) | 14/45 (31) | 16/74 (22) | 29/151 (19) | 49/407 (12) | 26/301 (9) | 152/1029 (15) |
| 31 | 0/1 (0) | 2/7 (29) | 2/18 (11) | 0/15 (0) | 9/41 (22) | 17/105 (16) | 15/272 (6) | 23/339 (7) | 68/798 (9) |
| 32-33 | 0 (0) | 0/5 (0) | 2/8 (25) | 4/10 (40) | 6/30 (20) | 5/79 (6) | 11/235 (5) | 10/166 (6) | 38/533 (7) |
| ≥34 | 0 (0) | 0 (0) | 0/3 (0) | 0/4 (0) | 1/4 (25) | 0/12 (0) | 2/62 (3) | 0/24 (0) | 3/109 (3) |
| Total | 98/112 (88) | 1162/1341 (87) | 746/1098 (68) | 365/707 (52) | 284/725 (39) | 268/1011 (27) | 222/1542 (14) | 79/947 (8) | 3224/7483 (43) |
| Rate of Type 1 or Type 2 ROP | |||||||||
| ≤23 | 12/20 (60) | 115/208 (55) | 3/6 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 130/234 (56) |
| 24 | 27/36 (75) | 194/400 (49) | 41/112 (37) | 1/11 (9) | 0/2 (0) | 0/1 (0) | 0 (0) | 0/1 (0) | 263/563 (47) |
| 25 | 17/27 (63) | 124/322 (39) | 73/254 (29) | 17/72 (24) | 4/13 (31) | 1/3 (33) | 0 (0) | 0 (0) | 236/691 (34) |
| 26 | 10/18 (56) | 47/188 (25) | 46/280 (16) | 33/190 (17) | 9/94 (10) | 5/27 (19) | 0/3 (0) | 0/1 (0) | 150/801 (19) |
| 27 | 3/6 (50) | 22/119 (18) | 24/186 (13) | 15/181 (8) | 10/196 (5) | 7/149 (5) | 1/42 (2) | 0/5 (0) | 82/884 (9) |
| 28 | 0/3 (0) | 6/52 (12) | 9/132 (7) | 3/115 (3) | 6/171 (4) | 12/278 (4) | 2/195 (1) | 1/16 (6) | 39/962 (4) |
| 29 | 0/1 (0) | 4/29 (14) | 1/59 (2) | 3/64 (5) | 0/100 (0) | 3/206 (1) | 7/326 (2) | 0/94 (0) | 18/879 (2) |
| 30 | 0 (0) | 0/11 (0) | 0/40 (0) | 1/45 (2) | 2/74 (3) | 1/151 (1) | 2/407 (0) | 1/301 (0) | 7/1029 (1) |
| 31 | 0/1 (0) | 0/7 (0) | 0/18 (0) | 0/15 (0) | 1/41 (2) | 0/105 (0) | 0/272 (1) | 4/339 (1) | 5/798 (1) |
| 32-33 | 0 (0) | 0/5 (0) | 0/8 (0) | 0/10 (0) | 1/30 (3) | 0/79 (0) | 0/235 (0) | 0/166 (0) | 1/533 (0) |
| ≥34 | 0 (0) | 0 (0) | 0/3 (0) | 0/4 (0) | 0/4 (0) | 0/12 (0) | 0/62 (0) | 0/24 (0) | 0/109 (0) |
| Total | 69/112 (62) | 512/1341 (38) | 197/1098 (18) | 73/707 (10) | 33/725 (5) | 29/1012 (3) | 12/1542 (1) | 6/947 (1) | 931/7483 (12) |
Abbreviation: ROP, retinopathy of prematurity.
The rates of severe ROP stratified by BW and GA are presented in Table 2. The overall rate of type 1 or 2 ROP was 12% (931 of 7483) in the G-ROP cohort. As with the rate of any ROP, the incidence of severe ROP varied considerably; the highest percentages of infants with severe ROP were in the lowest BW and GA strata. Among 6043 infants with a GA of 30 weeks or younger, 925 (15.3%) developed severe ROP, while severe ROP was noted in only 6 of 1440 infants (0.4%) with a GA older than 30 weeks. Among those infants born at 24 weeks or younger, almost half (393 of 797 [49.5%]) developed severe ROP. Among those infants with a BW of more than 1501 g and a GA of older than 30 weeks, severe ROP developed in 4 (0.75%).
The ROP diagnoses and PMA at diagnosis of the 459 infants who developed type 1 ROP are presented in Table 3. Most (314 of 459 [68.4%]) of these infants developed stage 3 ROP in zone II with plus disease at a mean (SD) PMA of 37 (2.6) weeks, while those with type 1 ROP in zone I underwent ROP treatment in 1 or both eyes on average a week or so earlier. Forty-one infants with stage 2 ROP in zone II with plus were also treated at a mean (SD) PMA of 37 (2.4) weeks. Only 19 infants (4.1%) with type 1 ROP were not designated as having plus disease.
Table 3. Characteristics of 459 Infants With Type 1 ROP.
| Type 1 ROP | No. (%) | Mean (SD) | ||
|---|---|---|---|---|
| BW, g | GA, wk | PMA at Diagnosis, wk | ||
| Stage 3 zone 2 with plus | 314 (68.4) | 741 (217) | 25 (1.6) | 37 (2.6) |
| Stage 3 zone 1 with plus | 70 (15.3) | 644 (156) | 25 (1.4) | 35 (1.8) |
| Stage 3 zone 1 without plus | 19 (4.1) | 613 (142) | 24 (1.3) | 35 (1.7) |
| Stage 2 zone 2 with plus | 41 (8.9) | 704 (178) | 25 (1.4) | 37 (2.4) |
| Stage 2 zone 1 with plus | 10 (2.2) | 651 (167) | 25 (0.8) | 35 (2.3) |
| Stage 1 zone 1 with plus | 5 (1.1) | 604 (110) | 24 (0.8) | 36 (2.6) |
Abbreviations: BW, birth weight; GA, gestational age; PMA, postmenstrual age; ROP, retinopathy of prematurity.
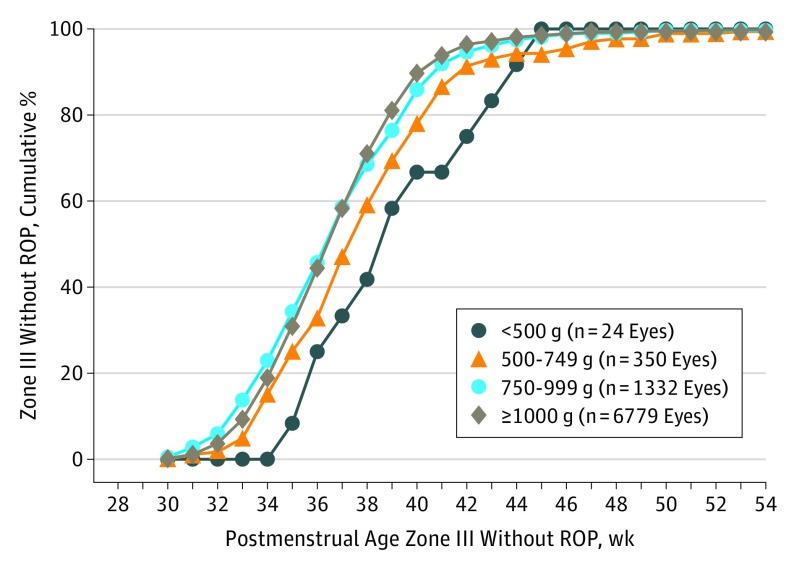
Figure 1 displays the cumulative proportion in 4 BW groups of eyes without ROP and with retinal vasculature that extended anteriorly into zone III across each PMA week. Based on the clinical examinations of the ophthalmologists at the 29 G-ROP hospitals, fewer than 10% of eyes were vascularized into zone III before 34 weeks PMA and half (49.4%) were vascularized into zone III by 37 weeks PMA.
Figure 1. Postmenstrual Age at the Time of First Diagnosis of Zone III Without Retinopathy of Prematurity (ROP) in 4 Birth Weight Categories.
The mean PMA at first observation of stage 1 ROP to stage 2 ROP and subsequently to stage 3 ROP was sequential (eFigure in the Supplement). Stage 1 ROP was first diagnosed at a mean (SD) PMA of 34.8 (2.9) weeks, stage 2 ROP at a mean (SD) PMA of 35.5 (2.7) weeks, and stage 3 ROP at a mean (SD) PMA of 36.4 (2.6) weeks.
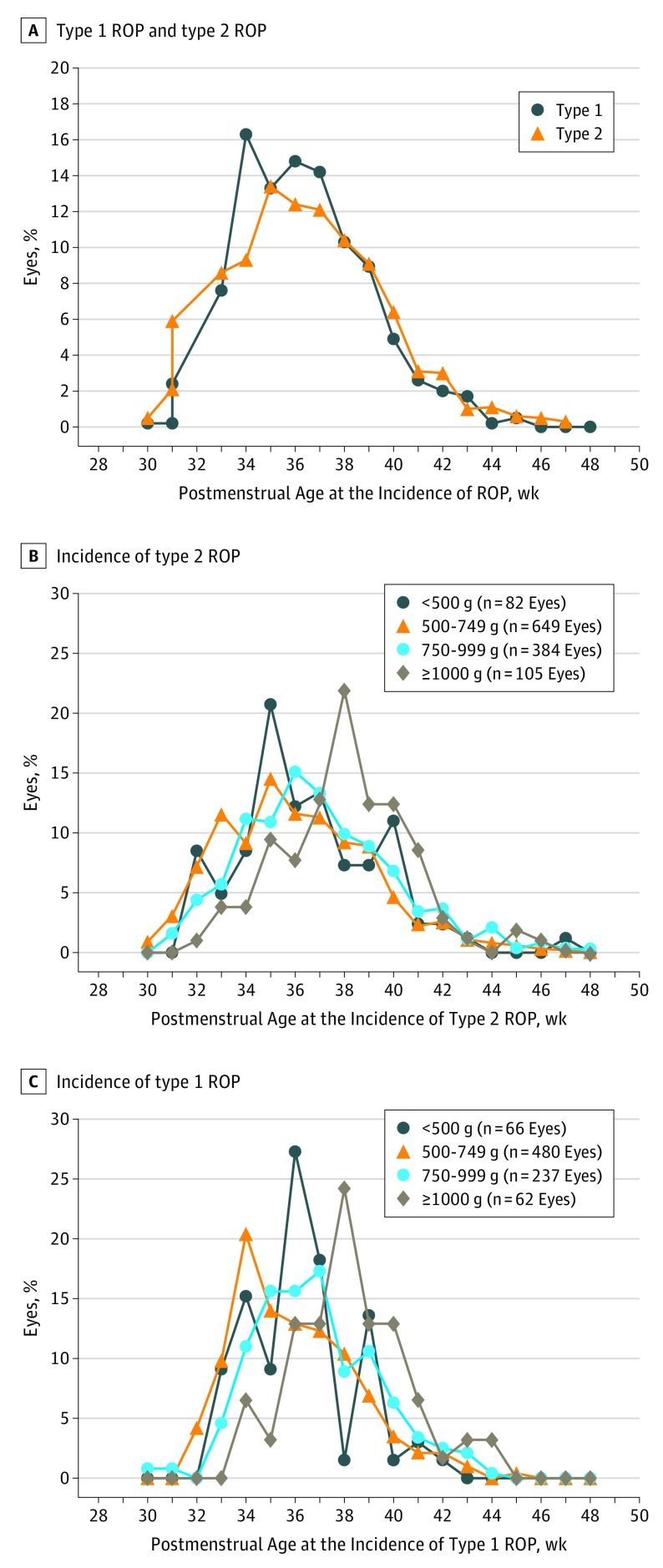
The PMAs at diagnosis of type 2 ROP and type 1 ROP are presented for the cohort overall (Figure 2A) and by 4 BW groups for type 2 ROP (Figure 2B) and type 1 ROP (Figure 2C). Overall, the mean (SD) PMA at first diagnosis of type 2 ROP was 36.6 (3.2) weeks, and the mean (SD) PMA of diagnosis of type 1 ROP was 36.4 (SD) weeks, with similar ranges noted in type 2 and type 1 ROP despite BW group.
Figure 2. Postmenstrual Age at Onset of Retinopathy of Prematurity (ROP).
Postmenstrual age at onset of (A) type 2 ROP and type 1 ROP, (B) type 2 ROP in 4 birth weight categories, and (C) type 1 ROP in 4 birth weight categories.
Discussion
This retrospective cohort analysis provides an informative overview of the incidence of ROP in at-risk premature infants in North America by including infants across the range of BW and GA that fall under the currently recommended screening levels, as well as larger or older infants who were examined because of concern on the part of neonatologists. We found that most ROP and almost all severe ROP, as currently defined, occurred in the less mature, lower-BW infants. Retinopathy of prematurity was noted in only 79 of 947 infants (8%) with a BW of 1501 g or more and in only 109 of 1440 infants (20%) with a GA of older than 30 weeks. Only 6 of 947 infants (1%) with a BW of 1501 g or more and 6 of 1440 (0.4%) with a GA of older than 30 weeks developed severe ROP in 1 or both eyes. In addition, 4 of 529 infants (0.75%) with both a BW of 1501 g or more and a GA of older than 30 weeks developed severe ROP.
This article provides detailed and broadly representative data presented on infants who underwent ROP examinations in the United States and Canada during January 2006 to December 2011. With the wide BW and GA range included for infants in this study, the population at risk for severe ROP can be more clearly targeted among infants who meet the current criteria for examinations than was previously possible using data from clinical trials and studies that specifically targeted higher-risk infants. Based on these findings, the current ROP screening guidelines, which rely primarily on BW and GA, could be reexamined with an aim to more accurately identify those few infants among the higher BW or more mature GA categories who develop severe ROP by using other demographic or postnatal indicators of greater risk. Examining such factors as the rate of weight gain in early infancy, sepsis, and respiratory status could decrease the number of examinations conducted or even eliminate examinations altogether for infants who are at very low risk for developing ROP and virtually none for severe ROP. Moreover, this analysis presents an accurate picture of the overall demand for ROP detection programs and prompts further examination of resource expenditures that are associated with ROP screening, including personnel, equipment, and other costs.
Our observations in this study provide a contrast to previous large studies in the US. These studies, including Cryotherapy for ROP (CRYO-ROP; 1986-1987),9 ETROP (2000-2002),10 and Telemedicine System for the Evaluation of Acute-Phase ROP (e-ROP; 2011-2013),11 aimed to detect severe disease in a select group of infants who were at high risk for severe ROP (ie, infants with BW<1251 g). When comparing the subgroup of infants less than 1251 g in the G-ROP Study with the cohorts of these previous studies, there does not appear to be a higher rate of ROP, although the mean (SD) BW of infants across these studies has gradually decreased from 954 (185) g in CRYO-ROP to 907 (205) g in ETROP, and then to 893 (210) g in G-ROP and 864 (212) g in e-ROP, in which the study periods partially overlapped. Similarly, the proportion of infants in these studies with a BW of less than 750 g has increased from 15.8% in CRYO-ROP to 24.9% in ETROP, and to 28.0% in G-ROP and 33.4% in e-ROP. In each of these studies, the overall incidence of ROP was approximately 65%, although we included several infants in the smallest BW categories of less than 500 g and less than 750 g, likely because of the increased survival of such infants since the time of the CRYO-ROP study. The relatively stable incidence of ROP in these studies in the presence of decreasing birth weights over the decades spanning the study periods likely indicates improved obstetrical and neonatal care, particularly in the neonatal intensive care unit, with better management and monitoring of supplemental oxygen use, as well as improved nutritional and supportive care.
Strengths and Limitations
Among the strengths of this study are the large sample of infants from a geographically and demographically diverse range of institutions in North America and the rigorous data abstraction, reporting, and quality-check procedures used in this multicenter study.6 However, there are also limitations to consider. The medical and ophthalmological data were collected retrospectively, and the procedures for determining important measures, such as BW, GA, and ROP staging, were not standardized across the centers. Retinal photography was not used to confirm ROP zone or the presence of plus disease, the judgment of which can vary among examiners.12,13 However, the data do represent the standard practices for such clinical measures during the study period, and the ophthalmologists who performed the retinal examinations were all fellowship-trained pediatric or retinal ophthalmologists using standardized International Classification of ROP terminology for the staging of ROP. Therefore, we do not believe using retrospective data introduced substantive bias into the analysis, the purpose of which was to gain insight into the incidence and time course of ROP as reflected in current clinical practice. The scheduling of follow-up retinal examinations was based on the clinical decisions of the attending ophthalmologists at the various participating centers and could influence the timing of diagnosis of the stages and types of ROP. Again, these decisions reflect current practices, and the large number of hospitals in the study helps to overcome any effects of variability in scheduling practices; therefore, we do not think the onset data are significantly biased. In fact, these data may actually increase the generalizability of the findings. The study period for the G-ROP Study was from January 2006 to December 2011. More recent changes in neonatal care practices, such as those that may have arisen following the publication of results of the large oxygen saturation target range studies14,15,16,17,18,19 and that may result in changes in the profiles of infants who develop ROP, would not be reflected in the current study. Finally, for similar reasons, the study results are not necessarily generalizable to other regions of the world, where differences in neonatal care influence the risk of ROP.20,21,22,23,24
Conclusions
This analysis from the G-ROP Study provides data on the overall ROP status of infants who undergo ROP examinations in neonatal intensive care units in the United States and Canada, providing further information for ophthalmologists who perform examinations with regard to the timing of ROP, including the typical developmental ages at which each ROP stage and severe ROP develop and the time course of normal retinal vascularization into zone III in premature infants who do not develop ROP. The stratified results offer ROP risk profiles across GA and BW groups, which are informative for neonatologists, ophthalmologists, and care coordinators who are responsible for ensuring timely follow-up of ROP examinations so that a disease that may benefit from prompt treatment is not missed. Moreover, the low-risk profile of larger BW and older GA infants who are undergoing ROP examinations supports further efforts to improve the specificity of risk assessment for these infants and ultimately consider the reevaluation of the current criteria for indicating the need for an ROP examination at centers in North America.
eFigure: Postmenstrual Age at Onset of Stage 1, Stage 2, and Stage 3 ROP
References
- 1.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(suppl 1):35-49. doi: 10.1038/pr.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77-82. doi: 10.1016/j.earlhumdev.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-1694. doi: 10.1001/archopht.121.12.1684 [DOI] [PubMed] [Google Scholar]
- 4.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity. preliminary results. Arch Ophthalmol. 1988;106(4):471-479. doi: 10.1001/archopht.1988.01060130517027 [DOI] [PubMed] [Google Scholar]
- 5.Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189-195. doi: 10.1542/peds.2012-2996 [DOI] [PubMed] [Google Scholar]
- 6.Binenbaum G, Tomlinson LA. Postnatal growth and retinopathy of prematurity study: rationale, design, and subject characteristics. Ophthalmic Epidemiol. 2017;24(1):36-47. doi: 10.1080/09286586.2016.1255765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binenbaum G, Ying GS, Tomlinson LA, Postnatal G. Validation of the Children’s Hospital of Philadelphia Retinopathy of Prematurity (CHOP ROP) model. JAMA Ophthalmol. 2017;135(8):871-877. doi: 10.1001/jamaophthalmol.2017.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn GE, Ying GS, Daniel E, et al. ; e-ROP Cooperative Group . Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014;132(10):1178-1184. doi: 10.1001/jamaophthalmol.2014.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer EA, Flynn JT, Hardy RJ, et al. ; The Cryotherapy for Retinopathy of Prematurity Cooperative Group . Incidence and early course of retinopathy of prematurity. Ophthalmology. 1991;98(11):1628-1640. doi: 10.1016/S0161-6420(91)32074-8 [DOI] [PubMed] [Google Scholar]
- 10.Good WV, Hardy RJ, Dobson V, et al. ; Early Treatment for Retinopathy of Prematurity Cooperative Group . The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics. 2005;116(1):15-23. doi: 10.1542/peds.2004-1413 [DOI] [PubMed] [Google Scholar]
- 11.Quinn GE, Barr C, Bremer D, et al. . Changes in course of retinopathy of prematurity from 1986 to 2013: comparison of three studies in the United States. Ophthalmology. 2016;123(7):1595-1600. doi: 10.1016/j.ophtha.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace DK, Quinn GE, Freedman SF, Chiang MF. Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J AAPOS. 2008;12(4):352-356. doi: 10.1016/j.jaapos.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanovitch TL, Freedman SF, Wallace DK. Vascular dilation and tortuosity in plus disease. Arch Ophthalmol. 2009;127(1):112-113. doi: 10.1001/archophthalmol.2008.527 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt B, Whyte RK, Roberts RS. Trade-off between lower or higher oxygen saturations for extremely preterm infants: the first benefits of oxygen saturation targeting (BOOST) II trial reports its primary outcome. J Pediatr. 2014;165(1):6-8. doi: 10.1016/j.jpeds.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 15.Khadawardi E, Al Hazzani F. Oxygen saturation and outcomes in preterm infants the BOOST II United Kingdom, Australia, and New Zealand collaborative groups. J Clin Neonatol. 2013;2(2):73-75. doi: 10.4103/2249-4847.116404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd L. SUPPORT and comparative effectiveness trials: what’s at stake? Hastings Cent Rep. 2015;45(1):44-45. doi: 10.1002/hast.417 [DOI] [PubMed] [Google Scholar]
- 17.Magnus D, Caplan AL. Risk, consent, and SUPPORT. N Engl J Med. 2013;368(20):1864-1865. doi: 10.1056/NEJMp1305086 [DOI] [PubMed] [Google Scholar]
- 18.Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105(1):55-63. doi: 10.1159/000356561 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt B, Whyte RK, Asztalos EV, et al. ; Canadian Oxygen Trial (COT) Group . Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309(20):2111-2120. doi: 10.1001/jama.2013.5555 [DOI] [PubMed] [Google Scholar]
- 20.Gilbert C, Wormald R, Fielder A, et al. . Potential for a paradigm change in the detection of retinopathy of prematurity requiring treatment. Arch Dis Child Fetal Neonatal Ed. 2016;101(1):F6-F9. doi: 10.1136/archdischild-2015-308704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zin AA, Magluta C, Pinto MF, et al. . Retinopathy of prematurity screening and treatment cost in Brazil. Rev Panam Salud Publica. 2014;36(1):37-43. [PubMed] [Google Scholar]
- 22.Zin A, Gole GA. Retinopathy of prematurity-incidence today. Clin Perinatol. 2013;40(2):185-200. doi: 10.1016/j.clp.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 23.Zin AA, Moreira ME, Bunce C, Darlow BA, Gilbert CE. Retinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implications. Pediatrics. 2010;126(2):e410-e417. doi: 10.1542/peds.2010-0090 [DOI] [PubMed] [Google Scholar]
- 24.Gilbert C, Fielder A, Gordillo L, et al. ; International NO-ROP Group . Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518-e525. doi: 10.1542/peds.2004-1180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure: Postmenstrual Age at Onset of Stage 1, Stage 2, and Stage 3 ROP