Key Points
Question
Does using a long-term benefit approach improve the selection of individuals for statin therapy in primary prevention compared with standard risk-based approaches?
Findings
In this cross-sectional study, a long-term benefit approach to statin eligibility identified nearly 1 in 6 individuals as having a high degree of expected long-term benefit of statins. This approach identified younger individuals with higher low-density lipoprotein cholesterol who would not be currently recommended for treatment.
Meaning
A long-term benefit approach may provide a more optimal approach for determining statin eligibility in primary prevention.
This cross-sectional study uses National Health and Nutrition Survey data to model a 10-year risk, 10-year benefit, and 30-year benefit approach to selecting individuals for statin therapy.
Abstract
Importance
A 10-year benefit-based approach to statin therapy in primary prevention includes younger individuals with higher low-density lipoprotein cholesterol (LDL-C) and prevents more cardiovascular events than a risk-based approach. However, a 10-year treatment duration likely underestimates the expected benefits of statins.
Objective
To model the impact of a 30-year benefit approach to select individuals for statin therapy.
Design, Setting, and Participants
This cross-sectional analysis of the National Health and Nutrition Survey (NHANES) data set included samples of the US population from the 2009-2010, 2011-2012, and 2013-2014 data collection cycles. Individuals between 40 to 60 years old who did not have atherosclerotic cardiovascular disease, diabetes, or LDL-C levels greater than 190 mg/dL and who were not taking statins were included. Data analysis took place from November 2017 to August 2018.
Exposures
We calculated 10-year risk of atherosclerotic cardiovascular disease and 10-year and 30-year absolute risk reduction (10-year ARR and 30-year ARR) of atherosclerotic cardiovascular disease for each individual.
Main Outcomes and Measures
Number of individuals meeting eligibility for statins based on 10-year (atherosclerotic) cardiovascular disease risk, 10-year ARR, or 30-year ARR.
Results
A total of 1688 individuals were included, representing 56.6 million US individuals. Statin eligibility based on 7.5% CVR10 was 9.5%; based on 2.3% 10-year ARR, 13.0%, and based on 15% 30-year ARR, 17.5%. The 10-year risk, 10-year benefit, and 30-year benefit approaches all led to similar acceptable mean absolute risk reductions at 30 years, with the benefit-based approaches better able to avoid treatment of individuals with low expected benefit. Individuals who met statin eligibility based solely on the 30-year ARR threshold of 15% or greater were younger (mean age, 50 [95% CI, 48-52] years) and more likely to be women (43% [95% CI, 26%-59%]) than those recommended with a 10-year ARR threshold of 2.3% or greater (mean age, 56 [95% CI, 54-57] years; 22% [95% CI, 10%-34%] women). This group also had lower 10-year risk (mean risk, 4.7% [95% CI, 4.4%-5.1%]) and higher LDL-C levels (mean level, 149 mg/dL [95% CI, 142-155 mg/dL]) than those recommended with a 10-year ARR threshold of 2.3% or greater (mean risk, 9.3% [95% CI, 8.3%-10.2%]; mean LDL-C levels, 110 [103-118] mg/dL). Preventable atherosclerotic cardiovascular disease events in 10 and 30 years were highest using the 30-year benefit approach (296 000 at 10 years and 2.03 million at 30 years) and lowest based on 10-year risk (204 000 at 10 years and 1.18 million at 30 years).
Conclusions and Relevance
A long-term benefit approach to statin eligibility identifies nearly 1 in 6 individuals as having a high degree of expected long-term benefit of statins, with a number needed to treat of less than 7. This approach identifies younger individuals with higher LDL-C levels who would not be currently recommended for treatment and may provide a more optimal approach for determining statin eligibility in primary prevention.
Introduction
Identifying which individuals should receive statins in primary prevention continues to be controversial. The current approach, based on 10-year estimates of cardiovascular risk,1 leads to overly broad recommendations for statins in older individuals,2 while missing many younger individuals who develop premature atherosclerotic cardiovascular disease (ASCVD).3,4 We have demonstrated that a 10-year benefit-based approach, based on the level of low-density lipoprotein cholesterol (LDL-C) as well as calculated risk, would include younger individuals with higher LDL-C levels and would prevent more cardiovascular events.5,6 However, a 10-year treatment duration likely underestimates the expected benefits of statins, especially among younger individuals at lower short-term risk. Once initiated, statins are frequently continued for considerably longer than 10 years, and the expectation, by both physicians and their patients, is that they will be administered throughout the life span or at least for several decades. Therefore, the absolute benefit of statins should consider the expected long-term duration of treatment. Accordingly, in this report, we estimate the absolute benefit of long-term statin preventive treatment and model the outcomes of using a 30-year benefit approach to select individuals for statin therapy.
Methods
We examined the long-term benefit of statins in primary prevention for 30 years. Data from the National Health and Nutrition Survey (NHANES) collected during the 2009-2010, 2011-2012, and 2013-2014 cycles were used to create a nationally representative sample of adults age 40 to 60 years without atherosclerotic cardiovascular disease who were not taking statins. We excluded participants with diabetes and LDL-C greater than 190 mg/dL (to convert to mmol/dL, multiply by 0.0259), because statins are currently recommended by guidelines for these individuals.
All individuals provided informed consent as part of the NHANES study. Local institutional review board approval was exempt for this analysis.
We calculated 10-year risk using the pooled-cohorts equation1 and 30-year risk using the Framingham 30-year risk algorithm,7 as previously described. To estimate the 10-year and 30-year absolute risk reduction (ARR), we multiplied 10-year and 30-year risk by a relative risk reduction (RRR) calculated with a formula provided in Ference et al8: RRR = 1 − exp(−0.249 − 0.0152 × [Duration of Treatment - 5]) × (Decrease in LDL-C in mmol/dL). We assumed that all individuals who met eligibility by any threshold would receive a moderate-intensity statin (which according to the 2013 American College of Cardiology/American Heart Association guidelines is expected to lead to a 40% mean decrease in LDL-C levels). In keeping with prior work, we used a 2.3% 10-year ARR as the threshold for 10-year benefit. This threshold was determined as the minimum acceptable benefit in individuals eligible for treatment based on the 2013 American College of Cardiology/American Heart Association guidelines (eg, a 7.5% 10-year risk). To determine a meaningful threshold for 30-year ARR, we calculated the 30-year ARR in all NHANES participants with LDL-C levels greater than 190 mg/dL, who are currently recommended for statin therapy because of expected high long-term benefit.1 To be conservative, we used the 25th-percentile 30-year ARR from this group of individuals as the threshold for initiating statins (eg, a 30-year ARR≥15% or a number needed to treat [NNT] in 30 years <6.7). We compared the numbers of individuals recommended for therapy, their characteristics, and the expected number of events prevented using a 7.5% 10-year risk, 10-year ARR, or 30-year ARR. Analyses using the risk-based approach use calculated absolute risks, whereas the benefit-based approaches use calculated absolute risk reductions. In sensitivity analyses, we also compared the 30-year benefit approach to lower risk thresholds of 5% and 2.5%. We also performed an additional analysis (eTable in the Supplement), where the risk-based number of eligible individuals included for the benefit approach was fixed at the number included by a given risk threshold (achieved by varying the 30-year ARR threshold as required).
All analyses incorporated the NHANES sampling weights to provide representative data for the US population. All analyses were performed in SAS version 9.4 (SAS Inc). Two-sided P values less than .05 were considered significant. Analyses were performed from November 2017 to August 2018.
Results
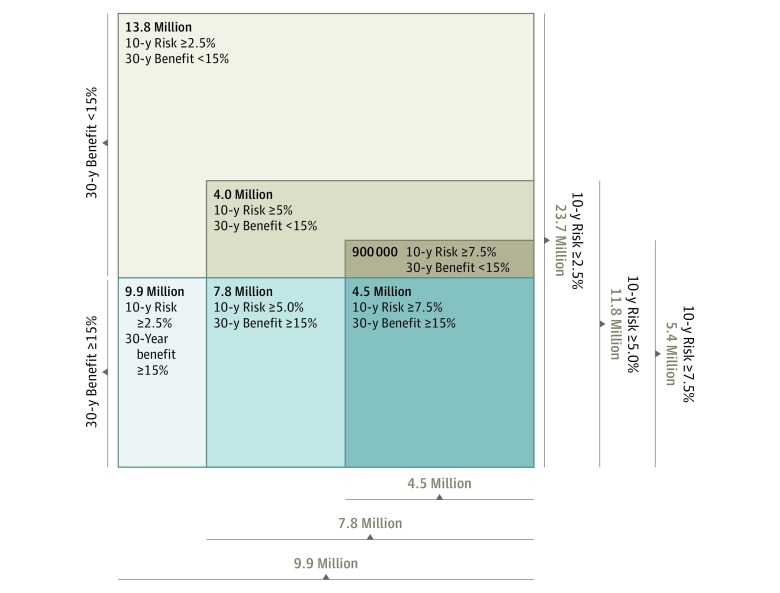
We included 1688 individuals (of whom 901 [53.3%] were women; overall mean [SD] age, 48.8 [6.1] years) who represent 56.6 million US individuals. Statin eligibility, as a proportion of the US population aged 40 to 60 years who are eligible for primary prevention and not currently taking statins, based on a 7.5% 10-year risk, was 5.4 million individuals (9.5%); based on a 2.3% 10-year ARR, it was 7.3 million individuals (13.0%), and based on a 15% 30-year ARR, it was 9.9. million individuals (17.5%). All 3 approaches led to similar acceptable mean 30-year ARR (mean [95% CI] ARR30: 10-year risk ≥7.5%, 22.1% [20.5%-23.7%]; 10-year benefit ≥2.3%, 21.5% [20.1%-22.8%]; 30-year benefit ≥15%, 20.6% [19.7%-21.6%]), with the benefit-based approaches better able to avoid treatment of individuals with low expected benefit (maximum NNT: 10-year risk ≥7.5%, 17.5 patients; 10-year benefit ≥2.3%, 14.5 patients; 30-year benefit ≥15%, 6.7 patients; Table 1). A 7.5% 10-year risk approach identifies 4.5 million individuals (45%) at high long-term benefit (30-year ARR≥15%) but also includes 900 000 at lower benefit (maximum 30-year NNT, 17; Figure). Numbers of preventable events at 10 and 30 years were highest using 30-year benefit (mean, 2 033 000 events [95% CI, 1 945 000-2 132 000]) and lowest when determining eligibility based on 10-year risk (204 000 events [95% CI, 193 000-220 000]; Table 1).
Table 1. Characteristics of Individuals 40 to 60 Years Old Recommended for Lipid-Lowering Treatment as Primary Prevention, Stratified by Benefit Level.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| 10-y Risk ≥7.5% | 10-y Benefit ≥2.3% | 30-y Benefit ≥15% | |
| Total study population | 212 (12.6) | 265 (15.7) | 307 (18.2) |
| Representation of US population | 5.4 million (9.5) | 7.3 million (13.0) | 9.9 million (17.5) |
| Age, median (interquartile range), y | 54 (53-55) | 54 (53-55) | 52 (51-53) |
| Women, No. (%) [95% CI] | 28 (13) [8-17] | 42 (16) [11-20] | 74 (24) [18-30] |
| Clinical findings, mean (95% CI) | |||
| Low-density lipoprotein cholesterol, mg/dL | 136 (130-142) | 143 (139-147) | 147 (144-150) |
| High-density lipoprotein cholesterol, mg/dL | 46 (44-49) | 47 (45-49) | 46 (44-47) |
| Triglycerides, mg/dL | 150 (132-169) | 153 (138-169) | 159 (147-172) |
| Systolic blood pressure, mm Hg | 134 (130-138) | 131 (127-134) | 129 (127-132) |
| Antihypertensive therapy, No. (%) [95% CI] | 68 (32) [21-43] | 74 (28) [19-36] | 95 (31) [22-39] |
| Cigarette smoking, No. (%) [95% CI] | 125 (59) [48-69) | 140 (53) [44-61) | 144 (47) [37-56] |
| Risk calculations, mean % (95% CI) | |||
| 10-y Risk | 10.6 (10.1-11.2) | 9.5 (8.9-10.1) | 7.9 (7.4-8.4) |
| 30-y Risk | 37.5 (35.4-39.6) | 35.6 (33.7-37.5) | 33.7 (32.2-35.1) |
| 30-y Absolute risk reduction | 22.1 (20.5-23.7) | 21.5 (20.1-22.8) | 20.6 (19.7-21.6) |
| 30-y Number needed to treat | (2.2-17.5) | (2.2-14.5) | (2.2-6.7) |
| Preventable cardiovascular events, mean (95% CI) | |||
| In 10 years | 204 000 (193 000-220 000) | 264 000 (242 000-279 000) | 296 000 (276 000-316 000) |
| In 30 years | 1 188 000 (1 102 000-1 275 000) | 1 577 000 (1 475 000-1 673 000) | 2 033 000 (1 945 000-2 132 000) |
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259. To convert triglycerides to millimoles per liter, multiply by 0.0113.
Figure. Outcome of Lowering the 10-Year Atherosclerotic Cardiovascular Disease Risk Threshold on Capturing Individuals With High 30-Year Benefit.
A 10-year risk threshold of 7.5% captures 4.5 million individuals (45%) who are at high long-term benefit, while also including 900 000 at lower benefit (maximum number needed to treat at 30 years, 17). Reducing the 10-year risk threshold to 5% captures 7.8 million individuals (79%) at high benefit, while also including 4.0 million at lower benefit (maximum number needed to treat at 30 years, 27). A 2.5% 10-year risk threshold captures all 9.9 million (100%) at high benefit, but also includes 13.8 million at lower benefit (maximum number needed to treat at 30 years, 34).
Compared with a short-term benefit approach (10-year ARR≥2.3%), there were 82 additional participants (5.9% of the study population of 1688 individuals) who met the 30-year ARR threshold for long-term benefit (Table 2). We also note that 40 individuals (1.4% of the study population) who met the 10-year ARR threshold did not meet the 30-year ARR threshold (ie, they had acceptable 10-year benefit but not high expected long-term, [30-year] benefit). Compared with those recommended by 10-year benefit, individuals who met statin eligibility based solely on the 30-year ARR threshold of greater than or equal to 15% were characterized as being younger (mean age, 50 [95% CI, 48-52] years), more likely to be women (43% [95% CI, 26%-59%]), and at lower 10-year risk (mean risk, 4.7% [95% CI, 4.4%-5.1%]). In those selected based solely on 30-year benefit, LDL-C levels were at a mean of 149 (95% CI, 142-155) mg/dL, significantly higher than among those not recommended by either strategy (mean LDL-C level, 117 [95% CI, 115-118] mg/dL) or those recommended solely based on 10-year benefit (mean LDL-C level, 110 [95% CI, 103-118] mg/dL). Compared with a 10-year benefit approach, treatment of this group would be expected to prevent more than 564 000 additional events over 30 years, potentially eliminating 61% of the 919 000 events expected among these individuals.
Table 2. Discordance in Characteristics of Individuals Recommended for Lipid-Lowering Treatment, Stratified by Benefit Level.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Recommended Based on Neither Benefit | Recommendation With 10-y Benefit ≥2.3% Only | Recommendation With 30-y Benefit ≥15% Only | Recommended Based on Both Benefits | |
| Total | 1341 (79.4) | 40 (2.4) | 82 (4.9) | 225 (13.3) |
| Representation of US population | 45.9 million (81.2) | 800 000 (1.4) | 3.3 million (5.9) | 6.6 million (11.6) |
| Age, median (interquartile range), y | 48 (47-48) | 56 (54-57) | 50 (48-52) | 53 (52-54) |
| Women, No. (%) [95% CI] | 818 (61) [58-63] | 9 (22) [10-34] | 35 (43) [26-59] | 34 (15) [10-20] |
| Clinical findings, mean (95% CI) | ||||
| Low-density lipoprotein cholesterol, mg/dL | 117 (115-118) | 110 (103-118) | 149 (142-155) | 147 (143-151) |
| High-density lipoprotein cholesterol, mg/dL | 58 (57-59) | 60 (53-68) | 46 (43-50) | 45 (44-47) |
| Triglycerides, mg/dL | 107 (102-112) | 96 (75-117) | 158 (138-179) | 160 (143-177) |
| Systolic blood pressure, mm Hg | 116 (115-117) | 134 (127-141) | 128 (124-132) | 130 (127-134) |
| Antihypertensive therapy, No. (%) [95% CI] | 148 (11) [10-13] | 9 (23) [10-36] | 29 (35) [19-51] | 63 (28) [19-37] |
| Cigarette smoking, No. (%) [95% CI] | 188 (14) [11-17] | 20 (49) [27-71] | 28 (34) [18-50] | 119 (53) [43-62] |
| Risk calculations, mean % (95% CI) | ||||
| 10-y Risk | 2.0 (1.9-2.1) | 9.3 (8.3-10.2) | 4.7 (4.4-5.1) | 9.6 (8.9-10.3) |
| 30-y Risk | 12.6 (12.2-13.0) | 26.2 (25.0-27.4) | 27.7 (26.7-28.7) | 36.7 (34.8-38.7) |
| 30-y Absolute risk reduction | 6.8 (6.6-7.0) | 13.2 (12.7-13.7) | 17.0 (16.5-17.5) | 22.5 (21.1-23.8) |
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259. To convert triglycerides to millimoles per liter, multiply by 0.0113.
In sensitivity analyses, we compared the effect of lowering the 10-year risk threshold from 7.5% to 5% and 2.5% from the 15% 30-year ARR threshold (Figure). Lowering the threshold to 5% leads to treatment of 11.8 million of the eligible population (20.8%; median 30-year NNT, 6) and identifies 7.8 million individuals at high long-term benefit (79% of all 9.9 million at high benefit) but also includes 4.0 million at lower benefit (maximum 30-year NNT, 27). Further lowering the risk threshold to 2.5% leads to treatment of 23.7 million individuals (41.9% of the population; median 30-year NNT, 7) and captures all 9.9 million (100%) at high benefit but also includes 13.8 million at lower benefit (maximum 30-year NNT, 34). In both cases, lowering the 10-year risk threshold captures a greater proportion of individuals at high long-term benefit for treatment at the expense of including additional individuals at lower benefit (and therefore increasing the maximum NNT).
Discussion
In this analysis of 1688 NHANES participants representing the 56.6 million Americans with LDL-C levels less than 190 mg/dL who are free of cardiovascular disease and diabetes and not currently taking statins, we demonstrate that using a 30-year benefit-based approach identifies many additional low-risk individuals with a high calculated benefit of long-term statin therapy, without extending treatment to individuals at low benefit (in that the maximum NNT is 6.7). We show that using a 30-year ARR of 15%, which is analogous to treatment of only 6 to 7 individuals, to prevent 1 cardiovascular event over 30 years, 9.9 million individuals (17.5%) are eligible for statin therapy. Most importantly, we show that this group includes many younger individuals who are at low short-term risk and have moderately high LDL-C levels, who would currently not be recommended for statin therapy. Based on our models, treatment of this group would be expected to prevent more than half a million more events over 30 years compared with other approaches. Our results using a 30-year time horizon extend a prior analysis using a 10-year benefit-based approach and demonstrate that nearly 1 in 6 individuals have a high calculated long-term benefit from receiving statin therapy. We also show that there is no 10-year risk threshold that accurately identifies individuals with high long-term benefit without including large segments of the population at lower benefit.
These results have several important implications for the use of statins in primary prevention. First, when the expected long-term duration of therapy is considered, the NNT is quite low for statin therapy in primary prevention in a substantial number of individuals. Indeed, we identified a group of 3.3 million individuals who would only be identified using the 30-year benefit approach, with a mean 30-year NNT less than 6. Although 5-year NNTs are frequently quoted in the literature based on the duration of many randomized clinical trials on statin use, most physicians who initiate statin therapy expect patients to continue statin therapy for several decades and in many cases for life. Therefore, long-term NNT estimates are needed to appropriately determine the value of such therapy and to appropriately counsel patients. Second, as we have demonstrated previously, compared with the conventional risk-based approach,6 the benefit approach selects individuals who are younger, with moderately high LDL-C levels, but few other risk factors. The present results demonstrate that these gains would be substantially greater with an approach that uses longer-term as opposed to short-term anticipated benefits. Based on the current understanding of LDL-C and the causal role of apolipoprotein B in the development of atherosclerotic cardiovascular disease,8 the selection of younger individuals (including more women) with higher LDL-C levels demonstrates that a long-term benefit-based approach provides a more rational and optimal approach to selecting candidates for preventive therapy.
Given the evidence in favor of statins, as well as the highly favorable cost-effectiveness profile demonstrated by others at low risk thresholds (ie, <7.5% 10-year risk), it has been proposed that statins could be recommended in very broad segments of the population (eg, to treat all individuals aged 40-75 years). Indeed, this would address the known prevention paradox, in which the largest number of events occurs in the lowest-risk group of individuals because of their much larger numbers at risk. While such a strategy may be theoretically appealing, such a strategy would unlikely be practical for several reasons. First, there is already considerable concern from primary care physicians, cardiologists, and patients alike regarding the overuse of statins. Therefore, a treat-all strategy would likely be unacceptable to most stakeholders. Second, any treat-all strategy would necessarily have extremely high maximum NNT because of the inclusion of many individuals at low 10-year (or even 30-year risk) and/or low LDL-C levels. These individuals, especially those at low risk and low LDL-C levels are extremely unlikely to benefit from taking statins. Indeed, based on the available randomized clinical trial data and other lines of evidence, the benefit of statins appears to be mediated primarily if not entirely by the lowering of apolipoprotein B particles.9 Therefore, statins cannot be expected to have major benefits in individuals with very low levels of LDL-C levels, especially those in primary prevention. We would urge that other health promotion activities (eg, lifestyle change, smoking cessation, and hypertension treatment) should be emphasized in such individuals. Rather than advocate treatment of all individuals, a targeted approach based on the highest absolute calculated benefit balances the need to broaden the eligibility of statin therapy to those with the most to gain from long-term therapy while avoiding the treatment of large segments of the population with very little to gain. Such an approach, which is in keeping with the concept of precision medicine, may be more acceptable to both physicians and patients.
Limitations
Strengths of our analysis include the application of a well-established 30-year risk prediction model from a US community cohort to NHANES, a large, nationally representative cohort. Several limitations also warrant mention. First, there are no long-term relative reduction estimates of statin therapy from randomized clinical trials; therefore we assumed that the relative risk reductions observed in such trials remained constant over the duration of treatment. Furthermore, there is limited evidence regarding the long-term effect of statins on LDL-C, and there could be some attenuation in LDL-C-lowering at older ages. Nonetheless, given that estimates from mendelian randomization and extended follow-up of several randomized clinical trials suggest that the relative risk reductions of statins appear to increase over time, the expected benefits presented may be underestimated.10 Second, we used several thresholds for 10-year and 30-year risk as well as benefit to determine which patients should be eligible for therapy. Although we provide the rationale for each threshold selected, we also acknowledge that the selection of any specific threshold is relatively arbitrary. Indeed, additional work, including detailed cost-effectiveness modeling, is required to identify the best thresholds for clinical use; however, our approach based on absolute 30-year benefit can be easily adapted to any threshold selected. Third, we did not consider the potential harms of long-term statin therapy, including incident diabetes and serious muscle-associated adverse effects. However, these adverse effects are relatively uncommon and are frequently observed shortly after initiation. Therefore, among patients treated over the long term who initially tolerate treatment, adverse effects of statins would unlikely negate the large potential benefits expected for cardiovascular events. We acknowledge that formal modeling of disutilities from adverse effects are required to better ascertain the net benefit to the individual. Fourth, we did not perform a time-dependent analysis in which risk factor values are updated over time, which may have reduced the number of eligible individuals selected based on 10-year risk compared with the 30-year models, since patients could become eligible for treatment in the future as they age or develop risk factors. However, this also implies that the 30-year model selects certain individuals (who may eventually become eligible based on 10-year risk) earlier in life for treatment, which may be advantageous. Finally, we assumed that all participants would remain compliant throughout the duration of therapy, which will overestimate the expected benefits of statins in practice. However, this analysis represents the maximal potential benefit that could be achieved from long-term statin use.
Conclusions
In summary, a long-term benefit approach to statin eligibility using a 30-year ARR greater than or equal to 15% identifies approximately 17.5% of individuals as having a high degree of expected long-term benefit of statins, with a 30-year NNT of less than 7. A long-term benefit approach identifies younger, lower-risk individuals with high LDL-C levels for statin treatment and thus provides a more optimal approach for determining statin eligibility in primary prevention.
eTable. Comparisons of different risk and benefit thresholds for selection of individuals for statin therapy in primary prevention with the same number of eligible participants.
References
- 1.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935-2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. . Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431. doi: 10.1056/NEJMoa1315665 [DOI] [PubMed] [Google Scholar]
- 3.Sniderman AD, Thanassoulis G, Williams K, Pencina M. Risk of premature cardiovascular disease vs the number of premature cardiovascular events. JAMA Cardiol. 2016;1(4):492-494. doi: 10.1001/jamacardio.2016.0991 [DOI] [PubMed] [Google Scholar]
- 4.Singh A, Collins BL, Gupta A, et al. . Cardiovascular risk and statin eligibility of young adults after an MI: Partners YOUNG-MI Registry. J Am Coll Cardiol. 2018;71(3):292-302. doi: 10.1016/j.jacc.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thanassoulis G, Pencina MJ, Sniderman AD. The benefit model for prevention of cardiovascular disease: an opportunity to harmonize guidelines. JAMA Cardiol. 2017;2(11):1175-1176. doi: 10.1001/jamacardio.2017.2543 [DOI] [PubMed] [Google Scholar]
- 6.Thanassoulis G, Williams K, Altobelli KK, Pencina MJ, Cannon CP, Sniderman AD. Individualized statin benefit for determining statin eligibility in the primary prevention of cardiovascular disease. Circulation. 2016;133(16):1574-1581. doi: 10.1161/CIRCULATIONAHA.115.018383 [DOI] [PubMed] [Google Scholar]
- 7.Pencina MJ, D’Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119(24):3078-3084. doi: 10.1161/CIRCULATIONAHA.108.816694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ference BA, Ginsberg HN, Graham I, et al. . Low-density lipoproteins cause atherosclerotic cardiovascular disease: 1. evidence from genetic, epidemiologic, and clinical studies. a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ference BA, Kastelein JJP, Ginsberg HN, et al. . Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318(10):947-956. doi: 10.1001/jama.2017.11467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford I, Murray H, McCowan C, Packard CJ. Long term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy: 20-year follow-up of West of Scotland Coronary Prevention Study. Circulation. 2016;133(11):1073-1080. doi: 10.1161/CIRCULATIONAHA.115.019014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Comparisons of different risk and benefit thresholds for selection of individuals for statin therapy in primary prevention with the same number of eligible participants.