Key Points
Question
Does the addition of a laser-induced chorioretinal anastomosis (L-CRA) to intravitreal ranibizumab treatment for a central retinal vein occlusion modify outcomes?
Findings
In this randomized clinical trial including 58 participants randomized to receive L-CRA plus intravitreal ranibizumab injections or a sham procedure plus intravitreal ranibizumab injections, the addition of an L-CRA significantly reduced the number of ranibizumab injections required in the follow-up period from 7 months to 2 years. The intervention group had better visual acuity at 2 years.
Meaning
These results suggest that the inclusion of an L-CRA to current intravitreal treatment for central retinal vein occlusion can reduce the number of injections required and lessen the burden of therapy.
This randomized clinical trial determines the 2-year efficacy of laser-induced chorioretinal anastomosis procedure plus intravitreal ranibizumab therapy vs sham procedure plus intravitreal ranibizumab therapy for patients with macular edema caused by central retinal vein occlusion.
Abstract
Importance
Adding a laser-induced chorioretinal anastomosis (L-CRA) to current treatments for central retinal vein occlusion (CRVO) may improve outcomes and lessen therapy burdens.
Objective
To determine the 2-year efficacy of intravitreal ranibizumab with an L-CRA vs ranibizumab alone for patients with macular edema caused by CRVO.
Design, Setting, and Participants
In this randomized clinical trial conducted at a single university clinic from March 2012 to June 2015, 58 participants with macular edema caused by CRVO were randomized 1:1 to either an L-CRA or sham procedure at baseline. All participants received monthly intravitreal injections of ranibizumab, 0.5 mg. Data were analyzed from April 2017 to September 2017.
Interventions
Random assignment to L-CRA plus monthly injections of intravitreal ranibizumab, 0.5 mg, (combination group; n = 29) or to a sham L-CRA procedure plus monthly injections of intravitreal ranibizumab, 0.5 mg, (ranibizumab alone group; n = 29) for 6 months. From month 7 to month 24, participants were evaluated monthly and received an injection of ranibizumab if a loss of 5 or more letters of best-corrected visual acuity (BCVA) on ETDRS chart from previous highest score occurred or if there was evidence of residual macular edema on optical coherence tomography.
Main Outcomes and Measures
Mean number of injections from month 7 to month 24, change in BCVA, and change in central subfield thickness (CST).
Results
Of the 58 included participants, 38 (66%) were men, and the mean (SD) age was 68.6 (11.8) years; participants had a mean (SD) BCVA of 57.09 (11.87) ETDRS letters (Snellen equivalent, 20/73) and a mean (SD) CST of 738.36 (175.54) μm. A successful L-CRA was created in 24 of 29 participants (83%) in the combination group. The mean number of injections from month 7 to month 24 was 3.2 (95% CI, 2.5-3.8) in the combination group and 7.1 (95% CI, 6.0-8.0) in the ranibizumab alone group. The ratio of the number of injections in the combination group compared with the ranibizumab alone group was 0.46 (95% CI, 0.36-0.61; P < .001). Mixed-effects regression modeling showed a difference in mean BCVA at 2 years between the combination and ranibizumab alone groups (combination, 70.3 letters [Snellen equivalent, 20/40]; ranibizumab alone, 61.6 letters [Snellen equivalent, 20/60]; difference, 8.8 letters; 95% CI, 0.2-17.3; P = .05). There was also a difference in CST at 2 years between the combination and ranibizumab alone groups (mean CST: combination, 303.6 μm; ranibizumab alone, 394.5 μm; difference, 90.9 μm; 95% CI, 24.3-157.5; P = .01). Four participants (14%) in the combination group required a vitrectomy for early macular traction or vitreous hemorrhage.
Conclusions and Relevance
For macular edema caused by CRVO, an L-CRA significantly reduced the number of ranibizumab injections required.
Trial Registration
anzctr.org.au Identifier: ACTRN12612000004864
Introduction
Current treatments for central retinal vein occlusion (CRVO) target the sequelae of the obstruction to venous outflow—ie, either the macular edema or anterior segment neovascularization—and do not address causal pathology.1,2,3,4,5,6 We have previously investigated the technique of using a high-power density laser to create an anastomosis between the obstructed retinal venous system and an unobstructed choroidal vein as a means of bypassing the obstruction to venous outflow that exists in CRVO.7,8,9,10 These studies showed a significant improvement in best-corrected visual acuity (BCVA) for participants in whom a successful laser-induced chorioretinal anastomosis (L-CRA) was created compared with natural history.9 Combining intravitreal anti–vascular endothelial growth factor (VEGF) agents with an L-CRA could be complementary, with the L-CRA addressing the component of the CRVO-induced macular edema caused by the elevated central venous pressure (CVP) and the anti-VEGF agents treating the component caused by the up-regulated cytokines.11,12,13 This may reduce the burden of therapy for patients with this condition.
Methods
Study Design
This randomized clinical trial compared the efficacy of combining an L-CRA with intravitreal ranibizumab vs ranibizumab alone for participants with macular edema secondary to CRVO over a period of 24 months. The study was conducted at the Lions Eye Institute in Perth, Western Australia, Australia. Entry criteria, treatment schedules, and retreatment criteria were based on the Efficacy and Safety of Ranibizumab Injection in Patients With Macular Edema Secondary to Central Retinal Vein Occlusion (CRUISE) study.1 Institutional ethics committee approval was obtained from the Sir Charles Gairdner Hospital, and the study was performed according to the Declaration of Helsinki.14 All participants provided written informed consent. The trial protocol can be found in Supplement 1.
Eligible participants (assessed by I.L.M.) were randomly assigned at baseline to either a combination treatment of an L-CRA procedure plus intravitreal ranibizumab injections (combination group) or a sham L-CRA procedure plus intravitreal ranibizumab injections (ranibizumab alone group). Treatment assignments were compiled using a list of computer-generated pseudorandom numbers in permuted blocks of variable size. The random allocation sequence and assignation of treatment was performed by L.A.S., and the L-CRA and sham procedures were performed by I.L.M. The primary efficacy outcome was the number of injections required from month 7 to month 24. Secondary outcomes were change in BCVA and central subfield thickness (CST).
Participants
Participants were included if they were 18 years or older with treatment-naive CRVO for less than 9 months, a BCVA letter score of 73 to 24 on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart (Snellen equivalent, 20/40 to 20/320), and CST of 250 μm or greater on spectral-domain optical coherence tomography (SD-OCT) using Spectralis HRA+OCT (Heidelberg Engineering). Key exclusion criteria included significant anticoagulation, a myocardial infarction, or a cerebrovascular accident within the previous 3 months. Screening examinations involved a complete ocular examination, BCVA, SD-OCT, and fluorescein angiography.
All participants underwent either an L-CRA as previously described9,15 or a sham procedure at baseline (month 0) and then were evaluated monthly for the next 6 months (month 1 to month 7 [loading phase]) while receiving monthly intravitreal injections of ranibizumab, 0.5 mg, commencing at month 1. For the remaining duration of the study (month 7 to month 24 [maintenance phase]), participants continued to be evaluated monthly and received intravitreal ranibizumab if they met the following criteria: (1) greater than 50-μm increase in CST on SD-OCT compared with lowest previous measurement; (2) new or persistent cystic retinal changes, subretinal fluid, or persistent diffuse edema of 270 μm or greater in CST; or (3) loss of 5 or more letters on the ETDRS chart from the previous best measurement in conjunction with any increase in CST.
Retreatment eligibility was determined by I.L.M. and confirmed by a masked data and safety monitoring committee (F.K.C. and D.A.M.). Patients and the BCVA and OCT assessors were blinded to the treatment assigned. Retreatment eligibility criteria were confirmed in all cases.
Statistical Analysis
The study was designed as a superiority trial but analyzed using 2-sided hypothesis tests. The sample size was computed to be 58 and to have 80% power with a 2-sided α of .05 to detect a difference between groups, assuming the true ratio in mean number of injections between groups was 0.72 (4.3/6.0) in the second year and no more than 10% dropouts. Poisson regression was used to test the power of associations.
Efficacy end point analyses were based on the intent-to-treat population, with participants grouped according to their assigned treatment. Missing values were imputed using the last-observation-carried-forward principle. Sample size calculations were performed in PASS, and data management and statistical tests were managed using R (The R Foundation). To account for the correlations among repeated measures from the same individuals, mixed-effects regression models were used to investigate the effects of treatment and other covariates on the number of injections, BCVA, and CST over time. The number of injections was considered a count-response variable, with a Poisson distribution of the response assumed and a log-link function used in the regression analysis. Best-corrected visual acuity and CST were modeled as continuous response variables. Baseline measures for BCVA, CST, and CRVO duration were included in the modeling to further adjust for chance imbalances associated with the randomization process. Time was considered as a categorical variable and treated separately for the count and continuous models. For the number of injections required, 3 main periods were considered in the analysis: month 1 to month 7, month 7 to month 13, and month 13 to month 24. For BCVA and CST, the effect of treatment was investigated at 4 main time points of interests: month 1, month 7, month 13, and month 24. To allow for the treatment effect to vary over time, a treatment group × time interaction term was evaluated for inclusion in the final model by a linear contrast test.
Results
Baseline Characteristics and Participant Disposition
Between March 2012 and June 2015, 58 participants (38 men [66%]; mean [SD] age, 68.6 [11.8] years) with a mean (SD) BCVA of 57.09 (11.87) ETDRS letters (Snellen equivalent, 20/73) and a mean (SD) CST of 738.36 (175.54) μm were randomized to treatment either with a combination of an L-CRA procedure plus intravitreal injection of ranibizumab, 0.5 mg (n = 29), or to a sham procedure plus intravitreal injection of ranibizumab, 0.5 mg (n = 29). Participant demographic characteristics and baseline ocular characteristics were similar between the 2 groups (Table 1).
Table 1. Patient Demographic Characteristics and Baseline Ocular Characteristics.
| Characteristic | Combination Group (n = 29) | Ranibizumab Alone Group (n = 29) |
|---|---|---|
| Sex, No. (%) | ||
| Male | 22 (76) | 16 (55) |
| Female | 7 (24) | 13 (45) |
| Age, mean (SD), y | 67.8 (10.2) | 69.3 (13.3) |
| CRVO duration, mean (SD), wk | 6.2 (4.8) | 8.1 (7.0) |
| BCVA | ||
| ETDRS letter score, mean (95% CI) | 59.6 (55.1-64.0) | 54.6 (50.1-59.1) |
| Snellen equivalent | 20/63 | 20/83 |
| CST, mean (95% CI), μm | 713.4 (657.5-768.2) | 763.3 (687.4-839.3) |
Abbreviations: BCVA, best-corrected visual acuity; CRVO, central retinal vein occlusion; CST, central subfield thickness; ETDRS, Early Treatment Diabetic Retinopathy Study.
Of the 58 participants enrolled at baseline, 56 (97%) completed the 24 months of follow-up. One participant in the combination group died at month 6, and 1 participant in the ranibizumab alone group withdrew at month 20. One participant from each group refused further ranibizumab injections—one at month 4 and the other at month 5. Both continued to be monitored as per protocol (Figure 1). More than 90% follow-up was achieved at the major time points throughout the study. A successful L-CRA was created in at least 1 site in 24 of 29 participants (83%) in the combination group (15 participants with 2 sites and 9 participants with 1 site), with the remaining 5 unsuccessful (Figure 2).
Figure 1. CONSORT Flow Diagram.
L-CRA indicates laser-induced chorioretinal anastomosis.
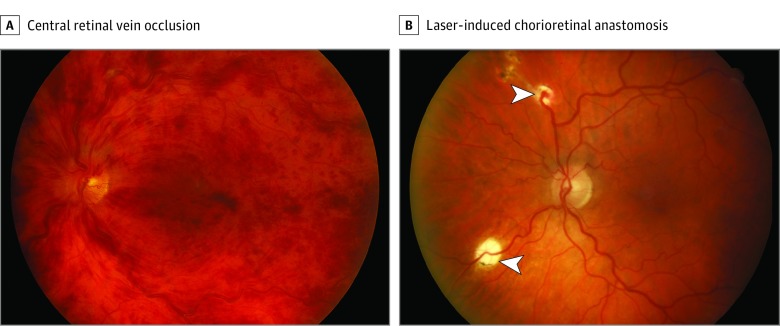
Figure 2. Combination Group Participant Before and After Treatment.
A, Example of central retinal vein occlusion that has been present for 6 weeks. The participant had a visual acuity of 20/100 OS prior to enrollment. B, Example of laser-induced chorioretinal anastomosis at month 12. There has been closure of the distal segment of the vein, and the area drained by this segment has been treated with laser prophylactically. The inferior anastomosis did not develop (arrowheads). Visual acuity improved to 20/25 OS.
Injections of Ranibizumab Required
Mixed-effects regression model results examining the effect of the L-CRA vs sham procedure on the number of injections required are shown in Table 2. A global test of the treatment group × time interaction term was found to be significant (χ22 = 23.64; P < .001) by a linear contrast. Therefore, the interaction term was retained in the final model, and the treatment effect difference in the number of injections required was allowed to vary with time.
Table 2. Treatment Exposurea.
| Phase | No. of Injections, Mean (95% CI) | Count Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Combination Group (n = 29) | Ranibizumab Alone Group (n = 29) | |||
| Loading phase (month 1 to month 6) | 5.5 (4.7-6.5) | 5.7 (4.9-6.7) | 0.96 (0.77-1.20) | .74 |
| Total maintenance phase (month 7 to month 24) | 3.2 (2.5-3.8) | 7.1 (6.0-8.0) | 0.46 (0.36-0.61) | <.001 |
| Early maintenance phase (month 7 to month 13) | 1.5 (1.1-2.0) | 2.4 (1.9-3.1) | 0.60 (0.41-0.88) | .01 |
| Late maintenance phase (month 13 to month 24) | 1.7 (1.3-2.2) | 4.6 (3.8-5.5) | 0.37 (0.26-0.51) | <.001 |
Based on regression analysis.
In the early maintenance phase (month 7 to month 13), the mean number of injections required was 1.5 in the combination group vs 2.4 in the ranibizumab alone group. The ratio of injections in the combination group compared with the ranibizumab alone group was 0.60 (95% CI, 0.41-0.88; P = .01).
In the late maintenance phase (month 13 to month 24), the mean number of injections required was 1.7 in the combination group vs 4.6 in the ranibizumab alone group. The ratio of injections in the combination group compared with the ranibizumab alone group was 0.37 (95% CI, 0.26-0.51; P < .001).
Overall, from month 7 to month 24, the mean number of injections required was 3.2 in the combination group vs 7.1 in the ranibizumab alone group (difference, 3.9; 95% CI, 2.7-5.1; P < .001). The ratio of injections in the combination group compared with the ranibizumab alone group was 0.46 (95% CI, 0.36-0.61; P < .001) (Table 2). Following the final mandatory intravitreal injection of ranibizumab at month 7, 10 participants (34%) in the combination group (all with functioning L-CRAs) compared with 1 participant (3%) in the ranibizumab alone group did not require any further injections (difference of proportions, 0.31; 95% CI, 0.09-0.53; P = .007).
Best-Corrected Visual Acuity
Between month 0 (L-CRA or sham procedure) and month 1 (commencement of monthly intravitreal injections of ranibizumab, 0.5 mg, from month 1 to month 7), there was a mean loss in BCVA of 5.2 ETDRS letters in the combination group and of 9.4 ETDRS letters in the ranibizumab alone group (eFigure 1 in Supplement 2). The results from the mixed-effects regression model examining the effect of L-CRA plus ranibizumab vs ranibizumab alone on BCVA are shown in Table 3. A global test of the treatment group × time interaction term was found to be nonsignificant by a linear contrast. Therefore, the interaction term was dropped from the final model, and the treatment effect difference between therapies was deemed invariant with time.
Table 3. Best-Corrected Visual Acuity (BCVA) Changes Across Treatment Groupsa.
| Category | Combination Group (n = 29) | Ranibizumab Alone Group (n = 29) |
|---|---|---|
| BCVA, Mean (95% CI), ETDRS Letter Score | ||
| Baseline | ||
| Estimated letter score | 59.6 (55.1-64.0) | 54.6 (50.1-59.1) |
| Snellen equivalent | 20/63 | 20/83 |
| Month 1 | ||
| Estimated letter score | 54.3 (47.5-61.1) | 45.6 (39.1-52.0) |
| Snellen equivalent | 20/83 | 20/121 |
| Month 7 | ||
| Estimated letter score | 70.5 (63.7-77.2) | 61.7 (55.3-68.2) |
| Snellen equivalent | 20/40 | 20/60 |
| Estimated letter score change from month 1 to month 7 | 16.2 (12.4-19.9) | 16.2 (12.4-19.9) |
| Month 13 | ||
| Estimated letter score | 70.7 (63.9-77.4) | 61.9 (55.5-68.4) |
| Snellen equivalent | 20/40 | 20/60 |
| Estimated letter score change from month 1 to month 13 | 16.4 (12.6-20.1) | 16.4 (12.6-20.1) |
| Month 24 | ||
| Estimated letter score | 70.3 (63.5-77.1) | 61.5 (55.1-68.0) |
| Snellen equivalent | 20/40 | 20/60 |
| Estimated letter score change from month 1 to month 24 | 16.0 (12.2-19.7) | 16.0 (12.2-19.7) |
| Treatment group difference in BCVA at months 7, 13, and 24 (95% CI) | 8.8 (0.2-17.3)b | |
| Change in BCVA From Baseline, No. (%) | ||
| Month 13 | ||
| Gain of ≥10 letters | 23 (79) | 16 (55) |
| Gain of 5-9 letters | 1 (3) | 3 (10) |
| Within 4 letters | 2 (7) | 2 (7) |
| Loss of 5-9 letters | 2 (7) | 0 |
| Loss of ≥10 letters | 1 (3) | 8 (28) |
| Month 24 | ||
| Gain of ≥10 letters | 24 (83) | 18 (62) |
| Gain of 5-9 letters | 0 | 2 (7) |
| Within 4 letters | 3 (10) | 0 |
| Loss of 5-9 letters | 0 | 2 (7) |
| Loss of ≥10 letters | 2 (7) | 7 (24) |
Abbreviation: ETDRS, Early Treatment Diabetic Retinopathy.
Based on regression analysis.
P = .05.
At month 13, the mean change in BCVA from month 1 for both groups was an increase of 16.4 ETDRS letters (95% CI, 12.6-20.1; P < .001). At month 24, the mean change from month 1 for both groups was an increase of 16.0 ETDRS letters (95% CI, 12.2-19.7; P < .001). The mean difference in BCVA at both month 13 and month 24 between treatment groups was 8.8 ETDRS letters (95% CI, 0.2-17.3; P = .05) (Table 3) (eFigure 1 in Supplement 2).
Central Subfield Thickness
Over 24 months, the mean difference in CST between the 2 groups was 90.9 μm (95% CI, −157.5 to −24.3; P = .01), in favor of the combination group (eTable and eFigure 2 in Supplement 2). A global test of the treatment group × time interaction term was not statistically significant.
Safety
In the 29 participants in the combination group, there were a potential 58 sites attempted for the L-CRA (2 per participant). Neovascularization (less than 1 disc area) was seen at 10 sites (17%), of which 5 regressed spontaneously, and the remaining 5 were treated with sectorial laser. Four participants (14%) required a vitrectomy, of which 3 were to relieve minor traction on the macula from avascular fibrous tissue emanating from the L-CRA site and 1 for a vitreous hemorrhage. All participants recovered without sequelae. None of these events occurred in the ranibizumab alone group. One participant in the combination group died at month 6 from a myocardial infarction 1 month after the last ranibizumab injection.
Discussion
This study demonstrated that in participants with a CRVO, creating an anastomotic connection between a retinal vein and a choroidal vein as a means of bypassing the obstruction to venous outflow significantly reduced the requirement for intravitreal ranibizumab injections over 2 years. There was also a significant increase in the number of participants not requiring further injections outside the mandatory loading phase. In the CRUISE study,1 on which the retreatment criteria for this study were based, the mean number of ranibizumab injections in the 6-month therapy as needed maintenance phase for the 0.5-mg group was 3.3, similar to the number required in our study for the ranibizumab alone group.2 Over the next 11 months up to month 24, the mean number of required injections was 1.7 for the combination group and 4.6 for the ranibizumab alone group (difference, 2.9; 95% CI, 2.0-3.8; P < .001), representing a 63% decrease in the need for injections in the combination group. Similar results to the ranibizumab alone group were seen in the Ranibizumab Intravitreal Injections in Patients With Visual Impairment Due to Macular Edema Secondary to Central Retinal Vein Occlusion (CRYSTAL) study,16 where 5 injections were required in the second year on an as needed regimen with stability of vision.
The injections commenced at 1 month from baseline, when patients underwent the L-CRA or sham procedure. This delay was to allow the anastomotic connection to develop, as this is dependent on the growth of a connecting vessel, and there is some circumstantial evidence that this is VEGF dependant.10,17 During this time, there was a decrease in mean BCVA in all groups, particularly in the ranibizumab alone group, likely because of increasing macular edema (eFigures 1 and 2 in Supplement 2), and this reflects changes seen in other natural history studies, although to our knowledge, none have had mean durations of CRVO as short as this study.18 By month 1, both the duration and the BCVA of CRVO in the ranibizumab alone group were similar to the CRUISE study baseline, with similar subsequent improvements over the next 12 months (Table 3).2
The BCVA results at month 24 indicate that for both groups, with regular monthly monitoring with injections performed on an as needed basis, visual acuity gains were maintained during the second year of treatment. A difference in BCVA between the 2 groups remained for the duration of the study, with the combination group achieving 8.8 ETDRS letters (95% CI, 0.2-17.3; P = .05) greater than the ranibizumab alone group at 24 months (Table 3) (eFigure 1 in Supplement 2).
In CRVO, the obstruction to venous outflow reduces retinal blood flow, leading to up-regulation of hypoxic-induced cytokines, predominantly VEGFA, and elevation of CVP, which can be up to 24-fold that of normal.11,12,13 The pathogenesis of the macular edema is likely to be multifactorial, with both the up-regulation of VEGF and the elevated CVP contributing. Whether the elevated CVP contributes directly to macular edema or acts by reducing arterial inflow and thereby increasing retinal hypoxia and the associated production of cytokines is undetermined. Elevated VEGF levels in the retina down-regulate the endothelial barrier’s proteins, and as the effect of the VEGF blockade wears off (intravitreal half-life of a 0.5-mg injection of ranibizumab is estimated to be 7.19 days19), the capillaries may leak more, and this is likely to be exacerbated by the elevated CVP.1,2,3,4,5,20,21 While blockade of the up-regulated VEGF is effective in the short term, both of these components need to be addressed to fully treat this condition in the longer term. Persistently elevated CVP in CRVO is associated with worse visual outcomes, greater degrees of retinal ischemia, and a higher incidence of anterior segment neovascularization.22 The results from the ranibizumab alone arm in this study and others16 indicate that BCVA gains can be maintained with strict interval monitoring and as needed treatment; however, this potentially may lead to patient fatigue with the burden of therapy and subsequent nonattendance. The results from real-world studies where recurrent injections are required for VEGF-mediated maculopathies would indicate that it is very difficult to achieve and maintain the same visual acuity gains that are seen in strict clinical trials in normal clinical practice, where patients may not be willing to attend and receive the same intensity of treatment.23,24,25,26 Other studies have suggested that patients with CRVO are more likely to experience reduced BCVA outcomes when the duration between follow-up visits is increased, and as natural history studies of CRVO have shown, the median time to macular edema resolution is between 23 and 29 months, so this does imply a considerable investment in time and resources.27,28
To achieve more sustainable results with less reliance on intravitreal therapy and ease the burden of treatment, the causative pathology in CRVO must be more adequately addressed. From the results of our study, the combination of ranibizumab and L-CRA appears to be complementary, with both modalities conferring separate benefits. The ranibizumab addresses the component of the CRVO-induced macular edema caused by the up-regulation of VEGF, and the L-CRA addresses the component due to the elevation in CVP. The complications of the procedure are manageable, provided there is close follow-up and prompt intervention. It was a protocol requirement for this study that a vitrectomy be done if any macular tractional effects became apparent, however minor, or if there was a vitreous hemorrhage sufficient to obscure the retinal details. No significant fibrovascular proliferation was seen in the participants treated and commencing regular ranibizumab injections 1 month after L-CRA creation, and this appears to have had a protective benefit.29
The direct costs of treating retinal vein occlusion with intravitreal therapy are significant, with CRVO consuming more resources than branch retinal vein occlusion.30 Reducing the injection load will confer savings in health expenditure and reduce the burden of therapy.
Limitations
This study has several limitations. Each group had a relatively small number of participants (29), and the 1-month delay in administering intravitreal anti-VEGF treatment does not reflect current practice. There is a possibility of bias with only a sole investigator involved (I.L.M.), although the outcome measures (ie, injection requirements, BCVA, and CST) were performed by personnel masked to patient group.
Conclusions
In conclusion, this study has shown that adding an L-CRA to current treatments with anti-VEGF agents for CRVO significantly reduces the number of injections required in the longer term. Best-corrected visual acuity improvement with L-CRA plus ranibizumab appears at least to be equivalent to as needed monthly ranibizumab treatment.
Trial protocol.
eTable. Central subfield thickness characteristics across treatment groups.
eFigure 1. Mean change in best-corrected visual acuity letter score from study baseline to 24 months.
eFigure 2. Mean change in central subfield thickness from baseline to 24 months.
Data sharing statement.
References
- 1.Brown DM, Campochiaro PA, Singh RP, et al. ; CRUISE Investigators . Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.e1. doi: 10.1016/j.ophtha.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Brown DM, Awh CC, et al. . Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041-2049. doi: 10.1016/j.ophtha.2011.02.038 [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Heier JS, Clark WL, et al. . Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155(3):429-437.e7. doi: 10.1016/j.ajo.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 4.Heier JS, Campochiaro PA, Yau L, et al. . Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119(4):802-809. doi: 10.1016/j.ophtha.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Heier JS, Clark WL, Boyer DS, et al. . Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121(7):1414-1420.e1. doi: 10.1016/j.ophtha.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 6.The Central Vein Occlusion Study Group A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion: the Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102(10):1434-1444. doi: 10.1016/S0161-6420(95)30848-2 [DOI] [PubMed] [Google Scholar]
- 7.McAllister IL, Constable IJ. Laser-induced chorioretinal venous anastomosis for treatment of nonischemic central retinal vein occlusion. Arch Ophthalmol. 1995;113(4):456-462. doi: 10.1001/archopht.1995.01100040072030 [DOI] [PubMed] [Google Scholar]
- 8.McAllister IL, Douglas JP, Constable IJ, Yu D-Y. Laser-induced chorioretinal venous anastomosis for nonischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol. 1998;126(2):219-229. doi: 10.1016/S0002-9394(98)00156-1 [DOI] [PubMed] [Google Scholar]
- 9.McAllister IL, Gillies ME, Smithies LA, et al. . The Central Retinal Vein Bypass Study: a trial of laser-induced chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmology. 2010;117(5):954-965. doi: 10.1016/j.ophtha.2009.10.026 [DOI] [PubMed] [Google Scholar]
- 10.McAllister IL, Gillies ME, Smithies LA, et al. . Factors promoting success and influencing complications in laser-induced central vein bypass. Ophthalmology. 2012;119(12):2579-2586. doi: 10.1016/j.ophtha.2012.06.047 [DOI] [PubMed] [Google Scholar]
- 11.Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105(3):412-416. doi: 10.1016/S0161-6420(98)93020-2 [DOI] [PubMed] [Google Scholar]
- 12.Jonas JB. Ophthalmodynamometric assessment of the central retinal vein collapse pressure in eyes with retinal vein stasis or occlusion. Graefes Arch Clin Exp Ophthalmol. 2003;241(5):367-370. doi: 10.1007/s00417-003-0643-7 [DOI] [PubMed] [Google Scholar]
- 13.Jonas JB, Harder B. Ophthalmodynamometric differences between ischemic vs nonischemic retinal vein occlusion. Am J Ophthalmol. 2007;143(1):112-116. doi: 10.1016/j.ajo.2006.09.019 [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.McAllister IL, Smithies LA, Previn V. Technique of laser chorioretinal anastomosis creation in central retinal vein occlusion and success rate with a new photocoagulator system. Retina. 2016;36(10):1971-1978. doi: 10.1097/IAE.0000000000001025 [DOI] [PubMed] [Google Scholar]
- 16.Larsen M, Waldstein SM, Priglinger S, et al. . Sustained benefits from ranibizumab for central retinal vein occlusion with macular edema: 24-month results of the CRYSTAL study. Ophthalmol Retina. 2018;2(2):134-142. doi: 10.1016/j.oret.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 17.Vijayasekaran S, Yu D-Y, McAllister I, Barry C, Constable I. Optimal conditions required for the creation of an iatrogenic chorioretinal venous anastomosis in the dog using argon green laser photocoagulation. Curr Eye Res. 1995;14(1):63-70. doi: 10.3109/02713689508999915 [DOI] [PubMed] [Google Scholar]
- 18.The Central Vein Occlusion Study Group Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115(4):486-491. doi: 10.1001/archopht.1997.01100150488006 [DOI] [PubMed] [Google Scholar]
- 19.Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012;154(4):682-686.e2. doi: 10.1016/j.ajo.2012.03.047 [DOI] [PubMed] [Google Scholar]
- 20.Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997;64(4):505-517. doi: 10.1006/exer.1996.0239 [DOI] [PubMed] [Google Scholar]
- 21.McAllister IL, Vijayasekaran S, Chen SD, Yu DY. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009;147(5):838-846, 846.e1-846.e2. doi: 10.1016/j.ajo.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 22.McAllister IL, Tan MH, Smithies LA, Wong WL. The effect of central retinal venous pressure in patients with central retinal vein occlusion and a high mean area of nonperfusion. Ophthalmology. 2014;121(11):2228-2236. doi: 10.1016/j.ophtha.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 24.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 25.Arnold JJ, Campain A, Barthelmes D, et al. ; Fight Retinal Blindness Study Group . Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122(6):1212-1219. doi: 10.1016/j.ophtha.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 26.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092-1101. doi: 10.1016/j.ophtha.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 27.Campochiaro PA, Hafiz G, Channa R, et al. . Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusions: two-year outcomes. Ophthalmology. 2010;117(12):2387-2394.e1, 5. doi: 10.1016/j.ophtha.2010.03.060 [DOI] [PubMed] [Google Scholar]
- 28.Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118(1):119-133.e1, 2. doi: 10.1016/j.ophtha.2010.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong KC, Barry C, McAllister IL. Intravitreal bevacizumab (Avastin) as a treatment of the neovascular complications of laser-induced chorioretinal anastomosis for nonischaemic central retinal vein occlusion. Clin Exp Ophthalmol. 2009;37(5):485-489. doi: 10.1111/j.1442-9071.2009.02063.x [DOI] [PubMed] [Google Scholar]
- 30.Taylor M, Serbetci E, Ferreira A, et al. . A United Kingdom–based economic evaluation of ranibizumab for patients with retinal vein occlusion (RVO). J Med Econ. 2014;17(6):423-434. doi: 10.3111/13696998.2014.909435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eTable. Central subfield thickness characteristics across treatment groups.
eFigure 1. Mean change in best-corrected visual acuity letter score from study baseline to 24 months.
eFigure 2. Mean change in central subfield thickness from baseline to 24 months.
Data sharing statement.