Key Points
Question
What is the outcome of patients with asymptomatic aortic stenosis (AS) followed up in a specialized heart valve clinic?
Findings
In this study using data from the Heart Valve Clinic International Database including 1375 patients from 10 heart valve clinics, left ventricular ejection fraction less than 60% and peak aortic jet velocity greater than 5 m/s were independent factors associated with all-cause and cardiovascular mortality in patients with asymptomatic severe AS. The adverse association of these factors with survival remains significant following aortic valve replacement, suggesting the need for earlier intervention.
Meaning
Taking into consideration the low procedural risk associated with aortic valve replacement, the potential benefit of earlier intervention should be considered in high-risk patients with asymptomatic severe AS.
This study determines the clinical outcomes of patients with asymptomatic aortic stenosis using data from the Heart Valve Clinic International Database.
Abstract
Importance
The natural history and the management of patients with asymptomatic aortic stenosis (AS) have not been fully examined in the current era.
Objective
To determine the clinical outcomes of patients with asymptomatic AS using data from the Heart Valve Clinic International Database.
Design, Setting, and Participants
This registry was assembled by merging data from prospectively gathered institutional databases from 10 heart valve clinics in Europe, Canada, and the United States. Asymptomatic patients with an aortic valve area of 1.5 cm2 or less and preserved left ventricular ejection fraction (LVEF) greater than 50% at entry were considered for the present analysis. Data were collected from January 2001 to December 2014, and data were analyzed from January 2017 to July 2018.
Main Outcomes and Measures
Natural history, need for aortic valve replacement (AVR), and survival of asymptomatic patients with moderate or severe AS at entry followed up in a heart valve clinic. Indications for AVR were based on current guideline recommendations.
Results
Of the 1375 patients included in this analysis, 834 (60.7%) were male, and the mean (SD) age was 71 (13) years. A total of 861 patients (62.6%) had severe AS (aortic valve area less than 1.0 cm2). The mean (SD) overall survival during medical management (mean [SD] follow up, 27 [24] months) was 93% (1%), 86% (2%), and 75% (4%) at 2, 4, and 8 years, respectively. A total of 104 patients (7.6%) died under observation, including 57 patients (54.8%) from cardiovascular causes. The crude rate of sudden death was 0.65% over the duration of the study. A total of 542 patients (39.4%) underwent AVR, including 388 patients (71.6%) with severe AS at study entry and 154 (28.4%) with moderate AS at entry who progressed to severe AS. Those with severe AS at entry who underwent AVR did so at a mean (SD) of 14.4 (16.6) months and a median of 8.7 months. The mean (SD) 2-year and 4-year AVR-free survival rates for asymptomatic patients with severe AS at baseline were 54% (2%) and 32% (3%), respectively. In those undergoing AVR, the 30-day postprocedural mortality was 0.9%. In patients with severe AS at entry, peak aortic jet velocity (greater than 5 m/s) and LVEF (less than 60%) were associated with all-cause and cardiovascular mortality without AVR; these factors were also associated with postprocedural mortality in those patients with severe AS at baseline who underwent AVR (surgical AVR in 310 patients; transcatheter AVR in 78 patients).
Conclusions and Relevance
In patients with asymptomatic AS followed up in heart valve centers, the risk of sudden death is low, and rates of overall survival are similar to those reported from previous series. Patients with severe AS at baseline and peak aortic jet velocity of 5.0 m/s or greater or LVEF less than 60% have increased risks of all-cause and cardiovascular mortality even after AVR. The potential benefit of early intervention should be considered in these high-risk patients.
Introduction
In the western world, calcific aortic stenosis (AS), the most common valvular heart disease, represents a major public health burden.1 Currently, there is no pharmacological treatment that prevents or slows the progression of AS.2 Surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) are the only therapies to significantly improve both survival and symptoms3,4 and are recommended in symptomatic patients with severe AS.5,6 The management of patients with asymptomatic severe AS, particularly the choice between early intervention vs watchful waiting, continues to be a matter of debate.7,8 Current guidelines advocate delaying AVR until symptoms or left ventricular (LV) systolic dysfunction develop.5,6 However, observational studies in 20109 and 201510 have suggested that early elective AVR might improve outcomes in patients with severe asymptomatic AS. This approach has been reinforced by continued advances in surgical techniques and aortic valve prostheses, the advent of TAVR, and the low perioperative mortality and morbidity rates achieved in valve centers of reference.11,12 However, preemptive surgery before onset of symptoms or LV systolic dysfunction is considered in only a selected group of patients after careful risk stratification. This is at least in part because the evidence for intervention in asymptomatic severe AS is derived from small, heterogeneous, retrospective, single-center studies, which have generally included the need for AVR (not always motivated by the development of symptoms or LV dysfunction) in the composite study end point.13,14,15,16,17,18,19,20 Moreover, decision making for AVR remains particularly difficult in older patients in whom it is sometimes unclear if the benefits of intervention outweigh the risk.5,6
In recent years, the establishment of multidisciplinary services delivered by experts in valvular heart disease has become the basis for the implementation of heart valve clinics.21 These clinics provide standardized care based on international evidence-based norms and facilitate large clinical registries, which may be used to further refine guideline recommendations and quality improvement. The Heart Valve Clinic International Database (HAVEC) is a multicenter registry created for prospective data collection of patients with echocardiographic confirmation of AS and other valve diseases.22 The objective of the present study was to determine the natural history and outcomes of patients with moderate or severe AS who are followed up in a heart valve clinic.
Methods
The data, analytic methods, and study materials can be made available to other researchers for purposes of reproducing the results or replicating the procedure after approval of the HAVEC group. Data are centrally collected at the Department of Cardiology, Centre Hospitalier Universitaire du Sart Tilman, Liège, Belgium. This retrospective analysis of clinically acquired data was approved by the respective institutional review boards of each participating center, and informed consent was waived because collected data were deidentified and retrospective.
Study Population
The HAVEC registry was assembled by merging data from prospectively gathered electronic institutional databases of 10 heart valve clinics, as defined by the European Society of Cardiology Working Group in Valvular Heart Diseases,13 collected between 2001 and 2014. The analyses were then performed retrospectively. Patients were eligible for this registry if they had AS diagnosed with the use of 2-dimension echocardiography at 1 of the participating centers and were followed-up according to available guidelines on a regular basis. Exclusion criteria included aortic valve area (AVA) greater than 1.5 cm2; class I indications for AVR (rest AS–related or exercise AS–related symptoms [ie, angina, syncope, and dyspnea] or LV ejection fraction [EF] less than 50%); concomitant congenital heart valve disease more than mild mitral, tricuspid, or pulmonic valve disease; or prior valve surgery. The study was conducted in accordance with the respective institutional guidelines, national legal requirements, and the revised Helsinki declaration.23
Doppler Echocardiography
Transthoracic echocardiography was performed as part of routine clinical practice using commercially available systems. The severity of AS was evaluated according to standard methods. Peak aortic jet velocity was derived from transaortic flow, recorded with continuous wave Doppler using a multiwindow approach. Peak and mean gradients were calculated using the simplified Bernoulli equation. The continuity equation was used to calculate AVA. Moderate and severe AS were defined as an AVA between 1.0 and 1.5 cm2 and less than 1.0 cm2, respectively. Left ventricular EF was estimated by the Simpson biplane method.
Follow-up
Follow-up was organized within each participating center according to available guidelines (every 6-12 months in patients with severe AS) (eTable 1 in Supplement 1). Data collection started after baseline evaluation until last available contact or death. Follow-up data were obtained by direct patient interview and clinical examination; telephone calls with physicians, patients, or next of kin; or review of autopsy records and death certificates. Information was collected regarding development of cardiac symptoms, subsequent AVR (performed for development of guideline indications: symptom onset, abnormal exercise test, peak aortic velocity greater than 5.5 m/s, or rapid progression of AS severity), and death.24 Exercise testing was performed in selected patients (572 of 1375 patients [41.6%]), especially when the symptomatic status was unclear. Cardiac deaths were classified as directly related to AS (ie, sudden death or heart failure) or to other cardiac pathology (ie, fatal myocardial infarction). All-cause mortality was the primary end point of the study; cardiovascular-related mortality was the secondary end point. Follow-up echocardiography data were obtained in all patients who underwent AVR to confirm the progression of moderate to severe AS (ie, AVA less than 1 cm2).
Statistical Analysis
Data are reported as means with standard deviations for continuous variables or numbers and percentages of individuals for categorical variables. Group comparisons for categorical variables were obtained with χ2 test and for continuous variables with Mann-Whitney U test if the normality of data was violated based on a Shapiro-Wilk test. Analyses of overall and cardiovascular mortality were performed by censoring data at the time of AVR. Multivariable analysis was then performed by including covariates selected on the basis of their known link to outcome in patients with AS (ie, age, sex, comorbidities, AS severity, and LVEF) into a Cox proportional hazard model. Peak aortic jet velocity (greater than or equal to 5 m/s) and LVEF (less than 60%) were also expressed as categorical variables.6,25 Survival curves were computed based on the Kaplan-Meier method. Regarding the prediction of all-cause and cardiovascular death, receiver operating characteristic curve analyses were performed, and areas under the curve (AUCs) were reported. The most accurate cutoff values (ie, best compromise between sensitivity and specificity) were obtained using Youden index. A P value less than .05 was considered statistically significant, and all P values were 2-tailed. Statistical analyses were performed using SPSS version 23 (IBM).
Results
A total of 1763 patients were included in the present registry, of whom 388 (22.0%) were excluded because of missing data regarding LVEF or AS severity. The characteristics of the remaining 1375 patients who fulfilled inclusion criteria are described in Table 1. The mean (SD; range) AVA was 0.94 (0.3; 0.30-1.50) cm2 and was less than 1 cm2 in 861 patients (62.6%) (Table 1).
Table 1. Comparison of Patients With Moderate vs Severe Aortic Stenosis (AS) at Baseline.
| Variable | Mean (SD) | P Value | ||
|---|---|---|---|---|
| All (N = 1375) | Moderate AS (n = 514) | Severe AS (n = 861) | ||
| Age, y | 71 (13) | 68 (13) | 72 (12) | <.001 |
| Male, No. (%) | 834 (60.7) | 337 (65.6) | 497 (57.7) | .004 |
| Height, cm | 167 (9) | 168 (9) | 166 (9) | .04 |
| Weight, kg | 75 (15) | 78 (15) | 73 (16) | <.001 |
| Body surface area, m2 | 1.8 (0.2) | 1.9 (0.2) | 1.8 (0.2) | <.001 |
| Systolic blood pressure, mm Hg | 140 (19) | 140 (18) | 140 (20) | .97 |
| Diastolic blood pressure, mm Hg | 78 (11) | 78 (10) | 77 (11) | .41 |
| Hypertension, No. (%) | 833 (60.6) | 327 (63.6) | 506 (58.8) | .07 |
| Diabetes, No. (%) | 245 (17.8) | 95 (18.4) | 150 (17.4) | .74 |
| Smoker, No. (%) | 415 (30.1) | 180 (35.0) | 235 (27.3) | .002 |
| Dyslipidemia, No. (%) | 722 (52.5) | 299 (58.1) | 423 (49.1) | <.001 |
| Chronic obstructive pulmonary disease, No. (%) | 104 (7.6) | 48 (9.3) | 56 (6.5) | .03 |
| β-Blockers, No. (%) | 482 (35.1) | 150 (29.2) | 332 (38.6) | <.001 |
| Angiotensin-converting enzyme inhibitor, No. (%) | 447 (32.5) | 177 (34.4) | 270 (31.4) | .31 |
| LV mass, g/m2 | 207 (73) | 209 (58) | 206 (81) | .51 |
| LVESV, mL | 39 (21) | 40 (22) | 39 (20) | .53 |
| LVEDV, mL | 103 (34) | 110 (35) | 100 (33) | <.001 |
| SV index, mL/m2 | 44 (11) | 46 (11) | 42 (11) | <.001 |
| LV ejection fraction, % | 65.5 (7.4) | 66 (6.9) | 65 (7.3) | .003 |
| Peak aortic velocity, m/s | 3.8 (0.8) | 3.3 (0.7) | 4.1 (0.7) | <.001 |
| Mean aortic pressure gradient, mm Hg | 37 (17) | 26 (12) | 44 (16) | <.001 |
| Aortic valve area, cm2 | 0.94 (0.3) | 1.20 (0.2) | 0.78 (0.1) | <.001 |
| Mitral E wave velocity, cm/s | 87 (28) | 84 (22) | 88 (31) | .02 |
| Mitral E/A ratio | 1 (0.6) | 1 (0.4) | 1 (0.6) | .80 |
| E/e’ ratio | 10.8 (5.7) | 10.6 (4.6) | 10.9 (6.4) | .28 |
Abbreviations: EDV, end-diastolic volume; ESV, end-systolic volume; LV, left ventricular; SV, stroke volume.
Outcome During Medical Management
Clinical follow-up information for patients in the 10 centers is shown in eTable 1 in Supplement 1. Echocardiographic data regarding the rate of progression of initially moderate to severe AS were not routinely available, although severe AS was documented in all patients with moderate AS at baseline who underwent AVR during follow-up. The mean (SD; range) follow-up time was 27 (24; 2-224) months. A total of 542 patients (39.4%) required AVR (SAVR, 429 [79.2%]; TAVR, 113 [20.8%]). The 2-year, 4-year, and 8-year overall survival rates for the entire cohort during medical management were 93% (1%), 86% (2%), and 75% (4%), respectively. The cardiovascular death–free survival rates were 96% (1%) at 2 years, 90% (1%) at 4 years, and 83% (3%) at 8 years. Of the 104 deaths during medical management, 57 (54.8%) were from a cardiovascular cause, including 38 from heart failure and 7 from sudden cardiac death. The incidence rate of sudden death was 2.5 cases per 1000 patient-years.
Patients With Severe AS at Entry
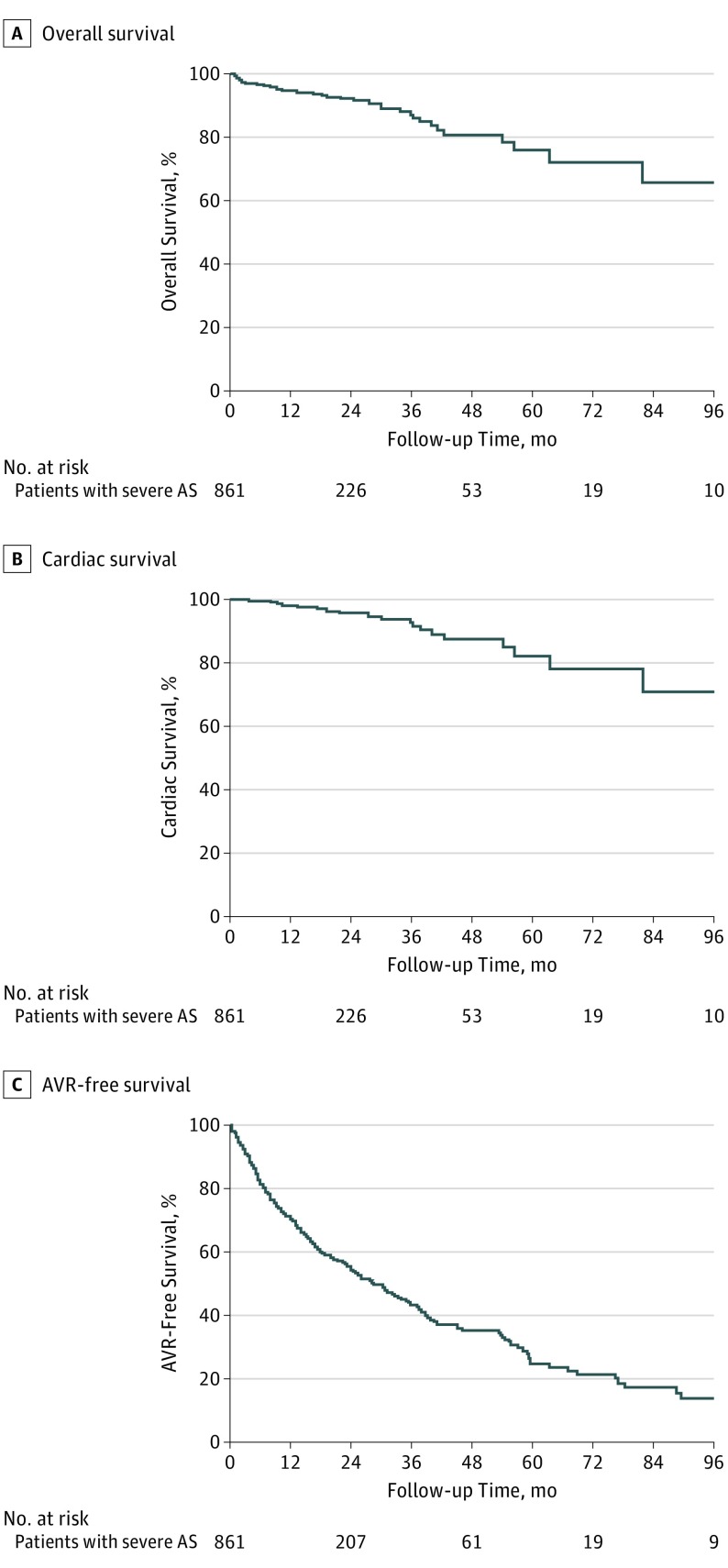
Among the 861 patients with severe AS at entry, the 2-year, 4-year, and 8-year overall survival rates were 92% (1%), 80% (3%), and 65% (8%), respectively (Figure 1A); the cardiovascular death–free survival rates at 2 years, 4 years, and 8 years were 96% (1%), 87% (3%), and 71% (9%), respectively (Figure 1B); and the 2-year, 4-year, and 8-year AVR-free survival rates were 54% (2%), 32% (3%), and 12% (3%), respectively (Figure 1C). Of the 64 deaths during medical management in patients with severe AS, 32 (50%) were from a cardiovascular cause, including 23 from heart failure, 4 from sudden cardiac death, 2 from myocardial infarction, 2 from stroke, and 1 from pulmonary embolism.
Figure 1. Kaplan-Meier Estimates for Events in Patients With Severe Aortic Stenosis (AS).
Kaplan-Meier analyses of overall survival (A), cardiovascular death–free survival (B), and aortic valve replacement (AVR)–free survival (C) for patients with severe AS at entry to the registry.
Aortic valve replacement was performed in 388 of 861 patients with severe AS (45.1%), with SAVR performed in 310 (79.9%) (Table 2). Indications for AVR were development of a class I indication in 366 patients (94.3%), a class IIa indication in 18 (4.6%), and a class IIb indication in 4 (1.0%). In these patients, the mean (SD) time between inclusion and AVR was 14.4 (16.6) months, and the median (range) time was 8.7 (0-133) months. Combined coronary artery revascularization was performed in 82 patients (26.5%) at the time of SAVR.
Table 2. Comparison of Survivors vs Nonsurvivors in Patients With Severe Aortic Stenosis at Baseline.
| Variable | Mean (SD) | P Value | ||
|---|---|---|---|---|
| Survivor (n = 738) | Death Under Medical Treatment (n = 64) | Death After AVR (n = 59) | ||
| Age, y | 72 (12) | 78 (7)a | 72 (10)b | <.001 |
| Male, No. (%) | 425 (57.6) | 37 (58) | 35 (59) | .97 |
| Height, cm | 166 (9) | 167 (10) | 169 (8) | .11 |
| Weight, kg | 73 (16) | 73 (15) | 73 (12) | .94 |
| Body surface area, m2 | 1.81 (0.2) | 1.81 (0.2) | 1.82 (0.2) | .84 |
| Systolic blood pressure, mm Hg | 139 (19) | 149 (23)a | 142 (19) | .001 |
| Diastolic blood pressure, mm Hg | 77 (11) | 80 (10) | 78 (11) | .12 |
| Hypertension, No. (%) | 436 (59.1) | 43 (67) | 27 (46) | .04 |
| Diabetes, No. (%) | 119 (16.1) | 17 (27) | 14 (24) | .06 |
| Smoker, No. (%) | 194 (26.3) | 24 (37) | 17 (29) | .18 |
| Dyslipidemia, No. (%) | 381 (51.6) | 27 (42) | 15 (25) | <.001 |
| Chronic obstructive pulmonary disease, No. (%) | 42 (5.7) | 9 (14) | 5 (9) | .03 |
| β-Blockers, No. (%) | 282 (38.2) | 26 (41) | 24 (41) | .94 |
| Angiotensin-converting enzyme inhibitor, No. (%) | 225 (30.5) | 25 (39) | 20 (34) | .39 |
| LV mass, g/m2 | 202 (83) | 227 (67) | 218 (67) | .06 |
| LVESV, mL | 39 (21) | 39 (14) | 40 (16) | .96 |
| LVEDV, mL | 101 (34) | 95 (27) | 102 (29) | .49 |
| SV index, mL/m2 | 42 (11) | 41 (11) | 42 (11) | .72 |
| LV ejection fraction, % | 66 (7) | 60 (5)a | 64 (9)b | <.001 |
| Peak aortic velocity, m/s | 4.1 (0.7) | 4.2 (0.9) | 4.4 (0.8)a | .001 |
| Mean aortic pressure gradient, mm Hg | 43 (16) | 42 (17) | 49 (18)a,b | .02 |
| Aortic valve area, cm2 | 0.78 (0.15) | 0.77 (0.15) | 0.77 (0.16) | .72 |
Abbreviations: AVR, aortic valve replacement; EDV, end-diastolic volume; ESV, end-systolic volume; LV, left ventricular; SV, stroke volume.
Significant difference with survivors.
Significant difference with death under medical treatment.
Patients With Moderate AS at Entry
Among the 514 patients with moderate AS at baseline, 154 (30.0%) underwent AVR (SAVR, 110 [71.4%]; TAVR, 44 [28.6%]); 128 patients (83.1%) developed class I indications, 22 (14.3%) developed class IIa indications, and 4 (2.6%) developed class IIb indications. Echocardiography preceding AVR confirmed that the stenosis had progressed to the severe stage (AVA less than 1.0 cm2) in all patients. Combined coronary artery revascularization was performed in 34 patients at the time of AVR. The mean (SD) time between inclusion and AVR was 29.9 (24.4) months, and the median (range) time was 22.6 (0-98) months. The mean (SD) overall survival rate was 94% (1%) at 2 years, 89% (2%) at 4 years, and 78% (4%) at 8 years follow-up (eFigure 1 in Supplement 1). In these patients with moderate AS at baseline, AVR-free survival rates are provided in eFigure 2 in Supplement 1. Of the 40 deaths during medical management, 25 were cardiovascular in nature, including heart failure in 14 and sudden death in 3. Of note, 2 of 3 patients who died suddenly had confirmed severe AS on echocardiography.
Predictors of Outcome
For the entire cohort, age, dyslipidemia, chronic obstructive pulmonary disease, higher systolic blood pressure, peak aortic jet velocity, and LVEF were associated with all-cause mortality. Age, peak aortic jet velocity, and LVEF were also associated with cardiovascular death (eTable 2 in Supplement 1).
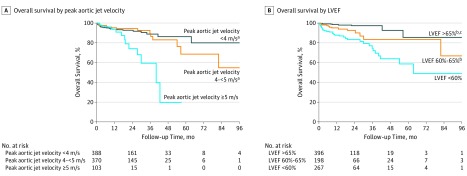
In patients with severe AS (Table 3), echocardiographic determinants of all-cause mortality identified in the multivariable analysis were peak aortic jet velocity greater than 5 m/s and LVEF. Independent determinants of cardiovascular mortality were age, diabetes, peak aortic jet velocity greater than 5 m/s, and LVEF. When peak aortic jet velocity and LVEF were taken as continuous variables, both were independently associated with cardiovascular mortality (Table 3). Using receiver operating characteristic curve analysis, the best cutoff values regarding the prediction of overall death were 59.6% for LVEF (AUC, 0.73; sensitivity, 81%; specificity, 56%) and 4.7 m/s for peak aortic jet velocity (AUC, 0.50; sensitivity, 30%; specificity, 80%). The AUCs for LVEF and peak aortic jet velocity were 0.68 and 0.59, respectively, for the prediction of cardiovascular death. Of note, there was a graded association of reduced survival with increased peak aortic jet velocity and with decreased LVEF (Figure 2). No EF threshold higher than 65% further affected survival. For peak aortic jet velocity, no additional prognostic information was obtained for velocities between 4 and 5 m/s. Similar data were obtained for AVA (eTable 3 and eFigure 3 in Supplement 1) for both total and cardiovascular mortality. In patients with initially moderate AS, the best cutoffs associated with the outcomes were 64% for LVEF and 3.0 m/s for peak aortic jet velocity (eTable 4 in Supplement 1).
Table 3. Multivariable Predictors of Mortality (Aortic Valve Replacement Censored) With Echocardiographic Data as Continuous and Categorical Variables in Patients With Severe Aortic Stenosis at Baseline.
| Predictor | All-Cause Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Continuous Variables | ||||
| Age, per 1 y | 1.05 (1.02-1.08) | .002 | 1.05 (1.00-1.10) | .03 |
| Systolic blood pressure, per mm Hg | 1.02 (1.01-1.03) | .004 | NA | NA |
| Diabetes | 1.34 (0.73-2.44) | .35 | 2.84 (1.24-6.55) | .01 |
| Dyslipidemia | 0.65 (0.38-1.12) | .12 | NA | NA |
| Chronic obstructive pulmonary disease | 2.47 (1.14-5.34) | .02 | NA | NA |
| Peak aortic velocity, per 0.1 m/s | 1.03 (0.99-1.07) | .11 | 1.01 (1.03-1.14) | .001 |
| LVEF, per 1% | 0.90 (0.86-0.94) | <.001 | 0.90 (0.85-0.96) | .002 |
| Categorical Variables | ||||
| Age, per 1 y | 1.05 (1.02-1.09) | .001 | 1.06 (1.01-1.11) | .02 |
| Systolic blood pressure, per mm Hg | 1.02 (1.01-1.03) | .003 | NA | NA |
| Diabetes | 1.38 (0.76-2.50) | .29 | 2.95 (1.26-6.90) | .01 |
| Dyslipidemia | 0.58 (0.34-1.00) | .051 | NA | NA |
| Chronic obstructive pulmonary disease | 2.56 (1.19-5.48) | .02 | NA | NA |
| Peak aortic velocity ≥5 m/s | 2.05 (1.01-4.16) | .046 | 6.31 (2.51-15.9) | <.001 |
| LVEF <60% | 5.01 (2.93-8.57) | <.001 | 4.47 (2.06-9.70) | <.001 |
Abbreviations: HR, hazard ratio; LVEF, left ventricular ejection fraction; NA, not applicable.
Figure 2. Kaplan-Meier Estimates for Events in Patients With Severe Aortic Stenosis According to Left Ventricular Ejection Fraction (LVEF) and Peak Aortic Jet Velocity.
Kaplan-Meier analyses of overall survival in patients with severe aortic stenosis at baseline as a function of peak aortic jet velocity (A) and LVEF (B).
aSignificant difference with peak aortic jet velocity of 5 m/s or greater.
bSignificant difference with LVEF less than 60%.
cSignificant difference with LVEF of 60% to 65%.
Post-AVR Outcomes
Thirty-day mortality following AVR was very low (n = 13 [0.9%]; SAVR, 7; TAVR, 6). During follow-up, a total of 69 patients who underwent AVR died (SAVR, 49; TAVR, 20), including 22 from a cardiovascular cause, of which 17 were from heart failure and 2 were from sudden death. The mean (SD) 2-year, 4-year, and 6-year postprocedural overall survival rates were 83% (2%), 75% (4%), and 68% (6%), respectively. Patients with severe AS at baseline and peak aortic velocity greater than 5 m/s had significantly lower mean (SD) postoperative survival rates than those with peak aortic velocity less than 5 m/s (2 years: 73% [8%] vs 84% [2%]; 4 years: 65% [10%] vs 78% [4%]; 6 years: 54% [13%] vs 70% [6%]; P = .03). Similarly, patients with severe AS at entry with reduced baseline LVEF less than 60% also had lower mean (SD) postoperative survival rates than those with baseline LVEF of 60% or greater (2 years: 67% [7%] vs 87% [5%]; 4 years: 63% [8%] vs 78% [4%]; 6 years: 63% [8%] vs 69% [7%]; P = .02). In multivariable analysis, age (hazard ratio [HR], 1.03; 95% CI, 1.01-1.06; P = .003), diabetes (HR, 2.62; 95% CI, 1.90-4.95; P = .003), dyslipidemia (HR, 0.2; 95% CI, 0.10-0.37; P < .001), and peak aortic velocity greater than 5 m/s (HR, 2.20; 95% CI, 1.16-4.18; P = .02) were independently associated with postoperative survival. Of note, LVEF less than 60% was not associated with reduced postoperative survival in multivariable analysis.
Discussion
The management of patients with asymptomatic AS has continued to challenge clinicians.6,21 A randomized clinical trial (Evaluation of Transcatheter Aortic Valve Replacement Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis; NCT03042104) has been initiated to compare outcomes of asymptomatic patients with severe AS who are randomized to transfemoral TAVR vs clinical and echocardiographic follow-up (ie, active surveillance). To our knowledge, a randomized surgical trial has not been performed, and current practice patterns vary widely. In the present registry, for patients with asymptomatic moderate or severe AS and preserved LVEF greater than 50% at baseline followed up in heart valve clinics over the intermediate term, the mean 2-year and 4-year overall survival rates under medical management were 93% and 86%, respectively. The crude rate of sudden death over the follow-up interval was low (0.65%) and represented approximately one-tenth of all cardiovascular deaths.
In patients with severe AS at entry, age, systolic blood pressure level, comorbidities (eg, chronic obstructive pulmonary disease), peak aortic jet velocity greater than 5 m/s, and LVEF less than 60% were associated with all-cause mortality. Age, peak aortic jet velocity of 5 m/s or greater, and LVEF less than 60% were also independently associated with cardiovascular death.
During follow-up, 34% of patients required AVR, and this rate rose to 59% at 4 years. Most AVRs were dictated by a class I indication (ie, symptom development), and most cardiovascular deaths were related to heart failure. The 30-day mortality following AVR in this series was very low (0.9%). After AVR, the negative effect of peak aortic jet velocity remained significant, while LVEF less than 60% was no longer associated with cardiovascular death. Interestingly, in patients with moderate AS at entry who progressed to severe AS and were referred for AVR, the baseline variables predicting worse outcomes were directionally similar (peak aortic jet velocity of 3.0 m/s or greater and LVEF less than 60%). Two of 3 patients with moderate AS at entry who had sudden cardiac death during follow-up had confirmed severe AS on surveillance echocardiography.
Approximately one-half of patients diagnosed with moderate or severe AS do not report symptoms.8,15 The clinically silent phase of severe AS is associated with a risk of sudden death ranging from 0.25% to 1.7% per year.18,19,25 Given the current low periprocedural mortality rates for SAVR and transfemoral TAVR, earlier intervention has been advocated, and to our knowledge, the current strategy of watchful waiting has not been examined in a large cohort of patients with asymptomatic moderate or severe AS monitored in specialized heart valve clinics. Delay in reporting symptoms is common in patients with AS.12 Considering an annual mortality rate of approximately 30% for patients with severe AS, once symptoms develop, early recognition of symptoms and timely referral to intervention are critical.3,4 It has been shown that when patients are regularly followed up within a heart valve clinic program, symptoms are recognized at an earlier and less severe stage, thus optimizing timing of AVR.12,26 Compared with previous studies, the low rate of sudden death, the good overall midterm survival rates, and the very low rate of 30-day mortality following AVR observed in the HAVEC registry likely reflect appropriate monitoring, planning, and high adherence to guidelines.13,14,15,16,17,18 However, our data highlight the need for additional efforts with probably closer follow-up in these patients, since the occurrence of overt heart failure remains a significant problem even in heart valve centers of excellence.
Comorbidities are frequent in elderly individuals with AS, and AS increases the mortality from myocardial infarction, stroke, trauma, or emergency noncardiac surgery.27,28,29,30,31 The HAVEC registry data highlighted that age and chronic obstructive pulmonary disease significantly worsen patients’ prognosis. Age has not been consistently reported as an outcome predictor in the literature. However, many older adults with severe AS are not candidates for surgical AVR because of high surgical risk, advanced age, frailty, or comorbid conditions.32 Some complications after transcatheter AVR (eg, vascular injuries) are more common in very elderly patients.3,4
Although supportive data are limited, an LVEF less than 50% is considered the appropriate threshold for defining LV systolic dysfunction in AS.5,6,33 In the HAVEC registry, patients with EF between 50% and 59% had less favorable outcomes and experienced more heart failure–related deaths than those with EF greater than 60%. These data reinforce observations from previous retrospective studies33,34 and provide support for adjusting the cutoff for LVEF (less than 60% instead of less than 50%) to define dysfunction and consider AVR in asymptomatic severe AS.
Despite limited evidence with a class IIa indication, asymptomatic patients with very severe AS (peak aortic jet velocity greater than 5 to 5.5 m/s) are often referred for AVR.5,6,14 Peak aortic jet velocity is recorded directly with the use of continuous Doppler interrogation and, unlike AVA, does not require calculations and has high reproducibility. Peak aortic jet velocity is a robust prognostic parameter in AS, with increasingly worse outcome from patients with mild to very severe (greater than 5 m/s) stenosis.14,15,19 This gradual effect of stenosis severity was challenged in a 2015 large multicenter retrospective Japanese study.10 However, the main limitations of this study were the inclusion of patients with LVEF less than 50% and the absence of standardized follow-up and treatment strategy. By contrast, the HAVEC registry confirmed previous observations regarding stenosis severity in a very large population of patients with asymptomatic AS evaluated and monitored in heart valve clinics. In fact, very severe obstruction (peak aortic jet velocity of 5 m/s or greater) was predictive of all-cause mortality and cardiovascular death regardless of treatment strategy in asymptomatic patients with AS.14 Although AVA encompassed a broad range of values from 0.3 to 1.50 cm2, it was also associated with outcomes in these patients (eFigure 3 in Supplement 1). An AVA less than 0.8 cm2 was associated with markedly increased risk of all-cause and cardiovascular mortality.
Limitations
Our study had limitations. Data from a centralized database (clinical and echocardiographic data at baseline and clinical data at follow-up) were obtained from each center. However, because of incomplete echocardiographic data at baseline and/or during follow-up, a total of 22% of the initially included patients were not included in the final study analysis. Follow-up echocardiographic data were also collected from all patients who underwent AVR to confirm the progression from moderate to severe AS (ie, AVA greater than 1 cm2). However, in the context of this study, we did not collect the echocardiographic parameters of AS severity and LV function at follow-up visits. This precluded the analysis of the rate of progression from moderate to severe AS. Although exercise testing was commonly performed (572 patients), some patients were considered asymptomatic based solely on questionnaire on symptom status (not available in all centers). The assessment of myocardial strain, which could identify patients with subclinical LV dysfunction,20 was not systematically performed. The reasons for which symptomatic patients died under medical management could not be ascertained.
Conclusions
This study shows that asymptomatic patients with severe AS followed up in heart valve clinics have a low risk of sudden death and good midterm survival. Asymptomatic patients with very severe AS (peak aortic jet velocity of 5 m/s or greater) or with LVEF less than 60% have higher all-cause and cardiovascular mortality even after successful AVR. These findings provide support for consideration of early elective AVR in these patients. Closer and more frequent (every 6 to 12 months) clinical and echocardiographic follow-up might be implemented in patients with moderate AS and a peak aortic jet velocity of 3.0 m/s or greater or LVEF less than 60%.
eTable 1. Number, follow-up duration, and clinical outcomes of patients by participating center.
eTable 2. Multivariable predictors of mortality (aortic valve replacement censored) with echocardiographic data as continuous and categorical variables in the entire population.
eTable 3. Multivariate analysis of cardiovascular mortality in patients with severe aortic stenosis with substitution of peak aortic velocity by aortic valve area (categorical data).
eTable 4. Baseline comparison between survivors and nonsurvivors in the moderate aortic stenosis group.
eFigure 1. Kaplan-Meier curves of overall survival (A) and freedom of cardiovascular mortality (B) in patients with moderate aortic stenosis.
eFigure 2. Kaplan-Meier curves of freedom of aortic valve replacement for moderate aortic stenosis.
eFigure 3. Kaplan-Meier curves overall survival (left) and freedom of cardiovascular-related mortality (right) as a function of aortic valve area (AVA).
Data Sharing Statement.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005-1011. doi: 10.1016/S0140-6736(06)69208-8 [DOI] [PubMed] [Google Scholar]
- 2.Rossebø AB, Pedersen TR, Boman K, et al. ; SEAS Investigators . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343-1356. doi: 10.1056/NEJMoa0804602 [DOI] [PubMed] [Google Scholar]
- 3.Kodali SK, Williams MR, Smith CR, et al. ; PARTNER Trial Investigators . Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686-1695. doi: 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 4.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Falk V, Bax JJ, et al. ; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739-2791. doi: 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(25):e1159-e1195. doi: 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 7.Lim WY, Ramasamy A, Lloyd G, Bhattacharyya S. Meta-analysis of the impact of intervention versus symptom-driven management in asymptomatic severe aortic stenosis. Heart. 2017;103(4):268-272. doi: 10.1136/heartjnl-2016-309830 [DOI] [PubMed] [Google Scholar]
- 8.Généreux P, Stone GW, O’Gara PT, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2016;67(19):2263-2288. doi: 10.1016/j.jacc.2016.02.057 [DOI] [PubMed] [Google Scholar]
- 9.Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121(13):1502-1509. doi: 10.1161/CIRCULATIONAHA.109.909903 [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi T, Morimoto T, Shiomi H, et al. ; CURRENT AS Registry Investigators . Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66(25):2827-2838. doi: 10.1016/j.jacc.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Smith CR, Mack MJ, et al. ; PARTNER 2 Investigators . Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 12.Zilberszac R, Lancellotti P, Gilon D, et al. Role of a heart valve clinic programme in the management of patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2017;18(2):138-144. doi: 10.1093/ehjci/jew133 [DOI] [PubMed] [Google Scholar]
- 13.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343(9):611-617. doi: 10.1056/NEJM200008313430903 [DOI] [PubMed] [Google Scholar]
- 14.Rosenhek R, Zilberszac R, Schemper M, et al. Natural history of very severe aortic stenosis. Circulation. 2010;121(1):151-156. doi: 10.1161/CIRCULATIONAHA.109.894170 [DOI] [PubMed] [Google Scholar]
- 15.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111(24):3290-3295. doi: 10.1161/CIRCULATIONAHA.104.495903 [DOI] [PubMed] [Google Scholar]
- 16.Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59(3):235-243. doi: 10.1016/j.jacc.2011.08.072 [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P, Magne J, Donal E, et al. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation. 2012;126(7):851-859. doi: 10.1161/CIRCULATIONAHA.111.088427 [DOI] [PubMed] [Google Scholar]
- 18.Monin JL, Lancellotti P, Monchi M, et al. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120(1):69-75. doi: 10.1161/CIRCULATIONAHA.108.808857 [DOI] [PubMed] [Google Scholar]
- 19.Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis: clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95(9):2262-2270. doi: 10.1161/01.CIR.95.9.2262 [DOI] [PubMed] [Google Scholar]
- 20.Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96(17):1364-1371. doi: 10.1136/hrt.2009.190942 [DOI] [PubMed] [Google Scholar]
- 21.Lancellotti P, Rosenhek R, Pibarot P, et al. ESC Working Group on Valvular Heart Disease position paper—heart valve clinics: organization, structure, and experiences. Eur Heart J. 2013;34(21):1597-1606. doi: 10.1093/eurheartj/ehs443 [DOI] [PubMed] [Google Scholar]
- 22.Dulgheru R, Pibarot P, Sengupta PP, et al. Multimodality imaging strategies for the assessment of aortic stenosis: viewpoint of the Heart Valve Clinic International Database (HAVEC) Group. Circ Cardiovasc Imaging. 2016;9(2):e004352. doi: 10.1161/CIRCIMAGING.115.004352 [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 24.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60(15):1438-1454. doi: 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya S, Hayward C, Pepper J, Senior R. Risk stratification in asymptomatic severe aortic stenosis: a critical appraisal. Eur Heart J. 2012;33(19):2377-2387. doi: 10.1093/eurheartj/ehs190 [DOI] [PubMed] [Google Scholar]
- 26.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231-1243. doi: 10.1016/S0195-668X(03)00201-X [DOI] [PubMed] [Google Scholar]
- 27.Arnold SV, Spertus JA, Vemulapalli S, et al. Quality-of-life outcomes after transcatheter aortic valve replacement in an unselected population: a report from the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2(4):409-416. doi: 10.1001/jamacardio.2016.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindman BR, Clavel MA, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otto CM, Prendergast B. Aortic-valve stenosis: from patients at risk to severe valve obstruction. N Engl J Med. 2014;371(8):744-756. doi: 10.1056/NEJMra1313875 [DOI] [PubMed] [Google Scholar]
- 30.Berry C, Lloyd SM, Wang Y, Macdonald A, Ford I. The changing course of aortic valve disease in Scotland: temporal trends in hospitalizations and mortality and prognostic importance of aortic stenosis. Eur Heart J. 2013;34(21):1538-1547. doi: 10.1093/eurheartj/ehs339 [DOI] [PubMed] [Google Scholar]
- 31.Tashiro T, Pislaru SV, Blustin JM, et al. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice. Eur Heart J. 2014;35(35):2372-2381. doi: 10.1093/eurheartj/ehu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26(24):2714-2720. doi: 10.1093/eurheartj/ehi471 [DOI] [PubMed] [Google Scholar]
- 33.Capoulade R, Clavel MA, Le Ven F, et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2017;18(12):1378-1387. doi: 10.1093/ehjci/jew288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8(4):e002917. doi: 10.1161/CIRCIMAGING.114.002917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number, follow-up duration, and clinical outcomes of patients by participating center.
eTable 2. Multivariable predictors of mortality (aortic valve replacement censored) with echocardiographic data as continuous and categorical variables in the entire population.
eTable 3. Multivariate analysis of cardiovascular mortality in patients with severe aortic stenosis with substitution of peak aortic velocity by aortic valve area (categorical data).
eTable 4. Baseline comparison between survivors and nonsurvivors in the moderate aortic stenosis group.
eFigure 1. Kaplan-Meier curves of overall survival (A) and freedom of cardiovascular mortality (B) in patients with moderate aortic stenosis.
eFigure 2. Kaplan-Meier curves of freedom of aortic valve replacement for moderate aortic stenosis.
eFigure 3. Kaplan-Meier curves overall survival (left) and freedom of cardiovascular-related mortality (right) as a function of aortic valve area (AVA).
Data Sharing Statement.