Key Points
Question
For extremely preterm infants, is targeting a lower oxygen saturation (85%-89%) compared with a higher saturation (91%-95%) associated with a difference in death or major disability by a corrected age of 24 months?
Findings
In a prospectively designed meta-analysis of individual participant data from 4965 infants in 5 randomized clinical trials, there was no significant difference in the primary composite outcome of death or major disability between those treated with lower vs higher oxygen saturations (53.5% vs 51.6%, respectively). Lower oxygen targets were associated with increased death and necrotizing enterocolitis but reduced retinopathy of prematurity treatment.
Meaning
Among extremely preterm infants, there was no significant difference between lower and higher oxygen saturation targets on a composite of death or major disability; secondary end points may need to be considered in decision making.
Abstract
Importance
There are potential benefits and harms of hyperoxemia and hypoxemia for extremely preterm infants receiving more vs less supplemental oxygen.
Objective
To compare the effects of different target ranges for oxygen saturation as measured by pulse oximetry (Spo2) on death or major morbidity.
Design, Setting, and Participants
Prospectively planned meta-analysis of individual participant data from 5 randomized clinical trials (conducted from 2005-2014) enrolling infants born before 28 weeks’ gestation.
Exposures
Spo2 target range that was lower (85%-89%) vs higher (91%-95%).
Main Outcomes and Measures
The primary outcome was a composite of death or major disability (bilateral blindness, deafness, cerebral palsy diagnosed as ≥2 level on the Gross Motor Function Classification System, or Bayley-III cognitive or language score <85) at a corrected age of 18 to 24 months. There were 16 secondary outcomes including the components of the primary outcome and other major morbidities.
Results
A total of 4965 infants were randomized (2480 to the lower Spo2 target range and 2485 to the higher Spo2 range) and had a median gestational age of 26 weeks (interquartile range, 25-27 weeks) and a mean birth weight of 832 g (SD, 190 g). The primary outcome occurred in 1191 of 2228 infants (53.5%) in the lower Spo2 target group and 1150 of 2229 infants (51.6%) in the higher Spo2 target group (risk difference, 1.7% [95% CI, −1.3% to 4.6%]; relative risk [RR], 1.04 [95% CI, 0.98 to 1.09], P = .21). Of the 16 secondary outcomes, 11 were null, 2 significantly favored the lower Spo2 target group, and 3 significantly favored the higher Spo2 target group. Death occurred in 484 of 2433 infants (19.9%) in the lower Spo2 target group and 418 of 2440 infants (17.1%) in the higher Spo2 target group (risk difference, 2.8% [95% CI, 0.6% to 5.0%]; RR, 1.17 [95% CI, 1.04 to 1.31], P = .01). Treatment for retinopathy of prematurity was administered to 220 of 2020 infants (10.9%) in the lower Spo2 target group and 308 of 2065 infants (14.9%) in the higher Spo2 target group (risk difference, −4.0% [95% CI, −6.1% to −2.0%]; RR, 0.74 [95% CI, 0.63 to 0.86], P < .001). Severe necrotizing enterocolitis occurred in 227 of 2464 infants (9.2%) in the lower Spo2 target group and 170 of 2465 infants (6.9%) in the higher Spo2 target group (risk difference, 2.3% [95% CI, 0.8% to 3.8%]; RR, 1.33 [95% CI, 1.10 to 1.61], P = .003).
Conclusions and Relevance
In this prospectively planned meta-analysis of individual participant data from extremely preterm infants, there was no significant difference between a lower Spo2 target range compared with a higher Spo2 target range on the primary composite outcome of death or major disability at a corrected age of 18 to 24 months. The lower Spo2 target range was associated with a higher risk of death and necrotizing enterocolitis, but a lower risk of retinopathy of prematurity treatment.
This meta-analysis uses individual participant data from 5 randomized clinical trials to compare the effects of lower vs higher oxygen saturation target ranges on death or major morbidity among infants born before 28 weeks’ gestation.
Introduction
Oxygen has been used in nurseries for more than 70 years. In the 1950s, it was shown that administering unrestricted oxygen to preterm infants significantly increased their risk of severe retinopathy of prematurity (ROP).1 Oxygen saturation as measured by pulse oximetry (Spo2), which is a noninvasive measure, is now almost universal in neonatal intensive care units. Lower oxygen levels (Spo2 target ≤90%) may reduce ROP,2 but no studies predating these investigations1,3 demonstrated impaired neurodevelopment or an increased risk of death. Higher oxygen levels (Spo2 target >90%) may increase adverse pulmonary sequelae at Spo2 levels higher than 95% in infants who remain dependent on oxygen for many weeks after birth.4,5
A total sample size of approximately 5000 infants was required to detect the small but clinically important hypothesized difference of 4% in the primary outcome of death or major disability between lower and higher Spo2 target ranges. To achieve this, the Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration6 was formed in 2003 with the investigators from 5 separate randomized clinical trials prospectively planning to undertake their individual trials using similar study designs, participants, interventions, comparators and outcomes, and agreeing to provide individual participant data at trial completion for inclusion in a meta-analysis. A previous Cochrane review7 reported the findings of an analysis of these 5 studies using aggregate data available from the published trial reports. This article reports the results from the prospectively planned meta-analysis of the individual participant data from these trials.
Methods
Data Sources and Search Strategy
The NeOProM Collaboration was a prospectively planned meta-analysis of individual participant data for the following 5 trials: the Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial (SUPPORT),8 which was conducted from 2005 through 2011 in the United States; the Canadian Oxygen Trial,9 which was conducted from 2006 through 2012; the Benefits Of Oxygen Saturation Targeting (BOOST) in New Zealand,10 which was conducted from 2006 through 2012; BOOST II in the United Kingdom,11 which was conducted from 2007 through 2014; and BOOST II in Australia,12 which was conducted from 2006 through 2013. These studies were considered eligible for inclusion in the meta-analysis prior to the results of any of the trials being known.13 The study protocol was published14 (Supplement 1) in January 2011 and registered on ClinicalTrials.gov. The statistical analysis plan was finalized in September 2015 and appears in Supplement 2. The conduct of each trial was approved by institutional review boards or ethics committees and written informed consent was obtained from participating parents.
Study Selection and Eligibility Criteria
All 5 studies15,16,17,18,19,20 were randomized, double-blind, multicenter trials with infants eligible if they were born before 28 weeks’ gestation and enrolled within 24 hours of birth. Infants were randomized within each trial to target either a lower (85%-89%) or higher (91%-95%) Spo2 range. To ensure that parents, caregivers, and outcome assessors remained masked to treatment allocation, each trial used Masimo pulse oximeters that had been modified to display and store oxygen saturations between 88% and 92% that were either 3% above or 3% below the actual values. True values were displayed if the actual Spo2 decreased below 84% or increased above 96%. Caregivers were instructed to adjust the concentration of inspired oxygen to maintain the displayed Spo2 between 88% and 92%, thus producing 2 treatment groups with actual target saturations of either 85% to 89% or 91% to 95% (eFigure 1 in Supplement 3).
During the trials, an artifact was identified in the calibration software of the oximeters that had the potential to influence the achieved oxygen saturation patterns.21 Three of the trials (BOOST II in the United Kingdom, BOOST II in Australia, and the Canadian Oxygen Trial) changed their oximeters to incorporate the revised oximeter software. Based on advice from their data and safety monitoring committees, 2 trials (BOOST II in the United Kingdom and BOOST II in Australia) were terminated by their respective trial steering committees after a pooled interim analysis of mortality data was undertaken22 in subgroups by oximeter software type when 81% and 95%, respectively, of their planned trial recruitment sample sizes had been met.
Data Extraction
A list of requested variables was sent to each trial group based on the statistical analysis plan prior to the sharing of any individual participant data for use in the combined meta-analysis. These variables included randomization and baseline characteristics (including subgroup variables) while infants were in the hospital as well as 18- to 24-month follow-up information from individual participants (a full list of prespecified variables appear in Supplement 3). Deidentified data were provided by the trial groups between March and April 2016. Data were checked for accuracy with published reports, trial protocols, and data collection sheets. Inconsistencies were discussed with individual investigators and discrepancies were resolved by consensus. Each trial verified its own finalized data set prior to inclusion in the study database. Data from the 5 included trials were collected and synthesized centrally after publication of the main results from each trial.
Key Outcome Definitions
The primary outcome was a composite of death or major disability at a corrected age of 18 to 24 months. Major disability comprised any of the following: Bayley Scales of Infant and Toddler Development version 3 (Bayley-III)23 cognitive or language score of less than 85; severe visual loss (cannot fixate or is legally blind with visual acuity <6/60 in both eyes); cerebral palsy with the Gross Motor Function Classification System level 2 or higher24; or deafness requiring hearing aids. When a Bayley-III assessment was unavailable, some trials used alternative sources of information for classifying cognitive delay such as a Bayley-II Mental Developmental Index score of less than 70 or another validated assessment tool (eg, Griffiths test), a pediatric assessment, or a parent-reported measure of neurodevelopmental impairment (eg, able to speak <5-10 words). To assess the statistical effects of inclusion of these alternate measures of disability, a prespecified supportive analysis of the primary outcome also was undertaken (Figure 1 and Supplement 3).
Figure 1. Participant Flow Diagram.
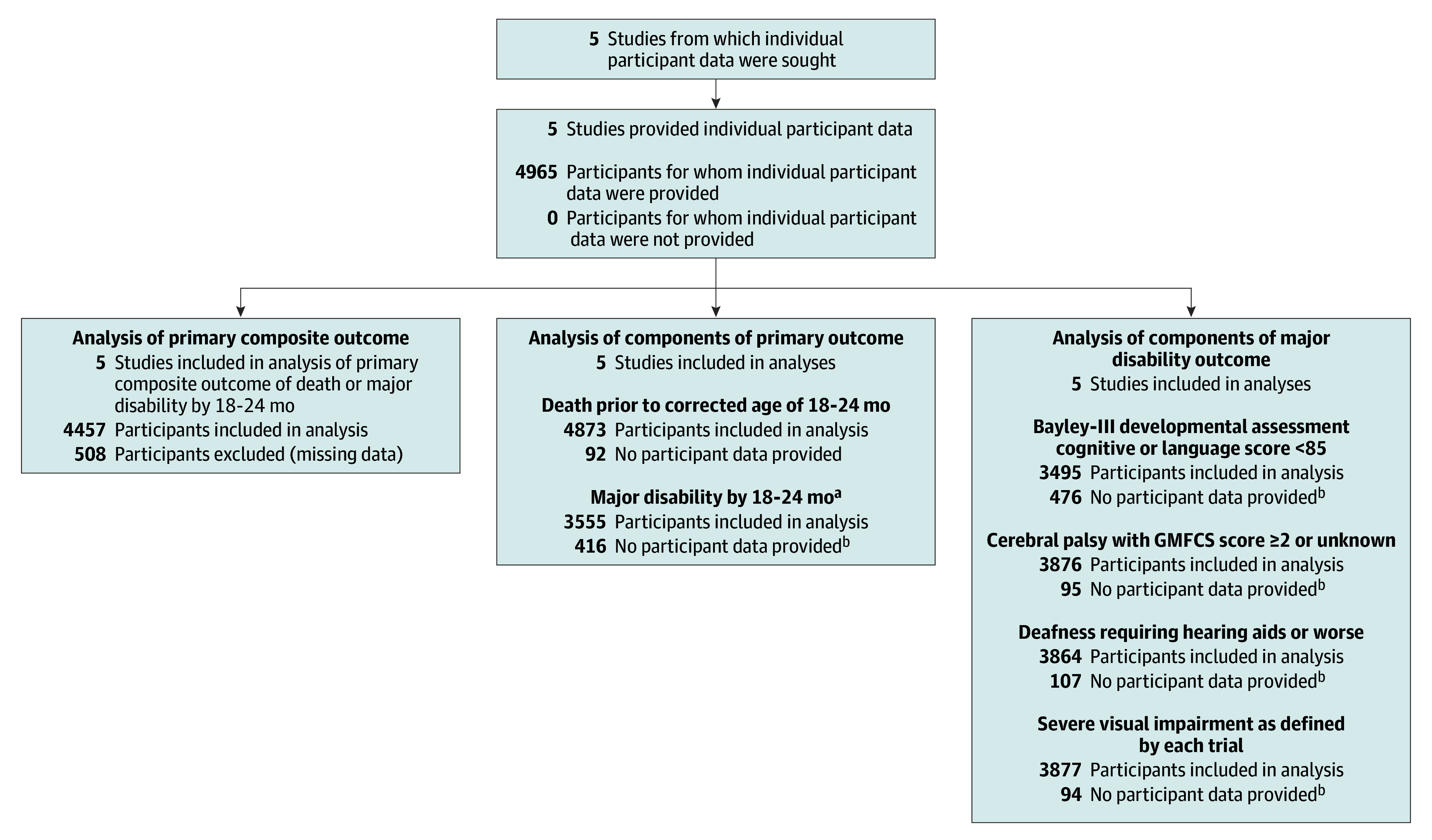
aMajor disability was prespecified (published in the Neonatal Oxygenation Prospective Meta-analysis protocol; Supplement 1) and includes any of the following: Bayley-III developmental assessment cognitive score of less than 85, language score of less than 85, or both; severe visual impairment; cerebral palsy with Gross Motor Function Classification System (GMFCS)23 level 2 or higher, at age 18 to 24 months corrected for prematurity; or deafness requiring hearing aids.
bThe maximum number of infants available for major disability assessment at 18 to 24 months was 3971 because 902 infants were known to have died prior to the age of 18 to 24 months. There were an additional 92 infants with unknown death status at this time point who could not be assessed for major disability outcomes.
Secondary outcomes were the components of the primary outcome (death prior to corrected age of 24 months and major disability); death prior to postmenstrual age of 36 weeks; death prior to hospital discharge; the individual components of the major disability outcome (developmental delay, severe visual impairment, deafness, cerebral palsy); ROP treated by laser photocoagulation, cryotherapy, or antivascular endothelial growth factor injection in 1 or both eyes; severe necrotizing enterocolitis leading to abdominal surgery or death; oxygen treatment at postmenstrual age of 36 weeks; postmenstrual age when each of the following respiratory support measures ceased: endotracheal intubation, continuous positive airway pressure, oxygen treatment, or home oxygen (if received); patent ductus arteriosus (PDA) diagnosed by ultrasound and receiving any treatment; PDA receiving surgical treatment; z scores for infant body weight at postmenstrual age of 36 weeks, at hospital discharge, and at corrected age of 18 to 24 months; 1 or more readmissions to the hospital by corrected age of 18 to 24 months; and time to death.
Assessing the Risk of Bias
The 5 trials were assessed for risk of bias using the Cochrane Collaboration domains25 and consensus was reached via discussion with the full study group.
Statistical Analysis
The preplanned total sample size was 5230 infants. Because 2 of the trials were stopped early, a meta-analysis of individual participant data was undertaken of the 4965 infants recruited overall, which provided approximately 80% power (with a 2-sided P value of .05) to detect a minimum absolute risk difference of 4% in the primary composite outcome of death or major disability by a corrected age of 18 to 24 months, corresponding to a minimally important number needed to treat of 25 infants to prevent 1 major adverse outcome.14 This minimal difference was derived via discussion with clinical experts, and no formal assessments were undertaken.
The analysis was performed on an intention-to-treat basis using all data from each trial included in a single model. The I2 statistic26 was used to assess heterogeneity for all primary and secondary outcomes. No statistical methods were used to deal with the small proportion of missing data, but sensitivity analyses were undertaken for the primary outcome by using alternative measures of disability when Bayley-III outcomes were missing. Binary end points were analyzed using log binomial regression in a generalized estimating equations model with an exchangeable correlation structure to account for multiple births. Models were adjusted for trial as a fixed effect because the methods used for the prospective meta-analysis meant all 5 trials were very similar with respect to their included participants, interventions, and outcome definitions. Sensitivity analyses using random-effects models also were undertaken.
The results are presented as risk differences and relative risks (RRs) with 95% CIs and 2-sided P values. If these models failed to converge, Poisson models with a robust variance estimator were used. Continuous outcomes were analyzed using linear regression in models for generalized estimating equations and presented as mean differences. Time to death was assessed between treatment groups using proportional hazard models and displayed using Kaplan-Meier survival curves.27
Relative risks and hazard ratios were computed such that values greater than 1 favored the higher target group. Subgroup analyses for gestational age (<26 weeks vs ≥26 weeks), inborn (indicates infant was born in the treating center) or outborn, use of any antenatal corticosteroids, sex, small for gestational age (SGA; <10th percentile using either the prespecified charts from Kramer et al28 or the post hoc curves from Alexander et al29 as in the SUPPORT trial30), multiple birth, type of delivery (vaginal or cesarean), time of intervention commencement (<6 hours vs ≥6 hours after birth), and type of oximeter software (original vs revised) were prespecified and performed for primary and secondary outcomes by including a treatment × subgroup interaction term in the model.
Two-sided P values of less than .05 were considered to indicate statistical significance, with no adjustment for multiple comparisons. Therefore, because of the potential for type I error, the prespecified secondary outcomes and the subgroup analyses should be considered exploratory. The statistical analyses were performed using SAS version 9.3 (SAS Institute Inc).
Results
Study Identification and Selection
Characteristics of the 5 studies appear in Supplement 1 and in eTable 1 in Supplement 3. Individual participant data from 4965 infants (2480 randomized to the lower and 2485 to the higher Spo2 target range), with a median gestational age of 26 weeks (interquartile range, 25-27 weeks) and a mean birthweight of 832 g (SD, 190 g) were included in the meta-analysis. Baseline characteristics of each of the included trials and the combined data appear in the Table. Data were available for 90% of infants for the protocol-defined primary outcome and for 96% of infants for the prespecified supportive analysis of the primary outcome, which used alternate measures of cognitive disability (Figure 1).
Table. Baseline Characteristicsa.
| SUPPORT15,16 (n = 1316) |
COT17 (n = 1201) |
BOOST NZ18 (n = 340) |
BOOST II UK19,20 (n = 973) |
BOOST II AUS19,20 (n = 1135) |
Spo2 Target | ||
|---|---|---|---|---|---|---|---|
| Lower (n = 2480) |
Higher (n = 2485) |
||||||
| Mothers at Birth | |||||||
| Use of antenatal corticosteroids, No. (%) | |||||||
| None | 50 (3.8) | 131 (10.9) | 38 (11.2) | 88 (9.0) | 106 (9.3) | 215 (8.7) | 198 (8.0) |
| Partial courseb | 326 (24.8) | 259 (21.6) | 89 (26.2) | 272 (28.0) | 293 (25.8) | 609 (24.6) | 630 (25.4) |
| Full course | 939 (71.4) | 807 (67.4) | 213 (62.6) | 607 (62.4) | 727 (64.1) | 1648 (66.5) | 1645 (66.3) |
| Type of delivery, No. (%) | |||||||
| Normal vaginal | 433 (32.9) | 462 (38.6) | 149 (43.8) | 593 (61.1) | 511 (45.0) | 1064 (43.0) | 1084 (43.7) |
| Instrumental vaginal | 0 | 3 (0.3) | 5 (1.5) | 0 | 18 (1.6) | 10 (0.4) | 16 (0.6) |
| Cesarean | 883 (67.1) | 732 (61.2) | 186 (54.7) | 378 (38.9) | 600 (52.9) | 1400 (56.5) | 1379 (55.5) |
| Infants at Birth | |||||||
| Birth weight, mean (SD), g | 830 (193) | 837 (193) | 879 (194) | 821 (185) | 825 (184) | 829 (187) | 836 (192) |
| Girls, No. (%) | 604 (45.9) | 546 (45.5) | 160 (47.1) | 456 (46.9) | 546 (48.1) | 1169 (47.1) | 1143 (46.0) |
| Gestational age, wk | |||||||
| Median (IQR) | 26.3 (25.3-27.1) |
26.0 (25.0-27.0) |
26.2 (25.2-27.0) |
26.1 (25.0-27.1) |
26.1 (25.1-27.0) |
26.0 (25.0-27.0) |
26.0 (25.0-27.0) |
| <26, No. (%) | 565 (42.9) | 512 (42.6) | 144 (42.4) | 431 (44.3) | 481 (42.4) | 1063 (42.9) | 1070 (43.1) |
| ≥26, No. (%) | 751 (57.1) | 689 (57.4) | 196 (57.6) | 542 (55.7) | 654 (57.6) | 1417 (57.1) | 1415 (56.9) |
| Small for gestational age, No. (%) | |||||||
| Defined by trial investigatorsc | 96 (7.3) | 105 (8.7) | 30 (8.8) | 147 (15.2) | 158 (13.9) | 267 (10.8) | 269 (10.8) |
| Defined by NeOProMd | 210 (16.0) | 105 (8.7) | 30 (8.8) | 113 (11.6) | 158 (13.9) | 302 (12.2) | 314 (12.6) |
| Apgar score at 5 min, median (IQR)e | 7 (6-8) | 7 (6-8) | 8 (6-9) | 7 (6-8) | 7 (6-8) | 7 (6-8) | |
| Admission temperature, mean (SD), °C | 36.2 (0.9) | 36.4 (0.9) | 36.4 (1.0) | 36.6 (0.9) | 36.0 (1.0) | 36.3 (1.0) | 36.3 (0.9) |
| Inborn, No. (%)f | 1316 (100) | 1105 (92.0) | 316 (92.9) | 854 (88.0) | 1049 (92.4) | 2327 (93.8) | 2313 (93.1) |
| Inspired oxygen concentration immediately prior to randomization, median (IQR), %e,g | 21 (21-25) | 21 (21-25) | 21 (21-24) | 21 (21-25) | 21 (21-25) | ||
| Infants at Randomization | |||||||
| Oximeter calibration software, No. (%) | |||||||
| Original | 1316 (100) | 564 (47.0) | 340 (100) | 228 (23.4) | 692 (61.0) | 1569 (63.3) | 1571 (63.2) |
| Revised | 0 | 563 (46.9) | 0 | 745 (76.6) | 443 (39.0) | 879 (35.4) | 872 (35.1) |
| Mixed | 0 | 74 (6.2) | 0 | 0 | 0 | 32 (1.3) | 42 (1.7) |
| Time intervention started <6 h, No. (%)e | 1283 (99.2) | 53 (4.4) | 56 (16.5) | 119 (10.5) | 752 (38.0) | 759 (38.3) | |
| Positive airway pressure, No. (%)e | |||||||
| With endotracheal tubeh | 835 (63.9) | 925 (77.0) | 230 (67.6) | 714 (63.0) | 1337 (67.3) | 1367 (68.5) | |
| Without endotracheal tubei | 449 (34.4) | 242 (20.1) | 109 (32.1) | 410 (36.2) | 621 (31.3) | 589 (29.5) | |
| Oxygen treatment without positive airway pressure, No. (%)e | 11 (0.8) | 3 (0.2) | 0 | 1 (0.1) | 9 (0.5) | 6 (0.3) | |
| No respiratory support, No. (%)e | 12 (0.9) | 31 (2.6) | 1 (0.3) | 9 (0.8) | 20 (1.0) | 33 (1.7) | |
Abbreviations: AUS, Australia; BOOST, Benefits Of Oxygen Saturation Targeting; COT, Canadian Oxygen Trial; IQR, interquartile range; NZ, New Zealand; Spo2, oxygen saturation as measured by pulse oximetry; SUPPORT, Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial; UK, United Kingdom.
Denominators include the total number of infants with a known outcome.
Mother did not receive the full 2 doses within 48 hours before birth.
Defined using trial-specific definitions.
Defined as less than the 10th percentile using charts from Kramer et al.28
Data were not available from BOOST II UK for this variable.
Indicates infant was born in the treating center.
Data were not available from SUPPORT for this variable.
Includes all forms of positive pressure ventilation.
Includes all other forms of respiratory support including continuous positive airway pressure and nasal cannula oxygen (high or low flow).
Primary Outcomes
There was no significant difference between a lower Spo2 target range (85%-89%) compared with a higher Spo2 target range (91%-95%) on the primary composite outcome of death or major disability at a corrected age of 18 to 24 months (53.5% with a lower Spo2 target vs 51.6% with a higher Spo2 target; risk difference, 1.7% [95% CI, −1.3% to 4.6%]; RR, 1.04 [95% CI, 0.98 to 1.09]; P = .21, I2 = 14%; Figure 2). A supportive analysis of the primary outcome, which included alternate measures of disability, also showed no significant between-group difference in the rate of death or major disability (51.2% with a lower Spo2 target vs 49.3% with a higher Spo2 target; risk difference, 1.7% [95% CI, −1.2% to 4.5%]; RR, 1.04 [95% CI, 0.98 to 1.09]; P = .20, I2 = 27%; Figure 2).
Figure 2. Effect of Oxygen Saturation as Measured by Pulse Oximetry (Spo2) Target Levels on Composite Primary Outcome of Death or Major Disability.
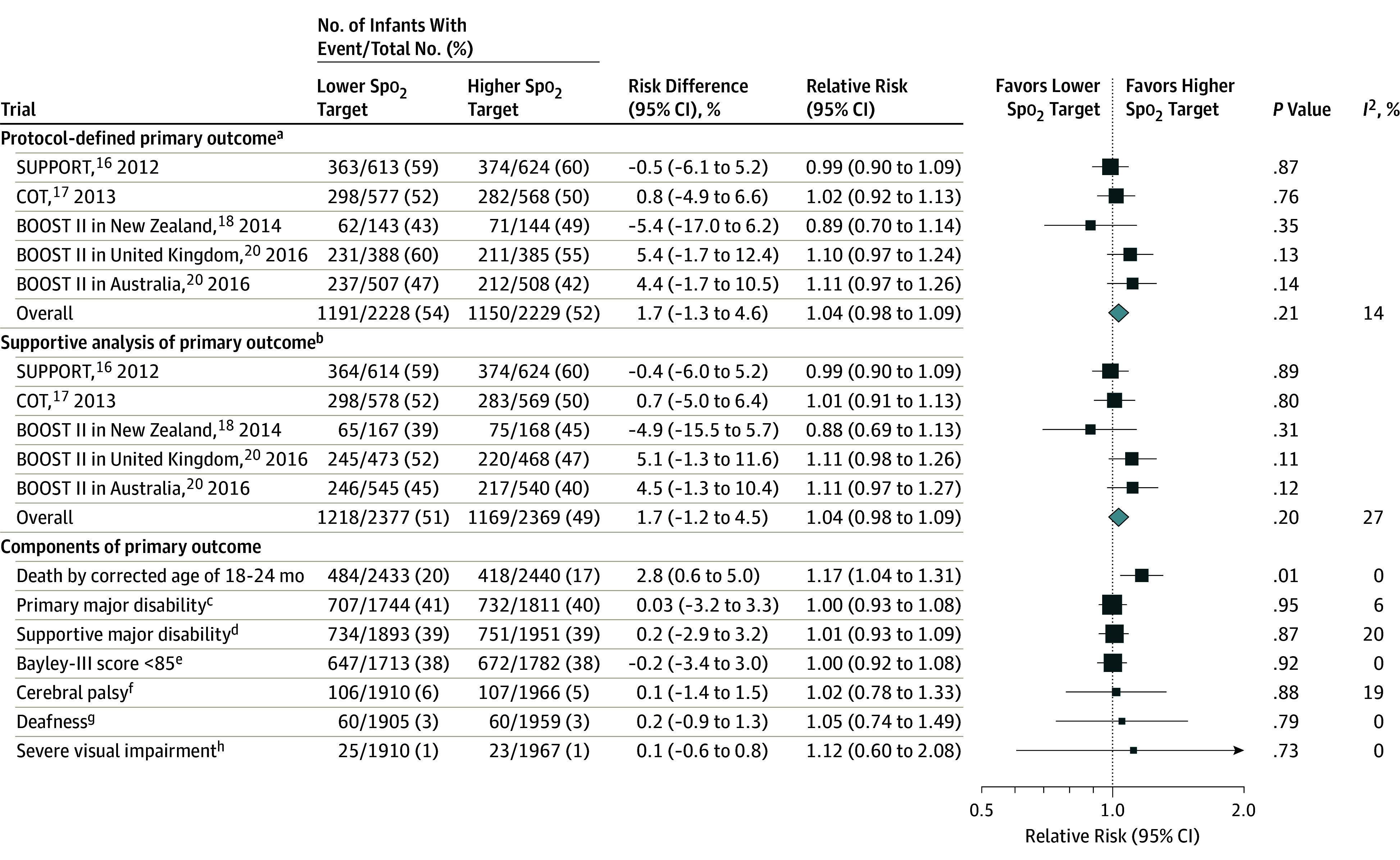
Box sizes correspond to precision; therefore, the more precise the larger the box. Precision was ascertained by calculating the inverse of the variance for each estimate.
aDefined as a composite outcome of death or major disability by the age of 18 to 24 months, which was corrected for prematurity and prespecified in the published Neonatal Oxygenation Prospective Meta-analysis protocol (Supplement 1).
bIncluded using alternative sources of information for classifying major disability as used within individual trials. This may have included a Bayley-II major disability score of less than 70, another validated assessment tool (eg, the Griffiths test), a pediatrician assessment, or parent-reported measure of neurodevelopmental impairment (eg, able to speak <5-10 words), or other measures.
cDefined per protocol.
dDefined using supplementary data as noted in the “b” footnote.
eDevelopmental assessment for cognition or language.
fDefined by Gross Motor Function Classification System23 level 2 or greater (higher levels = functioning more impaired) or cerebral palsy diagnosed but score unknown.
gRequiring hearing aids or worse.
hDefined by the trial investigators.
Secondary Outcomes
Of the 16 secondary outcomes, 11 were null, 2 significantly favored a lower Spo2 target, and 3 significantly favored a higher Spo2 target. An analysis of each component of the primary outcome (Figure 2) showed that the lower Spo2 target range was associated with a significantly increased incidence of death at a corrected age of 18 to 24 months (19.9% with a lower Spo2 target vs 17.1% with a higher Spo2 target; risk difference, 2.8% [95% CI, 0.6% to 5.0%]; RR, 1.17 [95% CI, 1.04 to 1.31]; P = .01, I2 = 0%), but not major disability or the components of major disability. The survival analysis also showed a significant increase in risk of death by a corrected age of 18 to 24 months for the lower target group (hazard ratio, 1.17 [95% CI, 1.03 to 1.34]; P = .02; eTable 2 and eFigure 2 in Supplement 3).
Other secondary outcome results appear in Figure 3. These results show infants in the lower target group had an increase in death at other time points (postmenstrual age of 36 weeks and at hospital discharge), severe necrotizing enterocolitis (9.2% with a lower Spo2 target vs 6.9% with a higher Spo2 target; risk difference, 2.3% [95% CI, 0.8% to 3.8%]; RR, 1.33 [95% CI, 1.10 to 1.61]; P = .003), and PDA treated with surgical ligation, but a lower rate of ROP treatment (10.9% with a lower Spo2 target vs 14.9% with a higher Spo2 target; risk difference, −4.0% [95% CI, −6.1% to 2.0%]; RR, 0.74 [95% CI, 0.63 to 0.86], P < .003) and oxygen treatment at a postmenstrual age of 36 weeks. There were no significant between-group differences for other secondary outcomes (Figure 2).
Figure 3. Effect of Oxygen Saturation as Measured by Pulse Oximetry (Spo2) Target Levels on Secondary Outcomes.
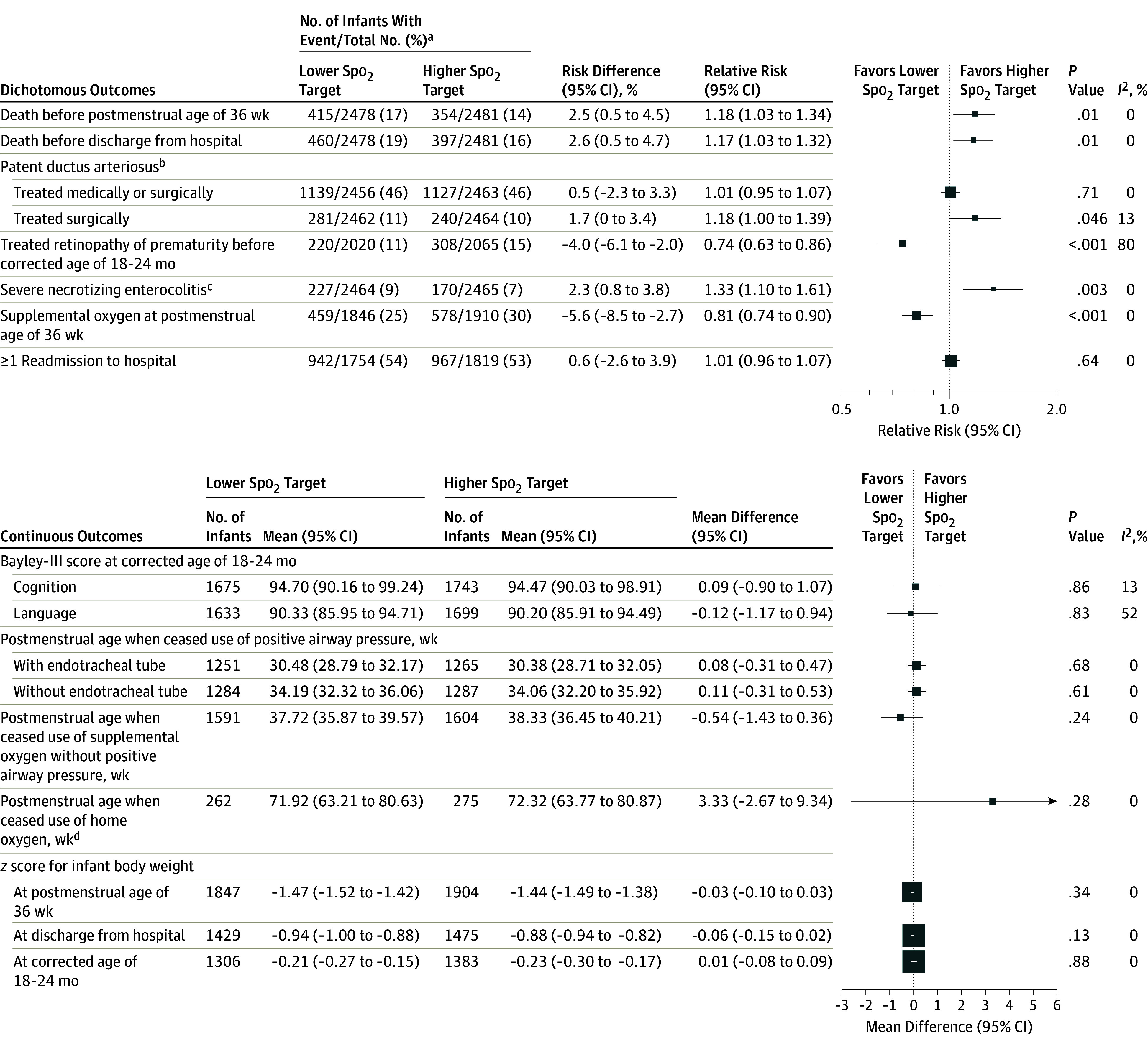
Box sizes correspond to precision; therefore, the more precise the larger the box. Precision was ascertained by calculating the inverse of the variance for each estimate.
aDenominators include the total number of infants with a known outcome.
bDiagnosed by ultrasound during initial hospitalization.
cTreated with surgery or leading to death during initial hospitalization.
dData on postmenstrual age when ceased use of home oxygen can only be calculated using the 537 infants who received home oxygen and for whom the postmenstrual age when ceased use is known.
Subgroup Analyses
There were no between-group differences for the primary outcome (death or major disability) for any of the prespecified subgroup analysis factors (gestational age, outborn, use of any antenatal corticosteroids, sex, SGA, multiple pregnancy, type of delivery, time intervention started, or oximeter software type; Figure 4). The number of prespecified subgroup analyses of secondary outcomes performed was large (n = 319; of which 17 [5%] were nominally significant), and the interaction P values were not formally adjusted for multiple subgroup comparisons and are thus considered exploratory.31
Figure 4. Subgroup Analyses of Primary Outcome Composite of Death or Major Disability.
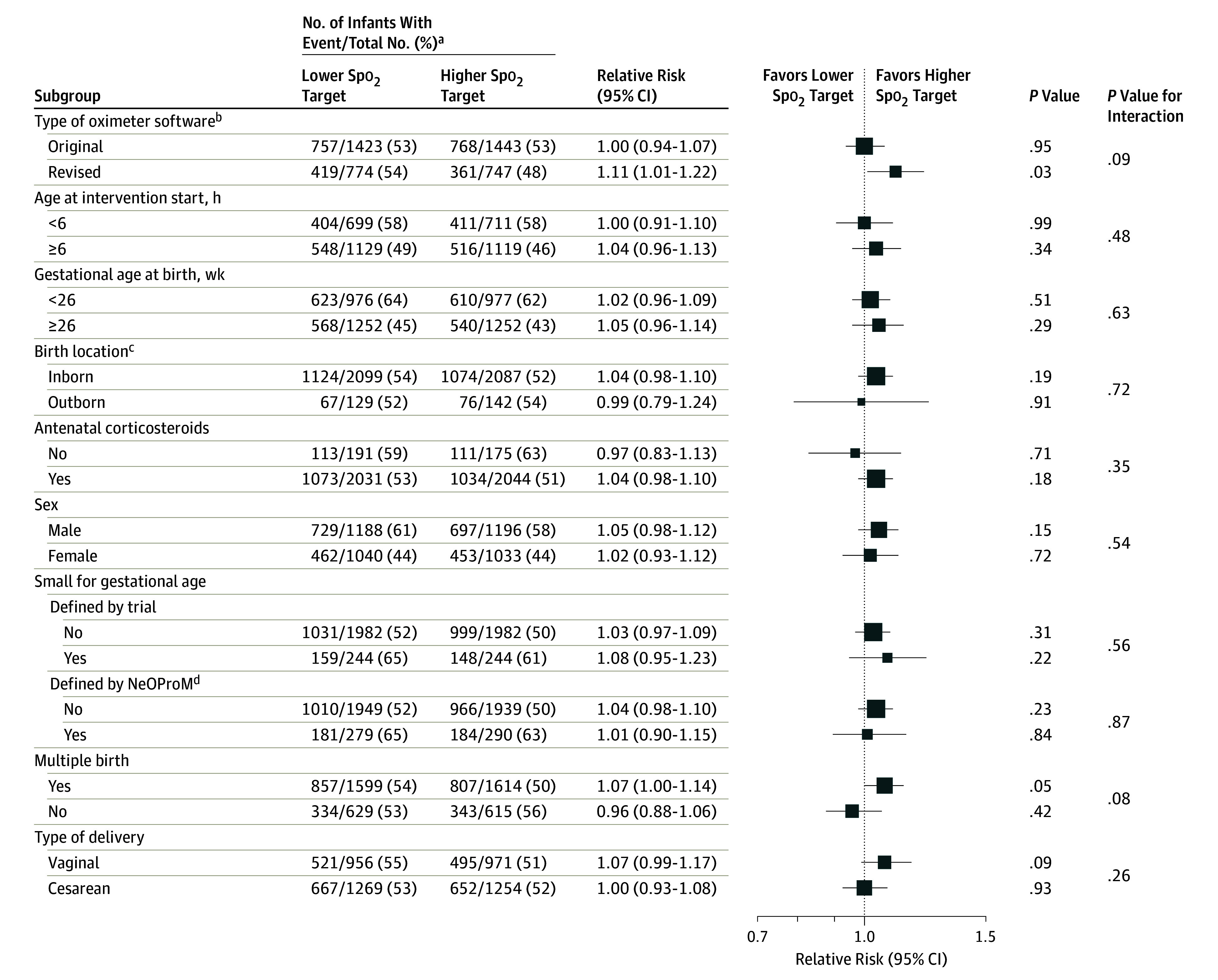
Box sizes correspond to precision; therefore, the more precise the larger the box. Precision was ascertained by calculating the inverse of the variance for each estimate. Spo2 indicates oxygen saturation as measured by pulse oximetry.
aDenominators include the total number of infants with a known outcome.
bExcluded 74 infants in the Canadian Oxygen Trial who were exposed to both the original and revised software.
cInborn defined as born inside the treating center; outborn, born outside the treating center (eg, transferred from another hospital).
dLess than 10th percentile using charts from Kramer et al.28
Subgroup analyses by oximeter software type (Figure 5) showed a significant difference in death by corrected age of 18 to 24 months for the original software (RR, 1.06 [95% CI, 0.91 to 1.23]; P = .47) vs the revised software (RR, 1.38 [95% CI, 1.14 to 1.68]; P = .001; P = .03 for interaction subgroup difference). A similar result was seen for death both before hospital discharge and before a postmenstrual age of 36 weeks.
Figure 5. Subgroup Analysis by Oximeter Software Type.
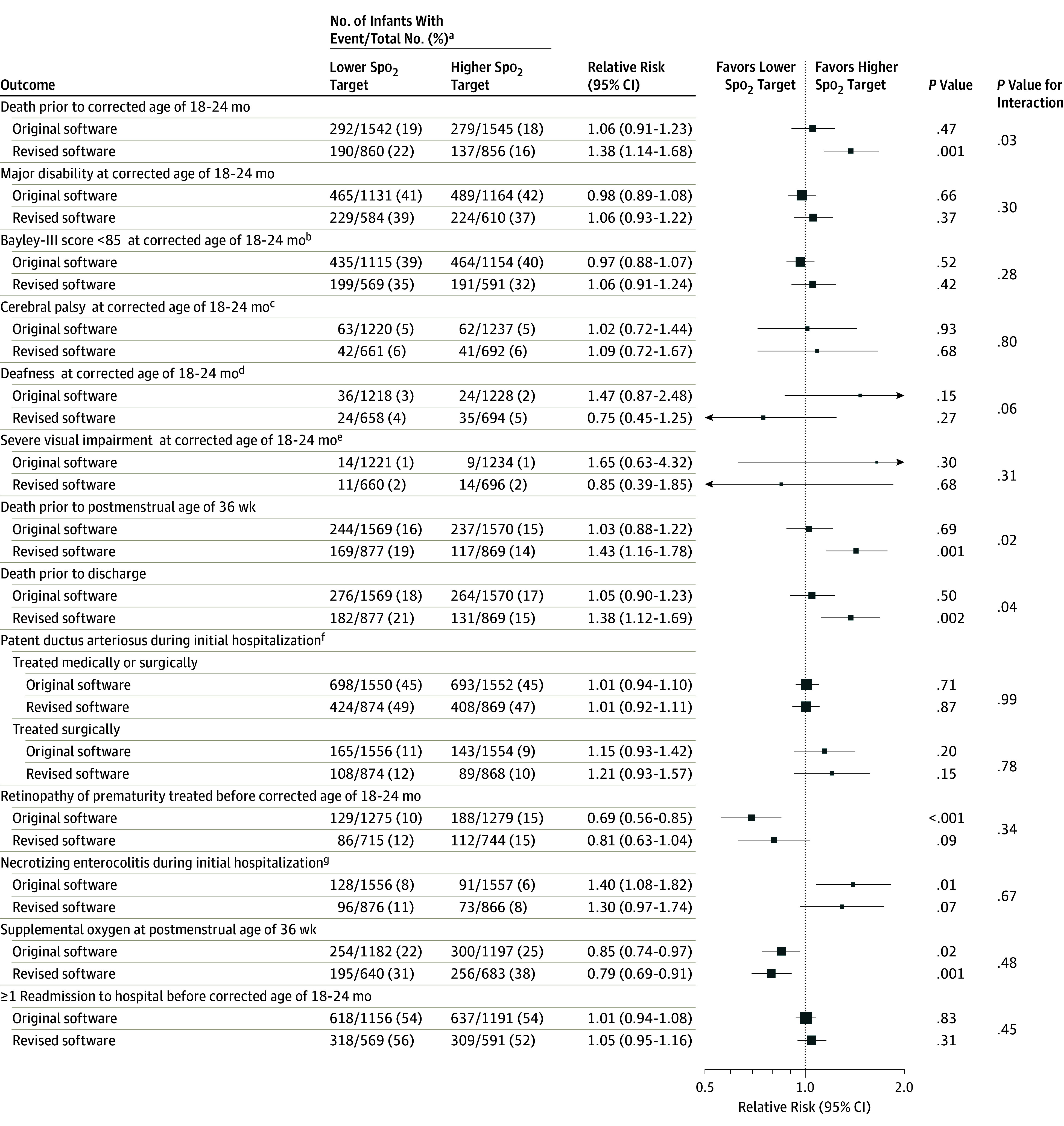
Box sizes correspond to precision; therefore, the more precise the larger the box. Precision was ascertained by calculating the inverse of the variance for each estimate. Spo2 indicates oxygen saturation as measured by pulse oximetry. This subgroup analysis excludes 74 infants in the Canadian Oxygen Trial who were exposed to both the original and revised software.
aDenominators include the total number of infants with a known outcome.
bDevelopmental assessment cognitive or language score of less than 85.
cDefined by Gross Motor Function Classification System23 level 2 or greater (higher levels = functioning more impaired) or cerebral palsy diagnosed but score unknown.
dRequiring hearing aids or worse.
eDefined by the trial investigators.
fDiagnosed by ultrasound.
gTreated with surgery or leading to death during initial hospitalization.
Other subgroup analyses of secondary outcomes appear in eTables 3-32 in Supplement 3. Even though there were differences in the subgroups for some of the outcomes using bivariable analyses, there was no overall pattern indicating that any particular subgroup of infants benefited more or less from the lower vs the higher Spo2 target.
There was no significant difference in the association with the lower Spo2 target for death at a corrected age of 18 to 24 months by known risk factors such as early gestational age, SGA, male sex, or infants born outside a tertiary center (eTables 15 and 33 in Supplement 3). The association with the lower oxygen target for severe necrotizing enterocolitis was greater for inborn infants and singletons (eTable 26 in Supplement 3).
For the outcome of ROP treatment, the association with the lower Spo2 target was larger among infants starting the intervention at an age of less than 6 hours (largely driven by SUPPORT results) and for those born via cesarean section (eTable 27 in Supplement 3). There was no difference in the association with the lower Spo2 target for PDA among infants treated surgically for any of the prespecified subgroup variables (eTable 25 in Supplement 3). The association with a lower Spo2 target at a postmenstrual age of 36 weeks was greater among SGA infants (eTable 30 in Supplement 3).
Sensitivity Analyses and Assessments of Bias and Heterogeneity
Sensitivity analyses exploring variations in the definition of the primary outcome (Figure 2) including a Bayley-III cognitive or language score of less than 70 or by other definition variations used by the individual trials did not change the primary outcome findings. Using a random-effects model (rather than a fixed-effect model) gave the same conclusions for all outcomes with the exception of PDA treated with surgical ligation, which became nonsignificant (eTable 34 in Supplement 3).
Overall, the 5 trials were assessed as being at low risk of bias for all domains7 (selection, performance or detection, attrition, and reporting biases) and had low levels of statistical heterogeneity for most outcomes. The outcome of ROP treatment had a high level of heterogeneity (I2 = 80%), which resulted from the substantially larger treatment effect of the lower Spo2 target on this outcome in the SUPPORT trial.
Discussion
In this prospectively planned meta-analysis of individual participant data involving clinical trials of extremely preterm infants, there was no significant difference between a lower Spo2 target range (85%-89%) and a higher Spo2 target range (91%-95%) from soon after birth on the primary composite outcome of death or major disability at a corrected age of 18 to 24 months. However, the lower target range was associated with more deaths and cases of severe necrotizing enterocolitis and less treated ROP, but was not associated with blindness.
When evaluating outcomes within a clinical trial sample or synthesizing results from several trials in a meta-analysis, the effects associated with treatment represent averages, and the true benefits and harms may differ from those in these analyses. Furthermore, tests of associations between treatment and secondary, albeit prespecified and important, outcomes (including the individual components of the composite primary outcome), should be considered exploratory and the results interpreted with caution. In particular, the statistically significant increased risk of death would not remain significant if adjusted for multiple testing. However, death was a major component of the composite primary outcome, and a clear difference in death, in either direction, was used to assess the need for early stopping in 2 trials.22 The current pooled estimated risk and 95% CIs for mortality from these trials thus provide the best currently available evidence to guide future clinical practice.
Prespecified subgroup analyses showed consistent results across trials for most outcomes, except for a larger association on treated ROP within the SUPPORT trial. The reasons for this result in the SUPPORT trial need to be explored more fully. One possible explanation for the heterogeneity is that most infants in the SUPPORT trial were randomized before birth; however, this hypothesis cannot be explored reliably in the other trials because they included too few infants recruited early.32
Mortality was increased in the lower Spo2 target group overall, in the first reported trial that used the original software exclusively,15 and in the prespecified subgroup analysis of original vs revised oximeter software. There has been considerable debate among the study investigators whether the change in oximeter software was responsible for this result.22,33,34,35,36
A subgroup analysis undertaken by the SUPPORT trial investigators found that, in their trial, mortality in the lower Spo2 target group was greater for SGA infants.30 A prespecified subgroup analysis using a common definition of SGA28 across the combined data set, and a post hoc analysis on the full data set using the same definition of SGA as used in the SUPPORT trial (curves by Alexander et al)29,30 did not confirm this relationship.
The main strength of this meta-analysis is that the 5 trials were planned prospectively to be similar in design and their investigators agreed to undertake a combined pooled meta-analysis of individual participant data based on a protocol developed in advance of any trial results.37,38 The statistical analysis plan was finalized after the trial results were known, but before any central receipt or synthesis of data. As would be expected with this study design, heterogeneity across the trials for most outcomes was low.
A previous Cochrane review7 had synthesized the aggregate data available from the published reports of the 5 trials. In contrast, these results were derived using raw individual participant data sourced directly from the trial investigators and combined centrally, making this the most comprehensive and rigorous analyses available of these data. The methods of the analyses used for the individual participant data also permitted adjustment for the correlation of multiple births; standardization of important outcomes across trials, including the definition of major disability; and enabled testing of the effect of differences in outcome definitions via sensitivity analyses. Even though the main findings are similar to some of the Cochrane Review results, the current meta-analysis of individual participant data has provided new insights into the consistency of results across multiple subgroups that indicate the findings should not be restricted to certain groups of infants such as those born SGA or at very early gestational ages. The 2016 guidelines from the American Academy of Pediatrics noted that their recommendations at that time were made “pending additional data, including the individual patient meta-analysis (NeOProM).”39 Thus these new findings should help inform these ongoing debates.
Implications for future research may include investigations of the effects of differences in alarm limits and targeting compliance40 and in the level of exposure to the intervention on outcomes; measures of Spo2 achieved, the proportion of time spent at various Spo2 levels on outcomes (eg, via prediction models adjusted for potential confounders), or both; the oximeter software change on mortality (eg, further explanation of why a larger association was seen in this subgroup); and, using automated methods to match the relatively narrow target ranges required.
Limitations
This study has several limitations. First, all 5 trials reported less separation in oxygen exposure between treatment groups than anticipated, largely because the lower Spo2 target groups had higher than intended saturation levels.17 Second, 2 trials (BOOST II in United Kingdom and Australia) were stopped early, which may have resulted in some overestimation of the effect on mortality in these trials.41 However, excluding truncated studies from meta-analyses can lead to substantial bias due to underestimation of overall treatment effects.42 Therefore, the best estimate of the association with treatment remains the overall combined results from the 5 trials.
Third, the lack of an association of Spo2 target range on blindness, but with a clear difference on ROP by treatment group, may change with longer follow-up, when less severe visual impairments may become apparent. Fourth, the potential for false-positive results based on multiple comparisons from 16 secondary outcomes and hundreds of subgroup analyses means that individual comparisons, although nominally significant, should be considered exploratory and interpreted cautiously. Fifth, even though the results are generalizable across the 5 trials, caution should be exercised not to extend these findings to other settings that do not have early screening for ROP, appropriate ROP treatment, or skilled nursing care regarding alarm limits. The trials studied Spo2 target ranges, not oximeter alarm limits, and these 2 concepts are not interchangeable.
Conclusions
In this prospectively planned meta-analysis of individual participant data from extremely preterm infants, there was no significant difference between a lower Spo2 target range compared with a higher Spo2 target range on the primary composite outcome of death or major disability at a corrected age of 18 to 24 months. The lower Spo2 target range was associated with a higher risk of death and necrotizing enterocolitis, but a lower risk of retinopathy of prematurity treatment.
Trial protocol
Statistical analysis plan
Baseline characteristics
Primary outcome
Secondary outcomes
Subgroup variables
eTable 1. Characteristics of randomized trials included in the NeOProM Collaboration
eFigure 1. Oximeter adjustment to maintain treatment allocation blinding
eTable 2. Overall survival analysis
eFigure 2. Cumulative incidence curve of death by treatment group to 3 months age
eTable 3. Subgroup numbers by trial
eTable 4. Death or major disability (primary analysis), by subgroups
eTable 5. Death or major disability (supportive analysis), by subgroups
eTable 6. Death or major disability (secondary analysis), by subgroups
eTable 7. Death or major disability (trialist defined), by subgroups
eTable 8. Major disability (primary analysis), by subgroups
eTable 9. Major disability (supportive analysis), by subgroups
eTable 10. Major disability (secondary analysis), by subgroups
eTable 11. Major disability (trialist defined), by subgroups
eTable 12. Cerebral palsy with GMFCS ≥ 2, by subgroups
eTable 13. Severe visual impairment (trialist defined), by subgroups
eTable 14. Deafness requiring hearing aids or worse, by subgroups
eTable 15. Death prior to 18‐24 months’ age corrected for prematurity, by subgroups
eTable 16. Death prior to 36 weeks’ postmenstrual age, by subgroups
eTable 17. Death prior to discharge, by subgroups
eTable 18. Bayley‐III language and/or cognitive <85, by subgroups
eTable 19. Bayley‐III cognitive <85, by subgroups
eTable 20. Bayley‐III language <85, by subgroups
eTable 21. Bayley‐III language or cognitive <70, by subgroups
eTable 22. Bayley‐III cognitive <70, by subgroups
eTable 23. Bayley‐III language <70, by subgroups
eTable 24. Patent ductus arteriosus (PDA) medically or surgically treated, by subgroups
eTable 25. Patent ductus arteriosus (PDA) surgically treated, by subgroups
eTable 26. Severe necrotizing enterocolitis (NEC), by subgroups
eTable 27. Treated retinopathy of prematurity (ROP), by subgroups
eTable 28. Positive airway pressure with endotracheal tube (ETT) at 36 weeks’ postmenstrual age (PMA), by subgroups
eTable 29. Positive airway pressure without endotracheal tube (ETT) at 36 weeks’ postmenstrual age (PMA), by subgroups
eTable 30. Supplemental oxygen without positive airway pressure at 36 weeks, by subgroups
eTable 31. Discharged home on oxygen, by subgroups
eTable 32. Re‐admission to hospital, by subgroups
eTable 33. Outcomes, by SUPPORT‐defined small for gestational age (SGA) subgroups
eTable 34. Primary and secondary outcomes using random effects models
References
References
- 1.Askie LM, Henderson-Smart DJ. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2001;4(4):CD001077. [DOI] [PubMed] [Google Scholar]
- 2.Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382(9902):1445-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84(2):F106-F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.STOP-ROP Investigators . Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial, I: primary outcomes. Pediatrics. 2000;105(2):295-310. [DOI] [PubMed] [Google Scholar]
- 5.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349(10):959-967. [DOI] [PubMed] [Google Scholar]
- 6.Cole CH, Wright KW, Tarnow-Mordi W, Phelps DL; Pulse Oximetry Saturation Trial for Prevention of Retinopathy of Prematurity Planning Study Group . Resolving our uncertainty about oxygen therapy. Pediatrics. 2003;112(6 pt 1):1415-1419. [DOI] [PubMed] [Google Scholar]
- 7.Askie LM, Darlow BA, Davis PG, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. 2017;4:CD011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Trials website . Surfactant Positive Airway Pressure and Pulse Oximetry Trial (SUPPORT). https://clinicaltrials.gov/ct2/show/NCT00233324. Accessed March 12, 2018.
- 9.Current Controlled Trials website . Efficacy and safety of targeting lower arterial oxygen saturations to reduce oxygen toxicity and oxidative stress in very preterm infants: the Canadian Oxygen Trial. http://www.isrctn.com/ISRCTN62491227. Accessed March 12, 2018.
- 10.Australian New Zealand Clinical Trials Registry website . A randomised phase III study to evaluate whether a lower versus a higher oxygen saturation target in infants of <28 weeks gestation is associated with a reduction in death or disability at 2 years of age. http://www.anzctr.org.au/ACTRN12605000253606.aspx. Accessed March 12, 2018.
- 11.Current Controlled Trials website . Which oxygen saturation level should we use for very premature infants? a randomised controlled trial. http://www.isrctn.com/ISRCTN00842661. Accessed March 12, 2018.
- 12.Australian New Zealand Clinical Trials Registry website . Which oxygen saturation level should we use for very premature infants? a randomised controlled trial to investigate the effect of two slightly different oxygen levels on the health of very premature infants. http://www.anzctr.org.au/ACTRN12605000055606.aspx. Accessed March 12, 2018.
- 13.Ghersi D, Berlin J, Askie L. Prospective meta-analysis. In: Cochrane Handbook for Systematic Reviews of Interventions: vol 5.1.0; 2011. http://www.handbook.cochrane.org. Accessed May 10, 2018.
- 14.Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W; NeOProM Collaborative Group . NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr. 2011;11(6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlo WA, Finer NN, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362(21):1959-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaucher YE, Peralta-Carcelen M, Finer NN, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367(26):2495-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt B, Whyte RK, Asztalos EV, et al. ; Canadian Oxygen Trial (COT) Group . Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309(20):2111-2120. [DOI] [PubMed] [Google Scholar]
- 18.Darlow BA, Marschner SL, Donoghoe M, et al. ; Benefits Of Oxygen Saturation Targeting-New Zealand (BOOST-NZ) Collaborative Group . Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. 2014;165(1):30-35.e2. [DOI] [PubMed] [Google Scholar]
- 19.Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. ; BOOST II United Kingdom Collaborative Group; BOOST II Australia Collaborative Group; BOOST II New Zealand Collaborative Group . Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094-2104. [DOI] [PubMed] [Google Scholar]
- 20.Tarnow-Mordi W, Stenson B, Kirby A, et al. ; BOOST-II Australia and United Kingdom Collaborative Groups . Outcomes of two trials of oxygen-saturation targets in preterm infants. N Engl J Med. 2016;374(8):749-760. [DOI] [PubMed] [Google Scholar]
- 21.Johnston ED, Boyle B, Juszczak E, King A, Brocklehurst P, Stenson BJ. Oxygen targeting in preterm infants using the Masimo SET radical pulse oximeter. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F429-F433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenson B, Brocklehurst P, Tarnow-Mordi W; UK BOOST II trial; Australian BOOST II trial; New Zealand BOOST II trial . Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364(17):1680-1682. [DOI] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment Inc; 2006. [Google Scholar]
- 24.Palisano RJ, Hanna SE, Rosenbaum PL, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther. 2000;80(10):974-985. [PubMed] [Google Scholar]
- 25.Higgins JPT, Altman DG, Sterne JAC; Cochrane Statistical Methods Group; Cochrane Bias Methods Group . Assessing risk of bias in included studies. In: Cochrane Handbook for Systematic Reviews of Interventions: vol 5.1.0; 2011. http://www.handbook.cochrane.org. Accessed May 10, 2018.
- 26.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 28.Kramer MS, Platt RW, Wen SW, et al. ; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System . A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E35. [DOI] [PubMed] [Google Scholar]
- 29.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163-168. [DOI] [PubMed] [Google Scholar]
- 30.Walsh MC, Di Fiore JM, Martin RJ, Gantz M, Carlo WA, Finer N. Association of oxygen target and growth status with increased mortality in small for gestational age infants: further analysis of the Surfactant, Positive Pressure and Pulse Oximetry Randomized Trial. JAMA Pediatr. 2016;170(3):292-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189-2194. [DOI] [PubMed] [Google Scholar]
- 32.Rich W, Finer NN, Gantz MG, et al. ; SUPPORT and Generic Database Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics. 2012;129(3):480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenson BJ. Oxygen targets for preterm infants. Neonatology. 2013;103(4):341-345. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B, Roberts RS, Whyte RK, et al. ; Canadian Oxygen Trial Group . Impact of study oximeter masking algorithm on titration of oxygen therapy in the Canadian oxygen trial. J Pediatr. 2014;165(4):666-71.e2. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JJ, Lakshminrusimha S, Polin RA. Oxygen-saturation targets in preterm infants. N Engl J Med. 2016;375(2):186-187. [DOI] [PubMed] [Google Scholar]
- 36.Whyte RK, Nelson H, Roberts RS, Schmidt B. Benefits of oxygen saturation targeting trials: oximeter calibration software revision and infant saturations. J Pediatr. 2017;182:382-384. [DOI] [PubMed] [Google Scholar]
- 37.Simes RJ; PPP and CTT Investigators . Prospective meta-analysis of cholesterol-lowering studies: the Prospective Pravastatin Pooling (PPP) Project and the Cholesterol Treatment Trialists (CTT) Collaboration. Am J Cardiol. 1995;76(9):122C-126C. [DOI] [PubMed] [Google Scholar]
- 38.Ioannidis J. Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med. 2017;51(20):1456-1458. [DOI] [PubMed] [Google Scholar]
- 39.Cummings JJ, Polin RA; Committee on Fetus and Newborn . Oxygen targeting in extremely low birth weight infants. Pediatrics. 2016;138(2):e20161576. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt B, Whyte RK, Roberts RS. Trade-off between lower or higher oxygen saturations for extremely preterm infants: the first benefits of oxygen saturation targeting (BOOST) II trial reports its primary outcome. J Pediatr. 2014;165(1):6-8. [DOI] [PubMed] [Google Scholar]
- 41.Bassler D, Briel M, Montori VM, et al. ; STOPIT-2 Study Group . Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303(12):1180-1187. [DOI] [PubMed] [Google Scholar]
- 42.Schou IM, Marschner IC. Meta-analysis of clinical trials with early stopping: an investigation of potential bias. Stat Med. 2013;32(28):4859-4874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
Baseline characteristics
Primary outcome
Secondary outcomes
Subgroup variables
eTable 1. Characteristics of randomized trials included in the NeOProM Collaboration
eFigure 1. Oximeter adjustment to maintain treatment allocation blinding
eTable 2. Overall survival analysis
eFigure 2. Cumulative incidence curve of death by treatment group to 3 months age
eTable 3. Subgroup numbers by trial
eTable 4. Death or major disability (primary analysis), by subgroups
eTable 5. Death or major disability (supportive analysis), by subgroups
eTable 6. Death or major disability (secondary analysis), by subgroups
eTable 7. Death or major disability (trialist defined), by subgroups
eTable 8. Major disability (primary analysis), by subgroups
eTable 9. Major disability (supportive analysis), by subgroups
eTable 10. Major disability (secondary analysis), by subgroups
eTable 11. Major disability (trialist defined), by subgroups
eTable 12. Cerebral palsy with GMFCS ≥ 2, by subgroups
eTable 13. Severe visual impairment (trialist defined), by subgroups
eTable 14. Deafness requiring hearing aids or worse, by subgroups
eTable 15. Death prior to 18‐24 months’ age corrected for prematurity, by subgroups
eTable 16. Death prior to 36 weeks’ postmenstrual age, by subgroups
eTable 17. Death prior to discharge, by subgroups
eTable 18. Bayley‐III language and/or cognitive <85, by subgroups
eTable 19. Bayley‐III cognitive <85, by subgroups
eTable 20. Bayley‐III language <85, by subgroups
eTable 21. Bayley‐III language or cognitive <70, by subgroups
eTable 22. Bayley‐III cognitive <70, by subgroups
eTable 23. Bayley‐III language <70, by subgroups
eTable 24. Patent ductus arteriosus (PDA) medically or surgically treated, by subgroups
eTable 25. Patent ductus arteriosus (PDA) surgically treated, by subgroups
eTable 26. Severe necrotizing enterocolitis (NEC), by subgroups
eTable 27. Treated retinopathy of prematurity (ROP), by subgroups
eTable 28. Positive airway pressure with endotracheal tube (ETT) at 36 weeks’ postmenstrual age (PMA), by subgroups
eTable 29. Positive airway pressure without endotracheal tube (ETT) at 36 weeks’ postmenstrual age (PMA), by subgroups
eTable 30. Supplemental oxygen without positive airway pressure at 36 weeks, by subgroups
eTable 31. Discharged home on oxygen, by subgroups
eTable 32. Re‐admission to hospital, by subgroups
eTable 33. Outcomes, by SUPPORT‐defined small for gestational age (SGA) subgroups
eTable 34. Primary and secondary outcomes using random effects models
References


