Key Points
Question
Does the timing of a loading dose of atorvastatin before percutaneous coronary intervention (PCI) affect 30-day major adverse cardiovascular events in patients presenting with acute coronary syndrome?
Findings
In this secondary analysis of a randomized clinical trial, the rate of 30-day major adverse cardiovascular events was 6.0% among patients who received loading doses of atorvastatin and 8.2% among patients who received placebo. This effect was more pronounced in patients with ST-elevation myocardial infarction and consistent, irrespective of the timing of loading dose atorvastatin before PCI.
Meaning
Loading doses of atorvastatin in patients with acute coronary syndrome undergoing PCI were not associated with any adverse effects and were associated with lower rates of major adverse cardiovascular events at 30 days, most clearly in patients with ST-elevation myocardial infarction.
Abstract
Importance
Loading doses of atorvastatin did not show reduction on clinical outcomes in the overall population of patients with acute coronary syndrome (ACS) enrolled in the Statins Evaluation in Coronary Procedures and Revascularization (SECURE-PCI) trial, but a potential benefit was identified in patients who subsequently underwent percutaneous coronary intervention (PCI).
Objectives
To determine whether periprocedural loading doses of atorvastatin are associated with decreased 30-day major adverse cardiovascular events (MACE) in patients with ACS undergoing PCI according to type of ACS and timing of atorvastatin administration before PCI.
Design, Setting, and Participants
Secondary analysis of a multicenter, double-blind, placebo-controlled, randomized clinical trial conducted at 53 sites that enrolled 4191 patients with ACS intended to be treated with PCI between April 18, 2012, and October 06, 2017.
Interventions
Patients were randomized to 2 loading doses of 80 mg of atorvastatin or matching placebo before and 24 hours after a planned PCI. By protocol, all patients (regardless of treatment group) received 40 mg of atorvastatin for 30 days starting 24 hours after the second dose of study medication.
Main Outcomes and Measures
The primary outcome was MACE through 30 days, composed by all-cause mortality, myocardial infarction, stroke, and unplanned coronary revascularization. Cox regression models adjusting for key baseline characteristics were used to assess the association between atorvastatin and MACE in patients undergoing PCI.
Results
From the overall trial population, 2710 (64.7%) underwent PCI (650 women [24.0%]; mean [SD] age, 62 [11.3] years). Loading atorvastatin was associated with reduced MACE at 30 days by 28% in the PCI group (adjusted hazard ratio [HR], 0.72; 95% CI 0.54-0.97; P = .03). Loading dose of atorvastatin was administered less than 12 hours before PCI in 2548 patients (95.3%) (45.1% < 2 hours and 54.3% between 2 and 12 hours). There was no significant interaction between treatment effect and timing of study drug administration. The treatment effect of loading atorvastatin was more pronounced in patients with ST-segment elevation myocardial infarction than in patients with non–ST-segment elevation ACS (adjusted HR, 0.59; 95% CI, 0.38-0.92; P = .02; HR, 0.85; 95% CI, 0.58-1.27; P = .43, respectively).
Conclusions and Relevance
In patients with ACS undergoing PCI, periprocedural loading doses of atorvastatin appeared to reduce the rate of MACE at 30 days, most clearly in patients with ST-segment elevation myocardial infarction. This beneficial effect seemed to be preserved and consistent, irrespective of the timing of atorvastatin administration, including within 2 hours before PCI.
Trial Registration
clinicaltrials.gov Identifier: NCT01448642.
This secondary analysis of a randomized clinical trial investigates whether periprocedural loading doses of atorvastatin are associated with decreased 30-day major adverse cardiovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention.
Introduction
Percutaneous coronary intervention (PCI) has been associated with a variable incidence of periprocedural myocardial infarction (up to frequencies greater than 10%), which is independently associated with higher subsequent mortality.1,2,3 Previous small trials and systematic reviews that investigated the effect of loading doses of statins before and after PCI have suggested that there may be a reduction in periprocedural myocardial infarction (MI) and in major adverse cardiovascular events (MACE).2,3,4,5,6
A well-powered study7 that addressed the effect of loading doses of statin in patients with acute coronary syndrome (ACS) treated with or without PCI did not show clinical benefit in the overall ACS population but did suggest a positive effect in patients undergoing PCI. It is unknown whether timing of a pre-PCI loading dose of atorvastatin was associated with a beneficial treatment effect. Thus, in this prespecified subgroup analysis, our main goal was to evaluate the association between timing of drug administration before PCI and 30-day MACE in patients with ACS undergoing PCI.
Methods
Study Design
The study design was published previously.7,8 Briefly, the Statins Evaluation in Coronary Procedures and Revascularization (SECURE-PCI) trial is a randomized, double-blind, multicenter trial that included patients aged 18 years and older with ACS (with or without ST elevation),7,8,9,10 before a planned invasive treatment within 7 days. The study was approved by each site’s institutional review board, and all patients provided written informed consent to enter the study.
The participants included were randomized to a loading dose of 80 mg of atorvastatin or matching placebo before and 24 hours after a PCI. The timing of the study medication varied according to the type of ACS. For patients with ACS without ST elevation, the pre-PCI dose was administered between 2 and 12 hours before the procedure. For patients with ST-elevation myocardial infarction (STEMI), the loading dose was administered as soon as possible before the primary PCI. In both cases, the second dose of 80-mg atorvastatin or matching placebo was administered 24 hours after the procedure. All patients (regardless of treatment assignment) were to receive 40 mg of atorvastatin daily after the procedure through 30 days, starting on the day after the administration of the second (post-PCI) loading dose of atorvastatin or placebo.
For patients who were randomized, received study medication, but did not undergo PCI within 24 hours, 2 strategies were recommended depending on the final decision regarding coronary intervention. If PCI was only postponed for more than 24 hours, patients received additional loading doses before the procedure and in addition to the dose 24 hours after the procedure. However, if the decision was not to perform PCI, then patients could receive a second dose of atorvastatin, 80 mg, (or placebo) at the discretion of the investigator (it was recommended by the protocol in cases where coronary artery disease was present on the angiogram).
Clinical Outcomes and Statistical Analysis
The primary outcome was the composite of all-cause mortality, acute MI, stroke, and unplanned coronary revascularization at 30 days. Secondary outcomes at 30 days included individual components of the primary outcome and also cardiovascular death, stent thrombosis, and target vessel revascularization. The treatment effect of loading doses of 80 mg of atorvastatin vs placebo was assessed using Cox regression model adjusted for key baseline characteristics and expressed by hazard ratios (HR) and 95% confidence intervals. All analyses considered a 2-tailed P value of .05 as statistically significant. A detailed description of the statistical analysis is provided in the eAppendix of Supplement 1.
Results
Patients
Of the 4191 patients included in the main trial, there were 2710 patients (64.7%) who underwent PCI. The number of patients lost to follow-up at 30 days was 24 (0.6%) overall and 11 (0.4%) in the PCI population. Baseline and procedural characteristics of patients with PCI and patients without PCI are shown in the Table and eTable 1 in Supplement 1.
Table. Baseline Participant Characteristics of Patients Undergoing PCI.
| Characteristic | No./Total No. (%) | |
|---|---|---|
| Atorvastatin (n = 1351) | Placebo (n = 1359) | |
| Age, mean (SD), y | 61.7 (11.1) | 62.3 (11.5) |
| Female | 300/1351 (22.2) | 350/1359 (25.8) |
| Diagnosis | ||
| STEMI | 417/1332 (31.3) | 448/1339 (33.5) |
| NSTEMI | 809/1332 (60.7) | 788/1339 (58.8) |
| Unstable angina | 106/1332 (8) | 103/1339 (7.7) |
| Previous use of chronic statin therapy (6 mo before randomization) | 365/1351 (27) | 364/1359 (26.8) |
| Medical history | ||
| Hypertension | 925/1351 (68.5) | 951/1359 (70) |
| Hypercholesterolemia | 479/1351 (35.5) | 483/1359 (35.5) |
| Diabetes mellitus | 415/1351 (30.7) | 426/1359 (31.3) |
| Tobacco use | 408/1351 (30.2) | 427/1359 (31.4) |
| Previous MI | 190/1351 (14.1) | 185/1359 (13.6) |
| Previous PCI | 152/1351 (11.3) | 165/1359 (12.1) |
| Previous CABG | 87/1351 (6.4) | 65/1359 (4.8) |
| Previous stroke | 38/1351 (2.8) | 45/1359 (3.3) |
| Renal impairment | 34/1351 (2.5) | 49/1359 (3.6) |
| Obesity | 203/1351 (15) | 216/1359 (15.9) |
| Time from randomization to study drug, No. | 1337 | 1337 |
| Mean (SD), h | 6.7 (31.4) | 5.9 (21.2) |
| Median (IQR), h | 0.1 (0-0.5) | 0.1 (0-0.6) |
| Time from hospital admission to PCI, No. | (n = 1351) | (n = 1359) |
| Mean (SD), h | 47.8 (66.6) | 45.3 (63.8) |
| Median (IQR), h | 20 (3-72) | 19 (3-64) |
| Time from randomization to PCI, No. | (n = 1351) | (n = 1359) |
| Mean (SD), h | 7.2 (88.8) | 9.1 (59.2) |
| Median (IQR), h | 3 (1-6) | 3 (1-6) |
| Other medical therapy | ||
| Aspirin | 1313/1351 (97.2) | 1331/1359 (97.9) |
| Clopidogrel/ticagrelor/prasugrel | 1310/1351 (97) | 1323/1359 (97.4) |
| β-blockers | 1121/1351 (83) | 1119/1359 (82.3) |
| ACE inhibitors or ARA | 1075/1351 (79.6) | 1034/1359 (76.1) |
| Enoxaparin | 531/1351 (39.3) | 555/1359 (40.8) |
| Nonfractioned heparin | 93/1351 (6.9) | 94/1359 (6.9) |
| Fondaparinux | 328/1351 (24.3) | 316/1359 (23.3) |
| PCI characteristics | ||
| Heparin used to support PCI | 1196/1349 (88.7) | 1199/1356 (88.4) |
| Stent | 1311/1350 (97.1) | 1333/1356 (98.2) |
| Bare-metal stent only | 1013/1350 (75) | 1020/1356 (75.2) |
| ≥1 Drug-eluting stent | 298/1350 (22.1) | 312/1356 (23) |
| Restenotic lesions | 32/1350 (2.4) | 42/1357 (3.1) |
| Multivessel PCI | 219/1350 (16.2) | 214/1356 (15.8) |
| Intravascular ultrasonography use | 28/1349 (2.1) | 22/1357 (1.6) |
| Balloon postdilatation | 651/1314 (49.5) | 645/1314 (49.1) |
| Stent deployment pressure (atm), mean (SD)a,b | 17.9 (3.4) | 17.9 (3.5) |
| Stents per patient, No. | ||
| 0 | 88/1349 (6.5) | 70/1356 (5.2) |
| 1 | 994/1349 (73.7) | 1023/1356 (75.4) |
| 2 | 236/1349 (17.5) | 233/1356 (17.2) |
| 3 or more | 31/1349 (2.3) | 30/1356 (2.2) |
| Procedural complications | 106/1350 (7.9) | 99/1357 (7.3) |
| Coronary dissection | 21/1349 (1.6) | 15/1357 (1.1) |
| Acute coronary artery occlusion during PCI | 4/1349 (0.3) | 2/1357 (0.1) |
| Coronary slow-flow phenomenon | 44/1349 (3.3) | 36/1357 (2.7) |
| Severe side-branch stenosis | 4/1349 (0.3) | 5/1357 (0.4) |
| Side-branch closure | 11/1349 (0.8) | 13/1357 (1) |
| Acute coronary perforation during PCI | 0/1349 | 4/1357 (0.3) |
| Coronary occlusion after PCI | 2/1349 (0.1) | 0/1357 |
| Temporary coronary occlusion during PCI | 4/1349 (0.3) | 4/1357 (0.3) |
| No reflow | 16/1349 (1.2) | 19/1357 (1.4) |
| Distal embolization | 18/1349 (1.3) | 11/1357 (0.8) |
| Acute stent thrombosis | 2/1349 (0.1) | 0/1357 |
Abbreviations: ACE, angiotensin-converting enzyme; ARA, adenosine regulating agent; CAGB, coronary artery bypass grafting; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
n = 598.
n = 589.
The median time from admission to PCI was 20 hours (interquartile range [IQR], 3-69 hours). Regarding the timing of study drug before PCI, a total of 1936 (71.4%) received the drug according to the timeframe of the protocol. The median time from admission to study drug administration was 0.67 hours (IQR, 0.05-2.56 hours), and the median time from study drug to PCI was 2.17 hours (IQR, 0.67-3.50 hours). The complete description of timing in both groups overall (atorvastatin and placebo) and according to type of ACS (STEMI and non–ST-elevation ACS [NSTE-ACS]) is detailed in eTable 2 in Supplement 1.
Primary Outcome
The treatment effect of loading atorvastatin on MACE at 30 days was different between the PCI (adjusted hazard ratio [HR], 0.72; 95% CI, 0.54- 0.97; P = .03) and non-PCI groups (HR, 1.44; 95% CI, 0.92-2.24; P = .11; P = .01 for interaction). The treatment effect of loading atorvastatin was more pronounced in patients with STEMI than in patients with NSTE-ACS (adjusted HR, 0.59; 95% CI, 0.38-0.92; P = .02; HR, 0.85; 95% CI, 0.58-1.27; P = .43, respectively; P = .22 for interaction) (Figure 1).
Figure 1. Analysis of Treatment Effect According to the Timing of Atorvastatin Before Percutaneous Coronary Intervention (PCI).
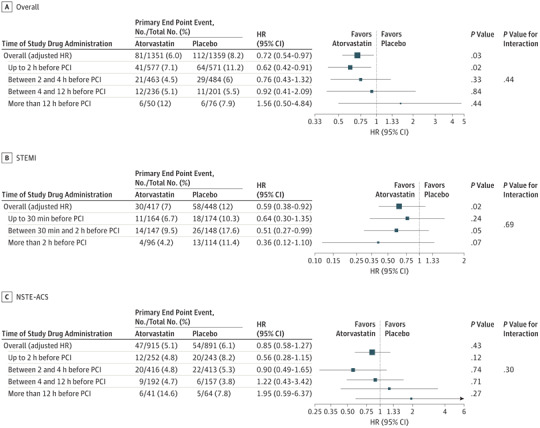
A, 52 Patients did not receive study drug before PCI (25 atorvastatin and 27 placebo). B, 22 Patients did not receive study drug before PCI (10 atorvastatin and 12 placebo). C, 28 Patients did not receive study drug before PCI (14 atorvastatin and 14 placebo).
The clinical benefit in the PCI group of patients was consistent, regardless of timing of use of study drug (Figure 1). The treatment effect of loading atorvastatin was more pronounced in patients with STEMI, including within 2 hours before PCI.
The association of the loading doses in the PCI population was also consistent according to previous use of statin or not (Figure 2). In patients undergoing coronary artery bypass grafting (n = 333) and medical management (n = 1144), there was no treatment effect of loading atorvastatin vs placebo (eTable 3 in Supplement 1).
Figure 2. Cumulative Incidence of Primary Outcome.
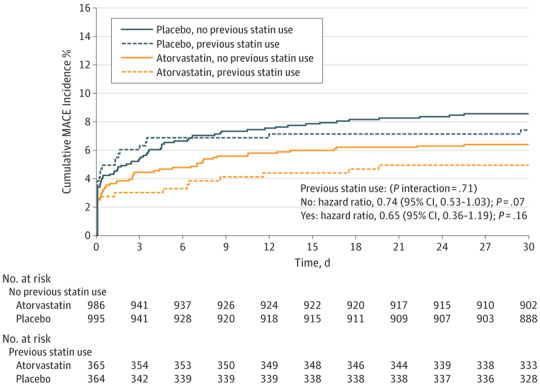
Composite of all-cause mortality, acute myocardial infarction, stroke, and unplanned coronary revascularization in the percutaneous coronary intervention (PCI) population according to previous use of statin or not (P = .71 for interaction between treatment effect and previous use of statin). MACE indicates major adverse cardiovascular event.
Secondary Outcomes
For each of the secondary outcomes in the overall PCI population, there were numerically fewer events in the atorvastatin arm than in the placebo group, with a nominally significant reduction in MI at 30 days (HR, 0.68; 95% CI, 0.47-0.99; P = .04). The detailed description of secondary outcomes in the PCI group according to type of ACS is shown in eTables 4 and 5 in Supplement 1.
Discussion
In this secondary analysis of a randomized clinical trial, we found that compared with placebo, loading doses of atorvastatin in patients with ACS undergoing PCI were associated with an overall reduction of 28% in the rates of MACE and a 32% reduction in MI. These results were primarily driven by patients presenting with STEMI. Importantly, we did not find any interaction between timing of atorvastatin administration and treatment effect. Finally, our results were consistent regardless of baseline statin status (naive or experienced).
Most of the evidence regarding periprocedural use of statin and subsequent outcomes derives from studies including patients with stable coronary disease and elective PCI.2,3 Analysis of pooled data from 1032 patients with ACS showed an aggregate 36% relative risk reduction in MACE at 30 days in the group using early and high dose of statin.3 Similar to the findings in SECURE-PCI, in the previous studies including only PCI-treated patients, the benefit of loading dose statin was consistent among the various scenarios of PCI indication (elective, NSTE-ACS, and STEMI) and also occurred in different timings of statin use.2,3 A meta-analysis of different regimens of statin use in patients with ACS undergoing PCI have shown important reduction in MACE at 30 days when statin was initiated before PCI.11 These results are aligned with our findings where patients with STEMI had the greatest treatment effect when loading atorvastatin was administrated before PCI, including within 2 hours of presentation.
The evaluation of the association according to timing is critical because only a few medications are routinely used before primary PCI. Our findings in a large cohort of patients with ACS undergoing PCI may influence clinical practice, especially in regards to upfront medications for patients with STEMI. We found that a loading dose of atorvastatin before PCI was associated with a reduction in important major cardiovascular outcomes, even if given within 2 hours before PCI. The mechanism behind this finding is not clear, although unlikely owing to low-density lipoprotein cholesterol level reduction. Mechanistic studies suggest that statins also possess effects beyond lipid lowering (pleotropic effects) that could act at the short term.12,13,14 This potential effect would be particularly more important in patients with more intense activation of inflammation and thrombotic processes as identified in our results, where the most pronounced effect occurred in patients presenting with STEMI.
Finally, the use of high-dose statin is recommended for patients with ACS, according to current evidence-based guidelines.9,10,15 However, the effect of early statin initiation in the ACS setting has not been clear, especially in patients undergoing primary or urgent PCI.10,15 Thus, this study provides important insight to help physicians in the decision-making process of starting statins in patients with the very early phase of ACS who are undergoing PCI. Because atorvastatin is a well-established and safe drug, our findings suggest that it is reasonable to consider loading doses of atorvastatin for patients with ACS undergoing PCI, particularly if presenting with STEMI.
Limitations
An important limitation in the analysis of the PCI population is associated with the fact that it was an analysis of a postbaseline subgroup of patients. Factors, such as immortal time (or survivor) bias, cannot be completely excluded when analyzing subgroups that are defined based on postbaseline characteristics. Nevertheless, this analysis was prespecified,8 and the findings were consistent with other studies designed to primarily assess the PCI population.2,3
Conclusions
In patients with ACS undergoing percutaneous coronary intervention, periprocedural loading doses of atorvastatin were associated with a reduced rate of MACE at 30 days, most clearly in patients with STEMI. This beneficial effect seemed to be preserved and consistent, irrespective of the timing of atorvastatin administration, including within 2 hours before PCI. Considering the safety of statins, treating patients with ACS, in particular with STEMI, with a loading dose of statin as early as possible before PCI is a reasonable approach that may improve short-term clinical outcomes.
eAppendix. Statistical Analysis
eTable 1. Baseline Participant Characteristics of Patients Not Undergoing PCI
eTable 2. Timing of Study Drug Administration According to Study Group and Type of Acute Coronary Syndrome
eTable 3. Treatment Effect According to the Type of Treatment in the Non-PCI Group
eTable 4. Exploratory Analysis in STEMI Patients Subgroup
eTable 5. Exploratory Analysis in Non-STE ACS Patients Subgroup
Data Sharing Statement
References
- 1.Stone GW, Mehran R, Dangas G, Lansky AJ, Kornowski R, Leon MB. Differential impact on survival of electrocardiographic Q-wave versus enzymatic myocardial infarction after percutaneous intervention: a device-specific analysis of 7147 patients. Circulation. 2001;104(6):642-647. doi: 10.1161/hc3101.093902 [DOI] [PubMed] [Google Scholar]
- 2.Winchester DE, Wen X, Xie L, Bavry AA. Evidence of pre-procedural statin therapy: a meta-analysis of randomized trials. J Am Coll Cardiol. 2010;56(14):1099-1109. doi: 10.1016/j.jacc.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 3.Patti G, Cannon CP, Murphy SA, et al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: a collaborative patient-level meta-analysis of 13 randomized studies. Circulation. 2011;123(15):1622-1632. doi: 10.1161/CIRCULATIONAHA.110.002451 [DOI] [PubMed] [Google Scholar]
- 4.Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G; ARMYDA Investigators . Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110(6):674-678. doi: 10.1161/01.CIR.0000137828.06205.87 [DOI] [PubMed] [Google Scholar]
- 5.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49(12):1272-1278. doi: 10.1016/j.jacc.2007.02.025 [DOI] [PubMed] [Google Scholar]
- 6.Di Sciascio G, Patti G, Pasceri V, Gaspardone A, Colonna G, Montinaro A. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54(6):558-565. doi: 10.1016/j.jacc.2009.05.028 [DOI] [PubMed] [Google Scholar]
- 7.Berwanger O, Santucci EV, de Barros E Silva PGM, et al. ; SECURE-PCI Investigators . Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: the secure-pci randomized clinical trial. JAMA. 2018;319(13):1331-1340. doi: 10.1001/jama.2018.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berwanger O, de Barros E Silva PGM, Dall Orto FTC, et al. Rationale and design of the Statins Evaluation in Coronary Procedures and Revascularization: the SECURE-PCI Trial. Am Heart J. 2018;198:129-134. doi: 10.1016/j.ahj.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 10.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362-e425. doi: 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 11.Navarese EP, Kowalewski M, Andreotti F, et al. Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2014;113(10):1753-1764. doi: 10.1016/j.amjcard.2014.02.034 [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA. 1998;279(20):1643-1650. doi: 10.1001/jama.279.20.1643 [DOI] [PubMed] [Google Scholar]
- 13.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37-44. doi: 10.1016/j.molmed.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nohria A, Prsic A, Liu PY, et al. Statins inhibit Rho kinase activity in patients with atherosclerosis. Atherosclerosis. 2009;205(2):517-521. doi: 10.1016/j.atherosclerosis.2008.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Statistical Analysis
eTable 1. Baseline Participant Characteristics of Patients Not Undergoing PCI
eTable 2. Timing of Study Drug Administration According to Study Group and Type of Acute Coronary Syndrome
eTable 3. Treatment Effect According to the Type of Treatment in the Non-PCI Group
eTable 4. Exploratory Analysis in STEMI Patients Subgroup
eTable 5. Exploratory Analysis in Non-STE ACS Patients Subgroup
Data Sharing Statement


