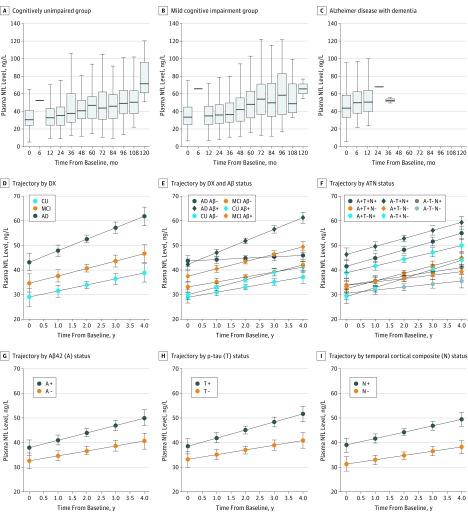
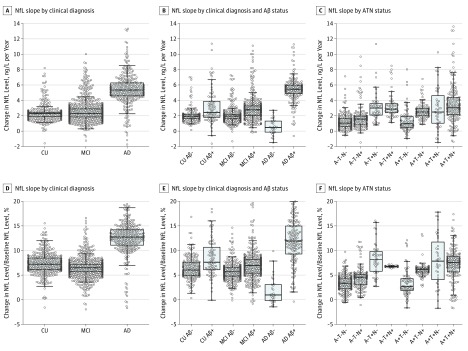
This cohort study uses data from the Alzheimer’s Disease Neuroimaging Initiative study to examine whether plasma neurofilament light, a noninvasive biomarker measurement, is associated with other markers of Alzheimer disease and other measures of neurodegeneration in patients with Alzheimer disease.
Key Points
Question
Is there an association between longitudinal plasma neurofilament light and hallmarks of Alzheimer disease?
Findings
In this cohort study of 1583 individuals in the multicenter Alzheimer’s Disease Neuroimaging Initiative study, longitudinal plasma neurofilament light level increased in association with several baseline and longitudinal hallmarks of Alzheimer disease. The increase in plasma neurofilament light level was associated with changes in other established measures of neurodegeneration in Alzheimer disease.
Meaning
The findings suggest that plasma neurofilament light can be used as a noninvasive biomarker to track neurodegeneration in patients with Alzheimer disease.
Abstract
Importance
Plasma neurofilament light (NfL) has been suggested as a noninvasive biomarker to monitor neurodegeneration in Alzheimer disease (AD), but studies are lacking.
Objective
To examine whether longitudinal plasma NfL levels are associated with other hallmarks of AD.
Design, Setting, and Participants
This North American cohort study used data from 1583 individuals in the multicenter Alzheimer’s Disease Neuroimaging Initiative study from September 7, 2005, through June 16, 2016. Patients were eligible for inclusion if they had NfL measurements. Annual plasma NfL samples were collected for up to 11 years and were analyzed in 2018.
Exposures
Clinical diagnosis, Aβ and tau cerebrospinal fluid (CSF) biomarkers, imaging measures (magnetic resonance imaging and fluorodeoxyglucose–positron emission tomography), and tests on cognitive scores.
Main Outcomes and Measures
The primary outcome was the association between baseline exposures (diagnosis, CSF biomarkers, imaging measures, and cognition) and longitudinal plasma NfL levels, analyzed by an ultrasensitive assay. The secondary outcomes were the associations between a multimodal classification scheme with Aβ, tau, and neurodegeneration (ie, the ATN system) and plasma NfL levels and between longitudinal changes in plasma NfL levels and changes in the other measures.
Results
Of the included 1583 participants, 716 (45.2%) were women, and the mean (SD) age was 72.9 (7.1) years; 401 had no cognitive impairment, 855 had mild cognitive impairment, and 327 had AD dementia. The NfL level was increased at baseline in patients with mild cognitive impairment and AD dementia (mean levels: cognitive unimpairment, 32.1 ng/L; mild cognitive impairment, 37.9 ng/L; and AD dementia, 45.9 ng/L; P < .001) and increased in all diagnostic groups, with the greatest increase in patients with AD dementia. A longitudinal increase in NfL level correlated with baseline CSF biomarkers (low Aβ42 [P = .001], high total tau [P = .02], and high phosphorylated tau levels [P = .02]), magnetic resonance imaging measures (small hippocampal volumes [P < .001], thin regional cortices [P = .009], and large ventricular volumes [P = .002]), low fluorodeoxyglucose–positron emission tomography uptake (P = .01), and poor cognitive performance (P < .001) for a global cognitive score. With use of the ATN system, increased baseline NfL levels were seen in A–T+N+ (P < .001), A+T–N+ (P < .001), and A+T+N+ (P < .001), and increased rates of NfL levels were seen in A–T+N– (P = .009), A–T+N+ (P = .02), A+T–N+ (P = .04), and A+T+N+ (P = .002). Faster increase in NfL levels correlated with faster increase in CSF biomarkers of neuronal injury, faster rates of atrophy and hypometabolism, and faster worsening in global cognition (all P < .05 in patients with mild cognitive impairment; associations differed slightly in cognitively unimpaired controls and patients with AD dementia).
Conclusions and Relevance
The findings suggest that plasma NfL can be used as a noninvasive biomarker associated with neurodegeneration in patients with AD and may be useful to monitor effects in trials of disease-modifying drugs.
Introduction
Alzheimer disease (AD) is characterized by amyloid β (Aβ) and tau aggregates in the brain. The start of a cascade is believed to begin with Aβ aggregation, which leads to spread of tau tangles and neuronal injury and progressive cognitive decline.1 Existing methods to monitor pathologic events in AD are based on imaging, either volumetric magnetic resonance imaging (MRI)2 or positron emission tomography (PET) of glucose metabolism Aβ and tau aggregates,3 or cerebrospinal fluid (CSF) biomarkers reflecting brain amyloidosis (Aβ42), neurodegeneration (total tau [t-tau]), and tau pathology (phosphorylated tau [p-tau]).4 Given that imaging biomarkers are expensive and have limited availability and CSF biomarkers involve lumbar puncture, there is a need for simple, noninvasive, inexpensive, and readily available biomarkers to track the neurodegenerative process in AD, both to monitor the disease in clinical practice and to facilitate drug development.
Neurofilament light (NfL) in CSF is a sensitive biomarker for neuroaxonal damage,5 and recent developments have allowed for measurement of NfL levels in blood samples.6 Plasma NfL is a candidate marker to track neurodegeneration in AD, in which levels are increased and correlate with future atrophy, hypometabolism, and cognitive decline.7 However, we are not aware of any study of longitudinal plasma NfL levels in AD. We tested longitudinal plasma NfL levels in a large number of people who were cognitively unimpaired (CU) and patients with mild cognitive impairment (MCI) or AD dementia enrolled in the Alzheimer Disease Neuroimaging Initiative (ADNI). The primary hypothesis was that plasma NfL levels would increase among people with baseline features of AD (clinical diagnosis, CSF biomarkers, and MRI and fluorodeoxyglucose [FDG]-PET features). We also tested whether a multimodal classification scheme with Aβ, tau, and neurodegeneration (ie, the ATN system8) was associated with differences in NfL level and whether changes in NfL level were associated with longitudinal changes in other measures.
Methods
Data were obtained from the ADNI database,9 which was launched in 2003 as a public-private partnership and is led by principal investigator Michael W. Weiner, MD. The ADNI participants have been recruited from more than 50 sites across the United States and Canada. For the present study, we used data accessed at the ADNI database on October 8, 2018. The study data and samples were collected from September 7, 2005, through June 16, 2016. Regional ethical committees of all institutions approved the ADNI study. All study participants gave written informed consent. This study was carried out according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.10
Participants
Our cohort consisted of all CU control individuals and patients with MCI and AD dementia with available plasma NfL measurements enrolled in the ADNI. Inclusion and exclusion criteria have been described.11 All ADNI participants were aged 55 to 90 years, had completed at least 6 years of education, were fluent in Spanish or English, and had no significant neurologic disease other than AD. The CU participants reported a Mini-Mental State Examination (MMSE) score of 24 or higher and a Clinical Dementia Rating Scale (CDR) score of zero. The MCI participants reported an MMSE score of 24 or higher, objective memory loss tested by delayed recall of the Wechsler Memory Scale Logical Memory II, a CDR score of 0.5, preserved activities of daily living, and absence of dementia. The patients with AD dementia fulfilled the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association criteria for probable AD,12 reported an MMSE score between 20 and 26, and a CDR score from 0.5 to 1.0.
Plasma NfL
Plasma NfL level was measured at the Clinical Neurochemistry Laboratory, University of Gothenburg, Mölndal Campus, Mölndal, Sweden, using an in-house ultrasensitive enzyme-linked immunosorbent assay on a single molecule array platform (Quanterix Corp).6 The lower limit of quantificaiton was 6.7 ng/L, and the upper limit of quantification was 1620.0 ng/L. All but one sample measured within the range spanned by the limits of quantification. The intra-assay coefficient of variation was 6.2% and the interassay coefficients of variation was 9.0% for the low-concentration quality control sample of 11.0 ng/L. The intra-assay coefficient of variation was 4.9% and the interassay coefficient of variation was 7.2% for the high-concentration quality control sample of 173.0 ng/L. The measurements were performed from January 1 through April 1, 2018, by a board-certified laboratory technician using a single batch of reagents.
Other Biochemical Markers
The CSF samples were obtained by lumbar puncture from a subset of participants. Data on the number of CSF samples (and other measures) are available in eTable 1 in the Supplement. Concentrations of Aβ42, t-tau, and p-tau were quantified using fully automated Elecsys assays (Roche Diagnostics).13,14
Neuroimaging
Structural brain images were acquired using 3-T MRI scanners with T1-weighted MRI scans using a sagittal volumetric magnetization-prepared rapid gradient echo sequence. FreeSurfer, version 5.1 (FreeSurfer) was used for quantification of regional thickness and volumes according to the 2010 Desikan-Killany atlas.15 We used volumetric data for hippocampus and lateral ventricle measures, whereas cortical thickness was used for the entorhinal cortex and for a meta-region of interest (temporal composite) involving entorhinal, inferior temporal, middle temporal, and fusiform cortex. Volumetric measures were adjusted for total intracranial volume using a previously described method whereby the imaging measure (y) is regressed on total intracranial volume (x) in the CU group and the adjusted imaging measure for all patients is subsequently computed as the residual value from the regression line.16 The adjusted imaging measure is interpreted as the deviation from the value expected in a healthy individual with the observed total intracranial volume. White matter lesion volume was quantified from fluid-attenuated inversion recovery images using an automated pipeline.17 The FDG-PET scans were acquired, and an FDG composite score was calculated as the mean uptake in left and right angular, temporal, and posterior cingulate regions.18
Cognitive Tests
Cognition was assessed using the MMSE, the 11-item version of the Alzheimer Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), and the CDR Scale Sum of Boxes (CDR-SB).
Cut Points
For Aβ positivity, we used a published cut point (CSF Aβ42 level, <880 ng/L).14 Cerebrospinal fluid t-tau positivity (>300 ng/L) and CSF p-tau positivity (>27 ng/L) were defined recently by maximizing the ability to predict progression in MCI (Oskar Hansson and K.B., unpublished data). For imaging measures and cognitive tests, we used the 90th percentile (toward the normal range, giving 90% sensitivity for AD) in the group with AD dementia to define positivity.16 As a sensitivity analysis, we also defined cut points at the mean level plus 2 SDs in Aβ-negative CU (or minus 2 SDs for hippocampal volume, cortical thickness, FDG-PET, and MMSE measures). Cut points are summarized in eTable 2 in the Supplement.
Statistical Analysis
We tested linear mixed-effects (LME) models with longitudinal NfL level as the response and different variables, including diagnostic group, baseline CSF biomarkers, MRI measures, and cognitive test findings. We evaluated models with dichotomous and continuous variables. Second, we repeated the LME models described above for each of the different diagnostic groups separately. Third, we evaluated LME models while simultaneously stratifying patients by amyloid pathology (A), defined as CSF Aβ42 level, <880 ng/L; tau pathology (T), defined as CSF p-tau level, >27 ng/L; and neurodegeneration (N), defined as a thin temporal cortex, using the 90% sensitivity for AD cut point (<2.75 mm) simultaneously in accordance with the ATN framework.8 Fourth, we tested correlations between longitudinal NfL level and longitudinal data for the other measures by computing the Pearson correlation between individual-specific random slopes from LME models for NfL level and random slopes from LME models similarly fit among the other measures.
All LME models included random intercepts and slopes and were adjusted for age and sex. Time was treated as a continuous variable. The models were fit using maximum likelihood estimation and differences in trajectories (ie, estimated change or slope) across groups were assessed using approximate F or t tests. When R2 values are presented for LME models, we refer to the marginal R2 (the proportion of the variance that is explained by the fixed effects). All statistical analyses were performed using the R programming language, version 3.4.3 (R Foundation), with LME analysis performed specifically using the nlme package, version 3.1. All tests were 2-sided with a significance level of P < .05.
Results
Of 1583 participants included in the study, 716 (45.2%) were women, and the mean (SD) age was 72.9 (7.1) years. We included 401 CU controls (median number of samples, 3; interquartile range, 2-4), 855 with MCI (median number of sample, 3; interquartile range, 2-4), and 327 with AD dementia (median number of samples, 1; interquartile range, 1-2), or a total of 4326 plasma NfL level measures from samples obtained annually up to 11 years after the baseline examination (Table 1). At baseline and irrespective of diagnostic group, NfL level was positively associated with age (r, 0.36; P < .001) but not with educational level or sex.
Table 1. Patient Demographics.
| Characteristic | Cognitively Unimpaired (n = 401) | Mild Cognitive Impairment (n = 855) | Alzheimer Disease (n = 327) |
|---|---|---|---|
| Age at baseline, mean (SD), y | 74.6 (5.6) | 72.4 (7.4) | 74.9 (7.8) |
| Male sex, No. (%) | 203 (50.6) | 485 (56.7) | 179 (54.7) |
| APOE ε4−/−, No. (%) | 290 (72.3) | 453 (52.9) | 153 (46.8) |
| APOE ε4+/−, No. (%) | 100 (24.9) | 326 (38.1) | 110 (33.6) |
| APOE ε4+/+, No. (%) | 11 (2.7) | 76 (8.8) | 64 (19.6) |
| Educational level, mean (SD), y | 16.3 (2.7) | 16.1 (2.7) | 15.1 (3.0) |
| CSF biomarkers at baseline, mean (SD), ng/L | |||
| Aβ42 level | 1191.0 (451.3) | 999.2 (445.5) | 674.5 (317.5) |
| p-tau Level | 21.8 (9.2) | 26.9 (14.5) | 36.9 (15.8) |
| t-tau Level | 236.7 (90.0) | 279.3 (130.3) | 369.8 (145.9) |
| Imaging measure at baseline, mean (SD)a | |||
| Hippocampus, mm3 | 7363.9 (899.7) | 6959.0 (1132.5) | 5751.5 (1003.2) |
| Entorhinal cortex, mm | 3.5 (0.3) | 3.4 (0.4) | 2.8 (0.5) |
| Temporal composite, mm | 2.78 (0.1) | 2.73 (0.2) | 2.53 (0.2) |
| Ventricular volume, mm3 | 34 304.8 (18 876.4) | 38 654.5 (22 123.9) | 49 411.1 (24 247.4) |
| FDG-PET composite, mean (SD) | 1.29 (0.12) | 1.24 (0.15) | 1.07 (0.15) |
| White matter lesions, mean (SD), mm3 | 6.42 (11.46) | 6.94 (8.94) | 8.45 (9.45) |
| Cognitive score at baseline | |||
| MMSE | 29.1 (1.1) | 27.9 (1.8) | 23.2 (2.0) |
| CDR-SB | 0.03 (0.1) | 1.3 (0.9) | 4.44 (1.7) |
| ADAS-Cog | 6.0 (3.0) | 9.4 (4.7) | 19.6 (6.7) |
| Plasma NfL, No. of samples | 1260 | 2610 | 456 |
| Baseline | 376 | 760 | 321 |
| Month | |||
| 6 | 1 | 1 | 0 |
| 12 | 178 | 465 | 101 |
| 24 | 152 | 451 | 31 |
| 36 | 28 | 329 | 1 |
| 48 | 134 | 245 | 2 |
| 60 | 100 | 101 | 0 |
| 72 | 106 | 108 | 0 |
| 84 | 95 | 80 | 0 |
| 96 | 64 | 54 | 0 |
| 108 | 23 | 14 | 0 |
| 120 | 3 | 2 | 0 |
Abbreviations: Aβ, amyloid β; ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; APOE, apolipoprotein E; CDR-SB, Clinical Dementia Rating Scale Sum of Boxes; CSF, cerebrospinal fluid; FDG-PET, fluorodeoxyglucose–positron emission tomography; MMSE, Mini-Mental State Examination; NfL, neurofilament light; p-tau, phosphorylated tau; and t-tau, total tau.
Imaging measures reported here are unadjusted by total intracranial volume.
Plasma NfL and Diagnosis
Baseline NfL level was higher in patients with MCI (37.9 ng/L) and AD dementia (45.9 ng/L) than in the CU controls (32.1 ng/L). The NfL level increased significantly in all groups, with greater rates among patients with MCI (2.7 ng/L per year; P = .22 vs CU controls) compared with CU controls (2.4 ng/L per year; P < .001) and greater rates among patients with AD dementia compared with the CU controls and patients with MCI (4.9 ng/L per year; P = .02 vs CU controls and P = .04 vs patients with MCI) (Figure 1A-D). Intercepts and rates were compared within diagnostic groups, stratified by Aβ status (defined by CSF Aβ42). The Aβ-positive CU controls had increased rates compared with the Aβ-negative CU controls, and patients with Aβ-positive MCI had increased baseline levels and increased rates compared with patients with Aβ-negative MCI. There were no significant differences between the Aβ-negative and Aβ-positive AD groups (Figure 1E and eTables 3 and 4 in the Supplement).
Figure 1. Plasma Neurofilament Light (NfL) Level by Diagnostic Group, Aβ-Status, and ATN Classification.
Observed data in different diagnostic (DX) groups (A-C). Estimated means and 95% CI in DX groups (D), with results from a linear mixed-effects model were adjusted for age (associated with greater NfL levels, β, 1.3; P < .001) and sex (P = .21). Estimated means and 95% CI of the means for different diagnostic groups (E) were stratified by amyloid β (Aβ) status (defined by cerebrospinal fluid [CSF] Aβ42). Estimated means and 95% CIs of the means in the ATN groups (F-I) were defined using CSF Aβ42 for A, CSF phosphorylated tau (p-tau) for T, and the temporal cortical composite for N in the ATN system. Trajectories were tested in linear mixed-effects models, adjusted for age and sex. The eTables 3 and 4 in the Supplement give details on DX group and Aβ status, and eTables 6 and 7 in the Supplement give details on the ATN classification. AD indicates Alzheimer dementia; CU, cognitively unimpaired; and MCI, mild cognitive impairment.
Plasma NfL by Baseline Biomarkers, Imaging Findings, and Cognition
Table 2 shows the associations between longitudinal NfL levels and the baseline variables of CSF Aβ42, t-tau level, and p-tau levels; hippocampal volume; entorhinal cortical thickness; ventricular volume; temporal cortical thickness; FDG-PET findings; white matter lesions; MMSE score; CDR-SB score; and ADAS-Cog score irrespective of the diagnostic group. All variables were associated with higher baseline NfL levels (both when used as continuous or dichotomous variables). All variables were also associated with more rapid increase in NfL levels when used continuously. Of the CSF biomarkers, Aβ42 had the strongest association with longitudinal NfL levels (β, –0.64; indicating that a 1-SD lower baseline Aβ42 level was associated with a 0.64-ng/L greater increase in NfL levels per year compared with a baseline Aβ42 level close to the mean). Of the imaging measures, hippocampal volume (β, –0.74) and the entorhinal cortical thickness (β, –0.78) had the largest association with NfL slope, whereas ADAS-Cog score had the largest association of the cognitive measures (β, 0.74). When used as dichotomous variables, the results were similar for baseline NfL level, but the associations with longitudinal NfL levels were reduced for some of the variables. The models had R2 values of 0.21 to 0.26 for continuous variables and R2 values of 0.20 to 0.24 for dichotomous variables (the highest R2 values were seen for hippocampal volume).
Table 2. Variables Associated With Longitudinal Plasma Neurofilament Light Measuresa.
| Measure | Continuous Variables | Dichotomous Variablesb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline NfL Level | NfL Rate | R2 | Baseline NfL Level | NfL Rate | R2 | |||||
| β | P Valuec | β | P Valuec | β | P Valuec | β | P Valuec | |||
| Aβ42 level | −3.11 | <.001 (<.001) | −0.64 | <.001 (.001) | 0.22 | 5.35 | <.001 (<.001) | 0.98 | .007 (.01) | 0.21 |
| t-tau Level | 2.99 | <.001 (<.001) | 0.49 | .01 (.02) | 0.21 | 5.29 | <.001 (<.001) | 1.25 | .001 (.004) | 0.21 |
| p-tau Level | 2.70 | <.001 (.002) | 0.52 | .009 (.02) | 0.21 | 5.34 | <.001 (<.001) | 1.37 | <.001 (.003) | 0.21 |
| Hippocampus volume | −4.56 | <.001 (<.001) | −0.74 | <.001 (<.001) | 0.26 | 7.66 | <.001 (<.001) | 1.22 | <.001 (.001) | 0.24 |
| Entorhinal cortex | −3.79 | <.001 (<.001) | −0.78 | .001 (.002) | 0.23 | 5.81 | <.001 (<.001) | 1.05 | .006 (.01) | 0.22 |
| Temporal composite | −4.35 | <.001 (<.001) | −0.62 | .005 (.009) | 0.24 | 7.75 | <.001 (<.001) | 0.86 | .04 (.06) | 0.23 |
| Ventricular volume | 3.55 | <.001 (<.001) | 0.55 | <.001 (.002) | 0.22 | 3.90 | .002 (.006) | 0.60 | .06 (.08) | 0.20 |
| FDG-PET composite | −3.79 | <.001 (<.001) | −0.51 | .008 (.01) | 0.23 | 6.78 | <.001 (<.001) | 1.01 | .004 (.01) | 0.24 |
| White matter lesions | 0.99 | .11 (.15) | 0.36 | .09 (.10) | 0.18 | 2.28 | .13 (.15) | 1.18 | .02 (.03) | 0.19 |
| MMSE score | −4.35 | <.001 (<.001) | −0.4 | .04 (.04) | 0.22 | 10.38 | <.001 (<.001) | 0.95 | .07 (.08) | 0.22 |
| CDR-SB score | 4.46 | <.001 (<.001) | 0.65 | .002 (.004) | 0.22 | 22 | <.001 (<.001) | 1.47 | .78 (.78) | 0.21 |
| ADAS-Cog score | 4.59 | <.001 (<.001) | 0.74 | <.001 (<.0001) | 0.24 | 17.79 | <.001 (<.001) | 4.65 | .23 (.25) | 0.23 |
Abbreviations: Aβ, amyloid β; ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; CDR-SB, Clinical Dementia Rating Scale Sum of Boxes; CSF, cerebrospinal fluid; FDG-PET, fluorodeoxyglucose-positron emission tomography; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; NfL, neurofilament light; p-tau, phosphorylated tau; and t-tau, total tau.
Variables associated with longitudinal NfL measures were tested in linear mixed-effects models adjusted for age and sex. All predictors were scaled to have zero mean and unit variance so that effect sizes are directly comparable with the additional change in NfL levels per year (or additional baseline NfL levels) expected with a 1-SD increase in the variable’s value. For dichotomous variables, normal is always represented as zero and pathological as 1; therefore, all dichotomous coefficients are positive, indicating the effect of being pathological for the given measure.
Cut points were a priori (CSF) or 90% sensitivity for Alzheimer disease (MRI and cognition).
P values in parentheses are corrected for multiple comparisons using the Benjamini-Hochberg procedure.
Plasma NfL by Baseline Biomarkers, Imaging Findings, and Cognition in Different Diagnostic Groups
eTable 5 in the Supplement shows prediction of the longitudinal NfL level within each diagnostic group using continuous variables. Among the CU controls, a longitudinal NfL level increase was most strongly associated with lower FDG-PET measures, a lower Aβ42 level, and a lower hippocampal volume and was also associated with a higher p-tau level and higher ventricular volume. Among patients with MCI, longitudinal NfL level increase was significantly associated with all measures (except FDG-PET and white matter lesions), with the strongest associations for hippocampal volume, temporal composite, and cognitive measures. Among patients with AD, longitudinal change in NfL levels was associated with ADAS-Cog. The models’ R2 values ranged from 0.17 to 0.25 for different variables and diagnostic groups.
Plasma NfL in Groups Stratified by Aβ, Tau, and Neurodegeneration
Using the ATN system, increased baseline NfL levels were seen in neurodegeneration (N+) cases, specifically in the A–T+N+, A+T–N+ (P < .001), and A+T+N+ (P < .001) groups. Longitudinal rates of plasma NfL levels were increased in N+ cases (A–T+N+ [P = .02], A+T–N+ [P = .04], and A+T+N+ [P = .002]) but were also high in the T+ only (A–T+N–) subgroup (Figure 1F through I and eTables 6 and 7 in the Supplement). Overall, the ATN model had an R2 of 0.25.
Variability in NfL Rates
Figure 2 shows the variability in patient-specific rates of NfL. When comparing clinical diagnoses, variability of slopes was highest in the AD group (SD of 3.4% of baseline levels vs 2.2% in patients in the MCI group and 1.9% in the CU group). When stratifying by diagnosis and Aβ status, variability was highest in the Aβ-positive groups (SD, 4.3% of baseline levels in the Aβ-positive AD group vs 2.8% in the Aβ-negative AD group; 2.9% in the Aβ-positive MCI group vs 1.8% in the Aβ-negative MCI group; and 3.7% of baseline in the Aβ-positive CU group vs 2.3% in the Aβ-negative CU group). When stratifying by ATN class, slope variability was highest in T+ participants (5.1% of baseline in A+T+N–, 3.3% in A+T+N+, and 3.3% in A–T+N–).
Figure 2. Distribution of Patient-Specific Plasma Neurofilament Light (NfL) Slopes.
The ATN system is described in the Statistical Analysis subsection of the Methods section. Aβ indicates amyloid β; AD, Alzheimer disease; CU, cognitively unimpaired; and MCI, mild cognitive impairment.
Longitudinal Changes in NfL Levels and Other Measures
In CU controls and patients with MCI and AD, greater rates of NfL were associated with accelerated reduction in FDG-PET measures (but not significantly in AD after correction for multiple comparisons), expansion of ventricular volume, reduction in MMSE scores, and increases in CDR-SB and ADAS-Cog scores. In addition, greater increases in NfL levels were associated with accelerated loss of hippocampal volume and entorhinal cortical thickness in CU controls and patients with MCI and with accelerated increases in t-tau level, p-tau level, and white matter lesions in patients with MCI (Table 3). In patients with MCI, there was also an association with a small increase in Aβ42 levels, but among the CSF biomarkers, the strongest association was seen for the t-tau levels.
Table 3. Correlations Between Changes in Plasma NfL Levels and Changes in Other Measuresa.
| Longitudinal Measure | Cognitively Unimpaired Controls | Patients With Mild Cognitive Impairment | Patients With Alzheimer Disease | |||
|---|---|---|---|---|---|---|
| ρ | P Valueb | ρ | P Valueb | ρ | P Valueb | |
| Aβ42 level | −0.1 | .12 (.16) | 0.09 | .01 (.03) | 0.05 | .45 (.45) |
| t-tau Level | 0.05 | .46 (.50) | 0.21 | <.001 (<.001) | 0.11 | .09 (.12) |
| p-tau Level | 0.03 | .58 (.58) | 0.13 | <.001 (.002) | 0.05 | .44 (.45) |
| Hippocampus volume | −0.18 | <.001 (.002) | −0.29 | <.001 (<.001) | −0.1 | .09 (.12) |
| Entorhinal cortex | −0.16 | .002 (.004) | −0.28 | <.001 (<.001) | −0.12 | .06 (.10) |
| Ventricular volume | 0.21 | <.001 (<.001) | 0.29 | <.001 (<.001) | 0.19 | <.001 (.001) |
| Temporal composite | −0.14 | .02 (.03) | −0.30 | <.001 (<.001) | −0.30 | <.001 (<.001) |
| Fluorodeoxyglucose composite | −0.14 | .01 (.02) | −0.26 | <.001 (<.001) | 0.14 | .03 (.06) |
| White matter lesions | 0.05 | .41 (.49) | 0.18 | <.001 (.001) | 0.08 | .29 (.35) |
| MMSE score | −0.13 | .009 (.02) | −0.36 | <.001 (<.001) | −0.24 | <.001 (<.001) |
| CDR-SB score | 0.3 | <.001 (<.001) | 0.36 | <.001 (<.001) | 0.28 | <.001 (<.001) |
| ADAS-Cog score | 0.19 | <.001 (.001) | 0.37 | <.001 (<.001) | 0.33 | <.001 |
Abbreviations: Aβ, amyloid β; ADAS-Cog, Alzheimer Disease Assessment Scale-Cognitive Subscale; CDR-SB, Clinical Dementia Rating Scale Sum of Boxes; CSF, cerebrospinal fluid; MMSE, Mini-Mental State Examination; NfL, neurofilament light; p-tau, phosphorylated tau; and t-tau, total tau.
Correlations between slopes for plasma NfL levels and slopes for other measures. Slopes for NfL levels and other measures were estimated in separate linear mixed-effects models (adjusted for age and sex) and then correlated with each other.
P values in parentheses are corrected for multiple comparisons using the Benjamini-Hochberg procedure.
Sensitivity Analyses
We repeated the main analyses without adjustment for age and sex (eFigures 1 and 2 and eTable 8 in the Supplement), which only had small effects on the associations between the variables and NfL levels. The main exception was that the overall R2 value of the models was reduced for some measures (Aβ42, t-tau, and p-tau levels and entorhinal cortex volume) when the covariates were not included.
For the analyses with dichotomous variables, we tested alternative strategies to define cut points (eTables 9 and 10 in the Supplement). There were slight differences in the results for the different cut point choices, suggesting that different cut point strategies may be used to optimize the associations between longitudinal NfL level and other measures for AD.
For analysis by clinical diagnosis and Aβ status, we removed an outlier patient with NfL rates higher than 30 ng/L and year. This patient had Aβ-negative AD, and the removal was associated with a decrease in the estimated group-level NfL rate compared with considering all patients. The group-level NfL rate still remained slightly above zero. It is difficult to draw strong conclusions about NfL level change in the Aβ-negative AD group because of the relatively few number of patients and follow-up visits.
Discussion
The findings supported our primary hypothesis, that plasma NfL levels increased in people with baseline AD-related features, and our secondary hypotheses, that changes in NfL levels differed by ATN class and were associated with longitudinal changes in other AD-associated measures. Compared with NfL levels in CU controls, the NfL level was increased at baseline in patients with MCI and AD dementia, and increased rates were found in patients with preclinical AD, prodromal AD, and AD dementia. Increased rates of NfL were also detected for people with abnormal baseline CSF biomarkers, MRI measures of atrophy, and reduced FDG-PET measures, and the associations were often strongest in patients with MCI. When stratifying by ATN class, the baseline NfL level was mainly increased in A–T+N+, A+T–N+, and A+T+N+; and rates were mainly increased in A–T+N–, A–T+N+, A+T–N+, and A+T+N+. Also, rates of NfL were correlated with rates of cognitive and imaging measures in all diagnostic groups and with CSF biomarkers in patients with MCI. Taken together, these findings suggest that the NfL level is a dynamic biomarker that changes throughout the course of AD and is sensitive to progressive neurodegeneration. This has important implications, given the unmet need for noninvasive blood-based methods to objectively track longitudinal neurodegeneration in AD. In general, groups with larger NfL slopes also had larger variability in patient-specific slopes.
The baseline associations replicated previous findings.7 The main novel aspect was the longitudinal analyses of NfL levels, which showed that baseline features of AD (including clinical diagnosis, CSF biomarkers, imaging measures, and cognitive test results) were associated with future increase in NfL levels and that longitudinal NfL levels were correlated with further change in other measures. The associations differed by clinical stage. For example, only some of the baseline measures (including lower Aβ42 level and higher p-tau level) were associated with longitudinal increase in NfL levels in CU controls, whereas most measures (atrophy, cognition, and CSF biomarkers) were associated with longitudinal increase in NfL level among patients with MCI, but only poor cognition (ADAS-Cog) was associated with longitudinal increase in NfL level among patients with AD dementia. When using dichotomous baseline variables, we replicated all findings in general but with some differences between different sets of cut points. For example, the a priori defined cut points for CSF biomarkers and the cut points at 90% sensitivity for AD for imaging and cognitive measures had stronger associations with the longitudinal NfL level compared with cut points defined using the data in Aβ-negative CU controls. Future studies can further refine optimal cut points for different measures. Worsening cognition and atrophy was correlated with an increase in NfL levels in all diagnostic groups, but the greatest correlations were seen in the MCI group. These results suggest that longitudinal NfL level can be used to dynamically track neurodegeneration throughout the preclinical stage and different clinical stages of AD.
Our ATN system explained about 25% of the variance in NfL level, which was similar to some of the best individual measures. Longitudinal NfL level was generally increased in patients who were classified as N+ (using temporal brain atrophy for the N-classifier; A–T+N+, A+T+N+, and A+T–N+) and in those who were isolated T+ (A–T+N–). The lowest slopes were seen in those who were T– and N–, independent of Aβ positivity (A+T–N– and A–T–N–). One interpretation of this is that NfL reflected neurodegeneration that occurred independent of Aβ pathology (of note, few patients had neurodegeneration because of causes other than AD, which may explain why Aβ positivity was associated with greater NfL slopes in all diagnostic categories). This result is similar to findings from other neurodegenerative conditions19,20 and brain injury due to other causes.6,21,22,23 In general, the concentrations of blood-based NfL appear to reflect the intensity of the neuronal injury. For example, for cardiac arrest, baseline serum NfL level was associated with neurological clinical outcome 6 months after the event.24
Limitations
One limitation is that we lacked tau–PET. We quantified tau using CSF biomarkers, which were associated with NfL levels, but previous studies suggest that tau-PET imaging is more closely associated with neurodegeneration compared with CSF tau biomarkers.25 For Aβ, we used CSF Aβ42. It may also be interesting to test Aβ-PET imaging in future studies. The analyses require a Quanterix single molecule array platform, which is still not readily available in most laboratories, and there to date are no in vitro diagnostics-approved methods for plasma NfL levels. There was a lack of long-term follow-up data in the AD dementia group because of drop out, whereas the estimates for long-term trajectories in the dementia group were uncertain. However, other data appeared to be missing at random, for which LME models were generally robust. We did not control for AD phenotype subtypes, and we cannot exclude the possibility that comorbidities (including vascular disease) contributed to the results. The study has potential for generalization in typical amnestic AD, but the value for extrahippocampal forms or the effect of vascular comorbidities is uncertain. For the recently proposed ATN classification,8 we acknowledge that it may be operationalized in many ways, which is beyond the scope of this study.
Conclusions
The findings suggest that plasma NfL level can be used as a noninvasive biomarker to track neurodegeneration in AD. Plasma NfL level may therefore be considered as a candidate tool to monitor effects on neurodegeneration in patients with AD, including in disease-modifying trials.
eTable 1. Longitudinal CSF, Imaging and Cognitive Data
eTable 2. Cut Points
eTable 3. Demographics by Diagnosis and Aβ Status
eTable 4. Effects of Diagnostic Group and Aβ Status on Plasma NFL
eTable 5. Predicting Longitudinal Plasma NfL in Different Diagnostic Groups
eTable 6. Demographics by ATN Class
eTable 7. Effects of ATN Classes on Plasma NfL
eTable 8. Predicting Longitudinal Plasma NfL Without Age and Sex as Covariates
eTable 9. Prediction of Plasma NfL Using Alternative Cut Points
eTable 10. Predicting Longitudinal Plasma NfL in Different Diagnostic Groups, Using Dichotomous Predictors
eFigure 1. Plasma NfL by Diagnostic Group Without Covariate Adjustment
eFigure 2. Plasma NfL by ATN Classification Without Covariate Adjustment
References
- 1.Scheltens P, Blennow K, Breteler MM, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505-517. doi: 10.1016/S0140-6736(15)01124-1 [DOI] [PubMed] [Google Scholar]
- 2.Jack CR Jr, Barnes J, Bernstein MA, et al. Magnetic resonance imaging in Alzheimer’s Disease Neuroimaging Initiative 2. Alzheimers Dement. 2015;11(7):740-756. doi: 10.1016/j.jalz.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laforce R Jr, Soucy J-P, Sellami L, et al. Molecular imaging in dementia: past, present, and future. Alzheimers Dement. 2018;14(11):1522-1552. doi: 10.1016/j.jalz.2018.06.2855 [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Zetterberg H. The past and the future of Alzheimer’s disease fluid biomarkers. J Alzheimers Dis. 2018;62(3):1125-1140. doi: 10.3233/JAD-170773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampel H, O’Bryant SE, Molinuevo JL, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14(11):639-652. doi: 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2015;3:135-140. doi: 10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer’s Disease Neuroimaging Initiative . Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557-566. doi: 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzheimer Disease Neuroimaging Initiative http://adni.loni.usc.edu. Accessed October 8, 2018.
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201-209. doi: 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 13.Bittner T, Zetterberg H, Teunissen CE, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517-526. doi: 10.1016/j.jalz.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 14.Hansson O, Seibyl J, Stomrud E, et al. ; Swedish BioFINDER study group; Alzheimer’s Disease Neuroimaging Initiative . CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470-1481. doi: 10.1016/j.jalz.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685-691. doi: 10.1002/jmri.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138(pt 12):3747-3759. doi: 10.1093/brain/awv283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Inf Process Med Imaging. 2009;21:239-251. doi: 10.1007/978-3-642-02498-6_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landau SM, Mintun MA, Joshi AD, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578-586. doi: 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs GG, Andreasson U, Liman V, et al. Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol. 2017;24(11):1326-e77. doi: 10.1111/ene.13389 [DOI] [PubMed] [Google Scholar]
- 20.Rohrer JD, Woollacott IOC, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87(13):1329-1336. doi: 10.1212/WNL.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Marchis GM, Katan M, Barro C, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol. 2018;25(3):562-568. doi: 10.1111/ene.13554 [DOI] [PubMed] [Google Scholar]
- 22.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791. doi: 10.1038/srep36791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moseby-Knappe M, Mattsson N, Nielsen N, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76(1):64-71. doi: 10.1001/jamaneurol.2018.3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattsson N, Schöll M, Strandberg O, et al. 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer’s disease. EMBO Mol Med. 2017;9(9):1212-1223. doi: 10.15252/emmm.201707809 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Longitudinal CSF, Imaging and Cognitive Data
eTable 2. Cut Points
eTable 3. Demographics by Diagnosis and Aβ Status
eTable 4. Effects of Diagnostic Group and Aβ Status on Plasma NFL
eTable 5. Predicting Longitudinal Plasma NfL in Different Diagnostic Groups
eTable 6. Demographics by ATN Class
eTable 7. Effects of ATN Classes on Plasma NfL
eTable 8. Predicting Longitudinal Plasma NfL Without Age and Sex as Covariates
eTable 9. Prediction of Plasma NfL Using Alternative Cut Points
eTable 10. Predicting Longitudinal Plasma NfL in Different Diagnostic Groups, Using Dichotomous Predictors
eFigure 1. Plasma NfL by Diagnostic Group Without Covariate Adjustment
eFigure 2. Plasma NfL by ATN Classification Without Covariate Adjustment