Key Points
Question
Does limiting sedation levels during hip fracture repair under spinal anesthesia reduce postoperative delirium overall or when stratified by baseline comorbidity?
Findings
In this randomized clinical trial that included 200 older patients randomized to receive lighter vs heavier sedation, limiting levels of sedation provided no significant overall benefit in reducing incident delirium. However, in a prespecified subgroup analysis, heavier vs lighter sedation levels doubled the risk of postoperative delirium in patients with low baseline comorbidities as defined by a Charlson comorbidity index score of 0.
Meaning
Limiting intraoperative sedation levels may reduce delirium in older patients with low baseline comorbidity.
Abstract
Importance
Postoperative delirium is the most common complication following major surgery in older patients. Intraoperative sedation levels are a possible modifiable risk factor for postoperative delirium.
Objective
To determine whether limiting sedation levels during spinal anesthesia reduces incident delirium overall.
Design, Setting, and Participants
This double-blind randomized clinical trial (A Strategy to Reduce the Incidence of Postoperative Delirum in Elderly Patients [STRIDE]) was conducted from November 18, 2011, to May 19, 2016, at a single academic medical center and included a consecutive sample of older patients (≥65 years) who were undergoing nonelective hip fracture repair with spinal anesthesia and propofol sedation. Patients were excluded for preoperative delirium or severe dementia. Of 538 hip fractures screened, 225 patients (41.8%) were eligible, 10 (1.9%) declined participation, 15 (2.8%) became ineligible between the time of consent and surgery, and 200 (37.2%) were randomized. The follow-up included postoperative days 1 to 5 or until hospital discharge.
Interventions
Heavier (modified observer’s assessment of sedation score of 0-2) or lighter (observer’s assessment of sedation score of 3-5) propofol sedation levels intraoperatively.
Main Outcomes and Measures
Delirium on postoperative days 1 to 5 or until hospital discharge determined via consensus panel using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) criteria. The incidence of delirium was compared between intervention groups with and without stratification by the Charlson comorbidity index (CCI).
Results
Of 200 participants, the mean (SD) age was 82 (8) years, 146 (73%) were women, 194 (97%) were white, and the mean (SD) CCI was 1.5 (1.8). One hundred participants each were randomized to receive lighter sedation levels or heavier sedation levels. A good separation of intraoperative sedation levels was confirmed by multiple indices. The overall incident delirium risk was 36.5% (n = 73) and 39% (n = 39) vs 34% (n = 34) in heavier and lighter sedation groups, respectively (P = .46). Intention-to-treat analyses indicated no statistically significant difference between groups in the risk of incident delirium (log-rank test χ2, 0.46; P = .46). However, in a prespecified subgroup analysis, when stratified by CCI, sedation levels did effect the delirium risk (P for interaction = .04); in low comorbid states (CCI = 0), heavier vs lighter sedation levels doubled the risk of delirium (hazard ratio, 2.3; 95% CI, 1.1- 4.9). The level of sedation did not affect delirium risk with a CCI of more than 0.
Conclusions and Relevance
In the primary analysis, limiting the level of sedation provided no significant benefit in reducing incident delirium. However, in a prespecified subgroup analysis, lighter sedation levels benefitted reducing postoperative delirium for persons with a CCI of 0.
Trial Registration
clinicaltrials.gov Identifier: NCT00590707
This randomized clinical trial evaluates the effect of lighter vs higher sedation levels during spinal anesthesia on incident delirium in patients who were undergoing hip fracture repair.
Introduction
Postoperative delirium (PD) is the most common complication after major surgery in older patients without cognitive impairment.1 Postoperative delirium carries with it personal, social, and economic burdens.2 This complication, with its associated costs, will assume increasing importance as the number of older patients in the US population continues to grow.
The mainstay of delirium management is prevention by control and/or elimination of modifiable risk factors. One such risk factor may be sedative medications, as both drug selection and dosage can be modified. The role of sedative and analgesic medications as iatrogenic risk factors for delirium in intensive care unit (ICU) and postsurgical patients is well described.3,4,5,6 The evidence suggests that the level of sedation varies substantially during surgery and that heavier sedation levels that are consistent with general anesthesia are commonplace intraoperatively.7 Managing intraoperative sedation may be an important modifiable risk factor; however, to our knowledge, few studies have been done in this area.
To help explain the contribution of sedation levels to PD, we undertook a randomized, 2-group, parallel superiority trial called A Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients (STRIDE). The primary aim of STRIDE was to compare the effect of lighter and heavier intraoperative propofol sedation levels on delirium incidence in older patients who were undergoing hip fracture repair. A secondary objective was a prespecified subgroup analysis to determine whether limiting sedation levels during spinal anesthesia reduces incident PD when stratified by baseline comorbidity.
Methods
Study Design and Participants
The research protocol was approved by the Johns Hopkins institutional review board (NA_00041873). The trial was registered at ClinicalTrials.gov (NCT00590707) (Supplement 1). All participants provided their written informed consent. STRIDE was conducted at a single clinical center, and all intraoperative management was provided by 4 anesthesiologists. A detailed description of the trial protocol was published previously in the supplemental material of Li et al.8
Patients who were 65 years or older who were undergoing hip fracture repair with spinal anesthesia and propofol sedation and who did not have preoperative delirium or severe dementia were randomized to receive either heavier (modified observer’s assessment of alertness/sedation score [OAA/S], 0-2) or lighter (OAA/S, 3–5) intraoperative sedation levels.9 An OAA/S range for each sedation level was felt to more accurately reflect the clinical picture because exact OAA/S targets cannot always be achieved. The inclusion criteria were (1) admission to Johns Hopkins Bayview Medical Center for surgical repair of a traumatic hip fracture, (2) being age 65 years or older, (3) a preoperative Mini-Mental State Examination (MMSE) score of 15 or higher,10 and (4) receiving spinal anesthesia. The exclusion criteria included (1) receiving general anesthesia, (2) an inability to speak or understand English, (3) severe chronic obstructive pulmonary disease or congestive heart failure, (4) refusal to give informed consent, (5) a nonparticipating attending surgeon, (6) hip fractures in both hips at the same admission, (7) a repair of another fracture concurrently with the hip fracture, (8) a prior hip surgery on the same hip that would be repaired in the current surgery, and (9) preoperative delirium.
Outcomes
Patients were assessed for delirium, delirium severity, and cognition preoperatively and on postoperative days 1 to 5 by research personnel who were masked to the randomization assignment. The diagnosis of delirium was made by a multidisciplinary consensus panel based on Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria using several data sources, including the confusion assessment method,11 the Delirium Rating Scale-Revised-98 (DRS),12 digit span, a review of medical records, and family/nursing staff interviews. The primary outcome was the incidence of delirium from postoperative day 1 to postoperative day 5 or hospital discharge, whichever occurred first. The secondary outcomes were MMSE scores and DRS severity and total scores during the first 5 postoperative days or until hospital discharge, whichever occurred first. The prespecified secondary objective was to assess the interaction between baseline comorbidity and propofol sedation levels in determining delirium incidence after hip fracture repair (see the protocol in the supplemental material of Li et al8).
STRIDE was powered to detect a 48.0% risk reduction in the primary outcome, PD in-hospital up to day 5 after surgery, from 39.6% in the heavier sedation arm to 20.6% in the lighter sedation arm with 80% power using a 2-sided test with a type I error of 0.05 through a sample of 200 participants in equal allocation who were randomly assigned to the 2 study arms.8 These power assumptions were based on our preliminary randomized clinical trial.13
Statistical Analysis
All analyses were conducted with the intention-to-treat principle. The incidence of delirium in the 2 intervention groups was compared using the time-to-event analytic approach. The cumulative incidence of PD was estimated by a Kaplan-Meier analysis, and the between-group difference of incidence was compared with the log-rank test. The relative risk of PD between intervention groups was evaluated with Cox proportional hazards models. The proportionality assumption was verified using Schoenfeld residuals and a proportionality test. The secondary outcomes included the tracking of daily MMSE scores, DRS severity, and total scores during the participant’s hospital stay using a nonparametric regression. Between-group comparisons were conducted with mixed-effects linear models.8 The binary outcome of PD that occurred in both intervention groups was evaluated by a logistic regression. All of the modeling approaches accounted for the age and MMSE score at baseline that was used in stratified randomization. Our only preplanned subgroup analyses explored the heterogeneity of intervention effects by stratifying the study groups according to the Charlson comorbidity index (CCI) score.14 The CCI score was not age corrected and was calculated as previously described,14 except that baseline a Clinical Dementia Rating score of 1 or more defined dementia. The treatment interaction with the CCI scores on the time-to-event outcome was tested with a Cox proportional hazards model and accounted for the covariates that were related to the outcome and the CCI scores. Treatment-CCI interactions on the binary outcome of PD were tested by a logistic regression similarly. In these subgroup analyses, we treated the CCI scores as categorical variables in exploratory analyses to identify parsimonious effect modification models. We tested them as a continuous variable that extended through the full range of CCI scores and as a “truncated” variable that combined all scores of 3 or more as more than 2. Baseline characteristics that are associated with PD and CCI scores were adjusted for in these effect modification models, in addition to baseline age and MMSE scores. All analyses were conducted with SAS, version 9.4 (SAS Institute).
Results
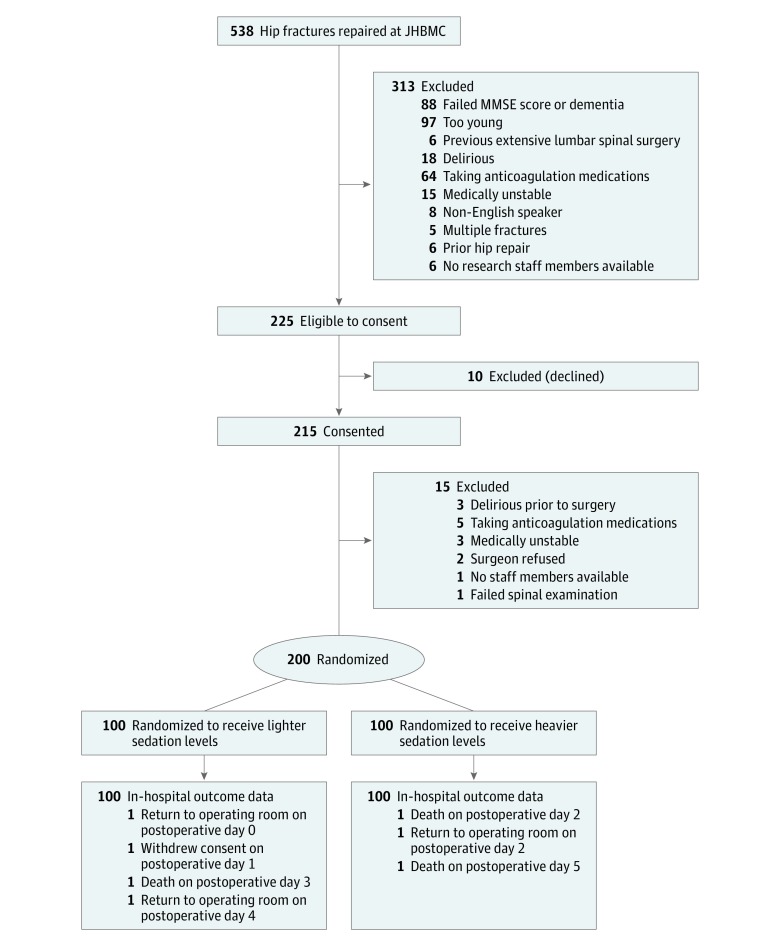
Figure 1 outlines the Consolidated Standards of Reporting diagram for STRIDE. Of 538 patients with a hip fracture who were screened from November 18, 2011, through May 19, 2016, 200 patients (37.2%) were randomized to receive either lighter or heavier sedation levels. The most common reasons for ineligibility were an age younger than 65 years (97 [18.0%]) and an MMSE score of less than 15 (88 [16.4%]). Of 225 eligible patients who were approached to provide consent, only 10 (4.4%) declined participation; 15 (6.7%) became ineligible between the time of consent and surgery.
Figure 1. Consolidated Standard of Reporting Diagram for STRIDE Study.
Patients were recruited between November 18, 2011, and May 19, 2016. JHBMC indicates John Hopkins Bayview Medical Center; MMSE, Mini-Mental State Examination.
Baseline Characteristics
Baseline demographics were similar in the 2 intervention groups (Table 1). Forty-eight participants (24%) had any education beyond high school. The baseline comorbidities, as assessed by CCI scores, and functional status were well-matched between the groups. Sixty-seven (33.5%) and 75 (37.5%) participants were capable of performing all physical self-maintenance scale items and the instrumental activities of daily living items, respectively.15 One-hundred sixteen (58%) had a smoking history, and 27 (13.5%) scored 2 or greater on the CAGE16 alcoholism questionnaire.
Table 1. Baseline Patient Characteristics by Depth of Sedation During Surgery.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total (N = 200) | Lighter (n = 100) | Heavier (n = 100) | |
| Age, mean (SD), y | 81.8 (7.7) | 81.6 (8.2) | 82.0 (7.2) |
| Body mass index (calculated as weight in kilograms divided by height in meters squared), mean (SD) | 25.0 (5.3) | 25.2 (5.2) | 24.8 (5.4) |
| Female | 146 (73.0) | 72 (72.0) | 74 (74.0) |
| White race/ethnicity | 194 (97.0) | 97 (97.0) | 97 (97.0) |
| Education level | |||
| Less than high school | 76 (38.0) | 37 (37.0) | 39 (39.0) |
| High school | 76 (38.0) | 37 (37.0) | 39 (39.0) |
| Some college | 28 (14.0) | 16 (16.0) | 12 (12.0) |
| College graduate or higher | 20 (10.0) | 10 (10.0) | 10 (10.0) |
| Employment | |||
| Retired/disabled | 162 (81.0) | 83 (83.0) | 79 (79.0) |
| Working full-time/part-time | 7 (3.5) | 3 (3.0) | 4 (2.0) |
| Homemaker | 31 (15.5) | 14 (14.0) | 17 (17.0) |
| Residence | |||
| Own home/family home | 172 (86.0) | 82 (82.0) | 90 (90.0) |
| Assisted living/nursing home | 7 (3.5) | 6 (6.0) | 1 (1.0) |
| Other | 21 (10.5) | 12 (12.0) | 9 (9.0) |
| Reside with | |||
| Alone | 85 (42.5) | 44 (44.0) | 41 (41.0) |
| Spouse/partner | 47 (28.0) | 21 (27.0) | 26 (29.0) |
| Family member (nonspouse) | 52 (30.5) | 23 (29.0) | 29 (32.0) |
| Nonfamily member | 1 (0.5) | 0 | 1 (1.0) |
| Other | 15 (3.0) | 12 (6.0) | 3 (0.0) |
| Status prior to surgery | |||
| Charlson comorbidity index score, mean (SD) | 1.5 (1.8) | 1.5 (1.8) | 1.6 (1.8) |
| American Society of Anesthesiologist rating ≥3 | 139 (69.5) | 63 (63.0) | 76 (76.0) |
| PSMS, mean (SD) | 4.61 (1.52) | 4.66 (1.48) | 4.56 (1.57) |
| IADL scale, mean (SD) | 5.85 (2.22) | 5.84 (2.17) | 5.86 (2.28) |
| Alcohol, CAGE score ≥2 | 27 (13.5) | 7 (7.0) | 20 (20.0) |
| Current cigarette smoker | 31 (15.5) | 18 (18.0) | 13 (13.0) |
| Baseline cognitive testing | |||
| Mini-Mental State Examination score, mean (SD) | 24.3 (3.7) | 24.4 (3.8) | 24.2 (3.6) |
| Geriatric Depression score, mean (SD) | 3.83 (3.48) | 3.86 (3.71) | 3.80 (3.25) |
| Clinical Dementia Rating score (n = 198/99/99) | |||
| 0 | 82 (41.4) | 46 (46.5) | 36 (36.4) |
| 0.5 | 94 (47.5) | 43 (43.4) | 51 (51.5) |
| 1 | 16 (8.1) | 7 (7.1) | 9 (9.1) |
| 2 | 6 (3.0) | 3 (3.0) | 3 (3.0) |
| Subsyndromal delirium | 13 (6.5) | 4 (4.0) | 9 (9.0) |
| Surgery characteristics | |||
| Emergency department to surgery, mean (SD), d | 1.2 (1.0) | 1.3 (1.2) | 1.2 (0.6) |
| Type of fracture | |||
| Femoral neck | 89 (44.5) | 42 (42.0) | 47 (47.0) |
| Inter/subtrochanteric | 111 (55.5) | 58 (58.0) | 53 (53.0) |
| Type of procedure | |||
| Hemiarthroplasty with/without cement | 69 (34.5) | 31 (31.0) | 38 (38.0) |
| Total hip arthroplasty with/without cement | 11 (5.5) | 5 (5.0) | 6 (6.0) |
| Cannulated screw | 9 (4.5) | 7 (7.0) | 2 (2.0) |
| Intramedullary nail | 110 (55.0) | 57 (57.0) | 53 (53.0) |
| Girdlestone | 1 (0.5) | 0 (0.0) | 1 (1.0) |
Abbreviations: CAGE, cut annoyed guilty eye; IADL, instrumental activities of daily living; PSMS, physical self-maintenance scale.
The baseline cognitive testing results were comparable between intervention groups. A Geriatric Depression Scale score of 6 or more occurred for 51 participants (25.5%),17 and 116 (58%) had a Clinical Dementia Rating score of 0.5 or more.18 The mean (SD) MMSE score for all participants was 24 (4). The type of fracture and surgical procedure, including the use of cement, did not differ between intervention groups (Table 1).
Intervention
Intraoperatively, the separation between groups was good (Table 2), as indicated by both modified OAA/S and EEG criteria (Bispectral index; BIS Brain Monitoring System, http://www.medtronic.com/covidien/products/brain-monitoring). The sedation scores demonstrate a clinical difference between lighter (OAA/S ~ 4 represents a lethargic response to calling a name in a normal tone) vs heavier sedation levels (OAA/S ~ 0 occurs when the participant does not respond to noxious stimuli). The bispectral index values reflect the same clinical differences as the OAA/S scores. As expected, the heavier sedation group received a higher total propofol dose. No intraoperative narcotics or benzodiazepines were administered to any patient. Patients in the heavier sedation group had longer surgical times secondary to prolonged awakening. Intraoperative mean arterial pressure was lower in the heavier sedation group secondary to the cardiovascular depressant effects of propofol. The overall level of spinal anesthesia was T9 ± 1.5 dermatomes. Most blood transfusions occurred postoperatively within 72 hours, with only 4 patients (2%) receiving intraoperative transfusions.
Table 2. Intraoperative and Postoperative Data by Depth of Sedation During Surgery.
| Characteristic | Mean (SD) | P Value | |
|---|---|---|---|
| Lighter (n = 100) | Heavier (n = 100) | ||
| Intraoperative | |||
| Modified Observer’s Assessment of Alertness/Sedation scale from incision to the end of surgerya | 4.1 (0.9) | 0.2 (0.4) | <.001 |
| Proportion of Modified Observer’s Assessment of Alertness/Sedation Scale recordings that fall in the desired range (0-2 for heavier levels and 3-5 for lighter sedation levels) from incision to the end of surgery, % | 89.9 | 97.7 | |
| Bispectral Index (n = 197/98/99) from incision to the end of surgeryb | 82.3 (9.4) | 57.0 (14.8) | <.001 |
| Total propofol dose, mg | 314.8 (185.0) | 739.1 (342.8) | <.001 |
| Total propofol dose by body weight, mg/kg | 4.6 (2.3) | 11.1 (4.4) | <.001 |
| Incision to end of surgery, min | 86.3 (31.0) | 96.8 (36.9) | .03 |
| Mean arterial pressure, mm Hg, (n = 199/99/100) from incision to the end of surgery | 75.7 (11.2) | 71.9 (9.5) | .01 |
| Estimated blood loss | 183.5 (154.5) | 201.3 (146.9) | .41 |
| Participants receiving an intraoperative RBC transfusion, No. (%) | 1 (1.0) | 3 (3.0) | .62 |
| Participants receiving a blood transfusion, within 72 h postoperative, No. (%)c | 37 (39.0) | 38 (39.2) | .99 |
| Postoperative | |||
| Mean daily morphine equivalents by weight, mg/kg | 0.13 (0.13) | 0.16 (0.19) | .14 |
| Mean daily pain score (Likert, 0-10) | 3.4 (2.6) | 3.9 (2.4) | .20 |
| Postoperative admission to ICU, No. (%) | 11 (11.0) | 13 (13.0) | .74 |
| Surgery until discharge, d | 4.1 (2.6) | 3.7 (2.5) | .27 |
| Discharge location, No. (%) | |||
| Rehabilitation center | 85 (85.0) | 89 (89.0) | .72 |
| Nursing home/assisted living | 1 (1.0) | 0 | |
| Own home/family home | 11 (11.0) | 9 (9.0) | |
| Other/death | 3 (3.0) | 2 (2.0) | |
Abbreviations: ICU, intensive care unit; RBC, red blood cells.
Sedation scores range from 0 (unresponsive) to 5 (wide awake).
Bispectral index values range from 40 to 60 (general anesthesia) to 100 (wide awake).
Of 192, there were 95 (49.5%) in the lighter sedation arm and 97 (50.5%) in the heavier sedation arm.
Patient Follow-up
Postoperative ICU admission rates, opioid consumption, and pain scores were similar in the 2 intervention groups (Table 2). All patients who were admitted to the ICU postoperatively had a 1-day length of stay. No patients in the ICU required intubation. The postoperative length of stay and hospital discharge location were comparable between intervention groups. There was no difference between groups in incidence or type of complications during the first 30 postoperative days.
Effect of Intervention on Incident PD
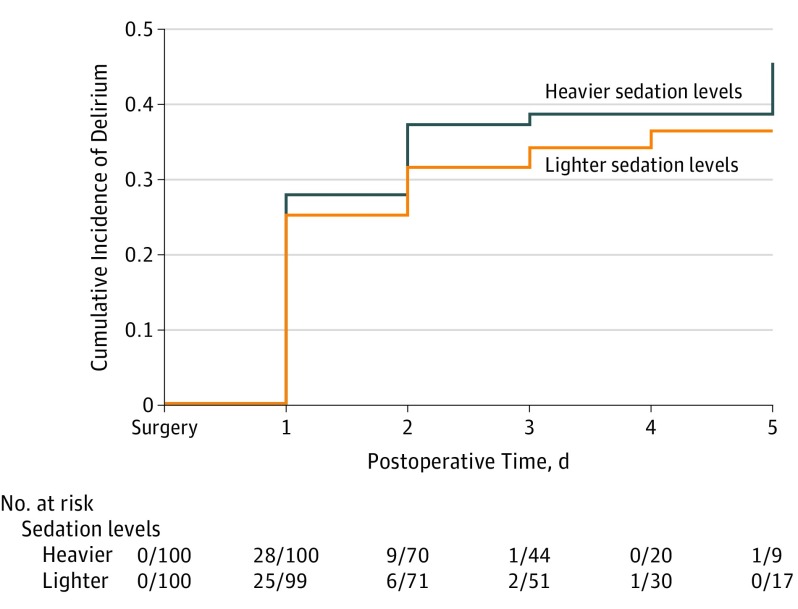
Without adjusting for comorbidities, the incidence of a delirium diagnosis at any time during postoperative days 1 to 5 was 39% (n = 39) in the heavier sedation group and 34% (n = 34) in the lighter sedation group (P = .46, by χ2 analysis). The overall incidence was 36.5% (n = 73). Although we observed consistently less PD in patients who received lighter sedation levels, the difference between groups was not statistically significant. An intention-to-treat analysis showed no statistical difference between groups in incident PD, total days of PD, or total days of PD + subsyndromal symptoms (Figure 2). Secondary outcomes showed that DRS severity was higher and MMSE scores were lower in the heavier sedation group on postoperative day 1 (eFigure in Supplement 2).
Figure 2. Kaplan-Meier Curve Showing Intention-to-Treat Analysis of Cumulative Incidence of Delirium During Postoperative Days 1 to 5 in the Lighter Sedation and Heavier Sedation Groups.
Log-rank P = .46.
Interaction Between Comorbidity and Intervention in Determining Incident PD
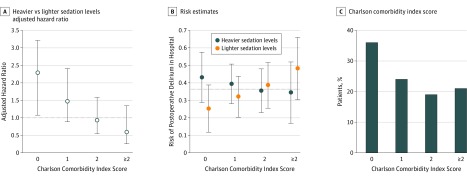
Incorporating a model that was adjusted for covariates that were associated with PD in the literature19,20 and this data set (age, MMSE score, fracture type, Geriatric Depression Scale score) revealed significant interaction between sedation levels and CCI in determining PD. Significant interactions occurred in the time-to-event outcome in the form of hazards ratios that were associated with the intervention from the Cox regression models as well as the risk in the binary outcome of ever receiving a diagnosis of PD (Figure 3). In patients with a baseline CCI score of 0, heavier sedation levels doubled the risk of PD (hazard ratio, 2.3; 95% CI, 1.1–4.9). In patients with higher baseline comorbidity, as indicated by a CCI score of more than 0, the level of sedation was not related to incident PD (eTables 1-4 in Supplement 2).
Figure 3. Charlson Comorbidity Index Scores.
A, The adjusted hazard ratio showed that patients with the least comorbidity (Charlson comorbidity index = 0) were 2.3 times more likely to experience postoperative delirium after receiving heavier sedation levels than after receiving lighter sedation levels. B, Risk estimates for in-hospital postoperative delirium by treatment group and the associated 95% confidence intervals. Sample sizes (incident cases/total cases) are indicated in eTable 1 in Supplement 2. B, The difference in delirium risk after heavier and lighter sedation levels was not significant in patients with greater preoperative comorbidity. The dashed horizontal line indicates a 36.5% risk of in-hospital postoperative delirium, which was the estimated overall risk from the entire STRIDE sample. C, Percentage of patients stratified by Charlson comorbidity index score.
Discussion
The overall intention-to-treat analysis, without considering comorbidity, showed no statistically significant benefits of lighter sedation levels during hip fracture repair under spinal anesthesia. However, secondary analyses that incorporated the consideration of comorbidities suggested that this lack of overall effect may be due to the heterogeneity of treatment effects (HTE) that was associated with baseline comorbidity. Preplanned secondary analyses for HTE showed that comorbidities may modify the treatment effect of sedation levels on PD risk in this older population with hip fracture. Lighter sedation levels were associated with lower PD incidence, but the difference from the heavier sedation group was statistically significant only in the subgroup with less comorbidity. At higher levels of comorbidity, the benefit of lowering PD risk by lighter sedation levels was attenuated. The selection of a sedation level can be an important decision in many patients as a means of decreasing PD. However, the benefits of lighter sedation levels may be overridden by baseline comorbidities.
The contribution of sedation depth to PD risk was examined in a previous randomized double-blind trial13 that used electroencephalography criteria (bispectral index) to determine lighter or heavier sedation levels. In that study, lighter sedation levels decreased the prevalence of PD by 50% compared with heavier sedation levels. However, the bispectral index is not routinely used to manage sedation because of its poor correlation with sedation scores, particularly in ICU patients.21 Additionally, several patients received multiple sedative agents, making the assessment of propofol-specific effects difficult. Furthermore, the total propofol dose was greater in the lighter sedation intervention arm of STRIDE than in the lighter sedation intervention arm of the previous study (4.6 ± 2.3 vs 2.5 ± 2.7 mg/kg, respectively). Our propofol doses were higher, having administered additional propofol to perform spinal anesthesia rather than other sedative agents to avoid any confounding effects. Although the exact propofol dosage that is required to transition to delirium is unclear,4 in vulnerable populations, propofol can precipitate delirium. Used as a sedative in the ICU, propofol is associated with rapidly reversible and persistent delirium.22 Furthermore, hallucinations and delirium are reported after patients receive propofol doses similar to those administered in the STRIDE lighter sedation treatment arm.23,24
The trial was not sufficiently powered to detect any intervention effect that would allow a greater than 20.6% PD risk in the lighter sedation arm. The small overall PD reduction that was observed with lighter sedation levels is far from the level that was hypothesized in the power calculation. However, the hypothesized study power is not totally unfounded, as the largest CCI subgroup, a CCI score of 0, exhibited effects from sedation depth that were similar to those in previous studies.13 The CCI score when treated as a continuous score modifies sedation depth effects on PD after adjusting for PD risk factors. At a CCI score of 0, lighter sedation levels were protective. The observation that higher CCI scores decreased the ability to prevent PD by modifying the depth of sedation is consistent with other studies that demonstrated that baseline vulnerabilities are the important independent drivers behind the development of PD.25 We previously reported that dementia is a strong risk factor for PD in patients with hip fractures.26 The high prevalence of baseline cognitive dysfunction in the STRIDE participants may have also influenced PD incidence, particularly at higher CCI scores. In addition, at a higher CCI, the estimate suggests a reverse situation in that heavier sedation levels might be protective. However, the trend was not significant and the estimate was unstable owing to small patient numbers at a high CCI. These factors limit our ability to investigate the reason for this reversal of effects and warrant additional studies. Several hypothesis that generate analysis were performed to elucidate the potential mechanisms that influenced the CCI interaction and intervention effect. The dementia CCI item alone did not predict PD after adjusting for baseline risk factors. When the CCI cardiovascular items were combined as a single score, there was significant intervention effect modification by this combined vascular score and risk of PD (χ2 = 4.07; P = .04), suggesting a cardiovascular mechanism.
Strengths and Limitations
STRIDE is an efficacy trial that examined whether lighter sedation levels produces clinically meaningful effects in reducing PD incidence in a high-risk population. To best answer this question, we controlled as many variables as possible. The STRIDE study strengths include using propofol as the only interventional drug. The high consent rate reduced selection bias. Compared with previous work, the methods were more rigorous. Four anesthesiologists anesthetized and managed all STRIDE cases in a consistent manner, and there were no protocol violations or group crossovers. Although a single-center study can lead to a loss of generalizability, we believe that protocol consistency and treated assignment administration outweighed this weakness. Additionally, hip fracture demographics are characterized by an older white female population.27 The STRIDE population reflects these demographics and its PD prevalence is consistent with recent multicenter trials.28,29,30 The perioperative delirium assessment was done in a systematic manner using standardized tools and a consensus panel. The randomization produced an excellent matching of participants in the 2 intervention groups. There were well-defined intraoperative differences in sedation levels between the intervention groups in terms of the propofol dose, OAAS, and bispectral index. Furthermore, bias was minimized in the delirium assessments and diagnostic process via a masking of assessors and consensus panel members to group assignments. The sample size was adequate to detect significant clinical effects.
One study weakness was the limiting of delirium testing to the first 5 postoperative days or until hospital discharge. Following hip fracture repair, the highest incidence of PD occurs on postoperative day 1,30,31 as confirmed in Figure 2. Furthermore, delirium duration in STRIDE was comparable with other studies that examined longer follow-up periods.32 Although some observations were cut short because of death, return to the operating room, or withdrawal, observations are available on every participant. Because most PD cases occurred on days 1 and 2 and the time-to-event approach treats the small numbers of observations that were cut short as censoring, the potential effect on our results would be minimal. Therefore, although statistical inaccuracy due to a shorter follow-up in STRIDE cannot be completely ruled out, the likelihood of inaccuracy is small. We did not measure delirium subtypes, which could represent an unintended consequence of the intervention. Only patients who could safely undergo spinal anesthesia were included. Although the study results cannot necessarily be generalized to all hip fracture patients, in the United States approximately 40% of all hip fracture repairs are performed with spinal anesthesia.26 The study results may not hold with anesthetic agents other than propofol. However, propofol is the most widely used drug for sedation. The STRIDE participants could reflect a healthier study population. However, the mean STRIDE CCI score33 and distribution of CCI scores were consistent with the literature.34 Several comorbidities that were included in the CCI calculation are associated with underlying cognitive dysfunction,35,36,37 but our multivariant analysis controlled for baseline MMSE scores. Because CCI is not a complete surrogate measure for baseline risk factors of delirium, the modeling results may have been limited. A prespecified subgroup analysis to look for HTE must be interpreted carefully. Heterogeneity and comorbidity in this population may have limited our ability to detect significant overall effects. However, heterogeneity is the expected clinical norm, and when assessing for the interaction with comorbidity, we did find significant treatment effects in the low-comorbidity subgroup. Besides in-hospital PD outcomes, it is important to determine the intervention effects on long-term functional and cognitive outcomes. These data are in the process of analysis and will be reported in a separate article.
Conclusions
In a subgroup analysis, the STRIDE results suggest that PD can be reduced in individuals with a CCI score of 0 by reducing sedation levels. The STRIDE trial suggests that the selection of sedation levels can be an important means of decreasing PD in many patients. However, the associated benefits of lighter sedation levels may be obscured by competing baseline comorbidities, placing patients at risk of developing PD. Given the STRIDE trial findings, the challenge for future research will be to determine the mechanisms and interactional relationships between comorbidities and precipitating risk factors for PD.
Trial Protocol
eTable 1. Raw numbers of patients diagnosed with delirium for main groups and sub-group analysis
eTable 2. Univariate analysis on associations between base line characteristics and incident postoperative delirium in hospital
eTable 3. Intervention Effect Modification by CCI on Post Operative Incident Delirium Risk; CCI Truncated Values >2
eTable 4. Intervention Effect Modification by CCI on Post Operative Incident Delirium Risk; CCI Continuous Values
eFigure. Mini-Mental State Exam score and Delirium Rating Scale-Revised ‘98 severity score at baseline and postoperative days 1–5 in the lighter-sedation and heavier-sedation groups
References
- 1.Gleason LJ, Schmitt EM, Kosar CM, et al. . Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. 2015;150(12):1134-1140. doi: 10.1001/jamasurg.2015.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi: 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande P, Ely EW. Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill. Crit Care Clin. 2006;22(2):313-327, vii. doi: 10.1016/j.ccc.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande P, Shintani A, Peterson J, et al. . Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-26. doi: 10.1097/00000542-200601000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297-1304. doi: 10.1007/s001340101017 [DOI] [PubMed] [Google Scholar]
- 6.Marcantonio ER, Goldman L, Mangione CM, et al. . A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139. doi: 10.1001/jama.1994.03510260066030 [DOI] [PubMed] [Google Scholar]
- 7.Sieber FE, Gottshalk A, Zakriya KJ, Mears SC, Lee H. General anesthesia occurs frequently in elderly patients during propofol-based sedation and spinal anesthesia. J Clin Anesth. 2010;22(3):179-183. doi: 10.1016/j.jclinane.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 8.Li T, Wieland LS, Oh E, et al. . Design considerations of a randomized controlled trial of sedation level during hip fracture repair surgery: a strategy to reduce the incidence of postoperative delirium in elderly patients. Clin Trials. 2017;14(3):299-307. doi: 10.1177/1740774516687253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86(4):836-847. doi: 10.1097/00000542-199704000-00014 [DOI] [PubMed] [Google Scholar]
- 10.Folstein MFFS, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- 12.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the delirium rating scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229-242. doi: 10.1176/jnp.13.2.229 [DOI] [PubMed] [Google Scholar]
- 13.Sieber FE, Zakriya KJ, Gottschalk A, et al. . Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85(1):18-26. doi: 10.4065/mcp.2009.0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 16.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252(14):1905-1907. doi: 10.1001/jama.1984.03350140051025 [DOI] [PubMed] [Google Scholar]
- 17.Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS). Clin Gerontol. 1986;5(1-2):165-173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 19.Smith PJ, Attix DK, Weldon BC, Monk TG. Depressive symptoms and risk of postoperative delirium. Am J Geriatr Psychiatry. 2016;24(3):232-238. doi: 10.1016/j.jagp.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber-Baldini AL, Zimmerman S, Morrison RS, et al. . Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003;51(9):1227-1236. doi: 10.1046/j.1532-5415.2003.51406.x [DOI] [PubMed] [Google Scholar]
- 21.Hajat Z, Ahmad N, Andrzejowski J. The role and limitations of EEG-based depth of anaesthesia monitoring in theatres and intensive care. Anaesthesia. 2017;72(suppl 1):38-47. doi: 10.1111/anae.13739 [DOI] [PubMed] [Google Scholar]
- 22.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658-665. doi: 10.1164/rccm.201310-1815OC [DOI] [PubMed] [Google Scholar]
- 23.Brown KE, Mirrakhimov AE, Yeddula K, Kwatra MM. Propofol and the risk of delirium: exploring the anticholinergic properties of propofol. Med Hypotheses. 2013;81(4):536-539. doi: 10.1016/j.mehy.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 24.Balasubramaniam B, Park GR. Sexual hallucinations during and after sedation and anaesthesia. Anaesthesia. 2003;58(6):549-553. doi: 10.1046/j.1365-2044.2003.03147.x [DOI] [PubMed] [Google Scholar]
- 25.Inouye SK, Zhang Y, Jones RN, Kiely DK, Yang F, Marcantonio ER. Risk factors for delirium at discharge: development and validation of a predictive model. Arch Intern Med. 2007;167(13):1406-1413. doi: 10.1001/archinte.167.13.1406 [DOI] [PubMed] [Google Scholar]
- 26.Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59(12):2306-2313. doi: 10.1111/j.1532-5415.2011.03725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman SM, Mendelson DA. Epidemiology of fragility fractures. Clin Geriatr Med. 2014;30(2):175-181. doi: 10.1016/j.cger.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 28.de Jonghe A, van Munster BC, Goslings JC, et al. ; Amsterdam Delirium Study Group . Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186(14):E547-E556. doi: 10.1503/cmaj.140495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carson JL, Terrin ML, Noveck H, et al. ; FOCUS Investigators . Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi: 10.1056/NEJMoa1012452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruber-Baldini AL, Marcantonio E, Orwig D, et al. . Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. J Am Geriatr Soc. 2013;61(8):1286-1295. doi: 10.1111/jgs.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duppils GS, Wikblad K. Acute confusional states in patients undergoing hip surgery. a prospective observation study. Gerontology. 2000;46(1):36-43. doi: 10.1159/000022131 [DOI] [PubMed] [Google Scholar]
- 32.Bellelli G, Mazzola P, Morandi A, et al. . Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014;62(7):1335-1340. doi: 10.1111/jgs.12885 [DOI] [PubMed] [Google Scholar]
- 33.Burgos E, Gómez-Arnau JI, Díez R, Muñoz L, Fernández-Guisasola J, Garcia del Valle S. Predictive value of six risk scores for outcome after surgical repair of hip fracture in elderly patients. Acta Anaesthesiol Scand. 2008;52(1):125-131. doi: 10.1111/j.1399-6576.2007.01473.x [DOI] [PubMed] [Google Scholar]
- 34.Johnson DJ, Greenberg SE, Sathiyakumar V, et al. . Relationship between the Charlson Comorbidity Index and cost of treating hip fractures: implications for bundled payment. J Orthop Traumatol. 2015;16(3):209-213. doi: 10.1007/s10195-015-0337-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yohannes AM, Chen W, Moga AM, Leroi I, Connolly MJ. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta-analysis of observational studies. J Am Med Dir Assoc. 2017;18(5):451.e1-451.e11. doi: 10.1016/j.jamda.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 36.Yaffe K, Ackerson L, Kurella Tamura M, et al. ; Chronic Renal Insufficiency Cohort Investigators . Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010;58(2):338-345. doi: 10.1111/j.1532-5415.2009.02670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggermont LH, de Boer K, Muller M, Jaschke AC, Kamp O, Scherder EJ. Cardiac disease and cognitive impairment: a systematic review. Heart. 2012;98(18):1334-1340. doi: 10.1136/heartjnl-2012-301682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Raw numbers of patients diagnosed with delirium for main groups and sub-group analysis
eTable 2. Univariate analysis on associations between base line characteristics and incident postoperative delirium in hospital
eTable 3. Intervention Effect Modification by CCI on Post Operative Incident Delirium Risk; CCI Truncated Values >2
eTable 4. Intervention Effect Modification by CCI on Post Operative Incident Delirium Risk; CCI Continuous Values
eFigure. Mini-Mental State Exam score and Delirium Rating Scale-Revised ‘98 severity score at baseline and postoperative days 1–5 in the lighter-sedation and heavier-sedation groups