Key Points
Question
Is there a role for prophylactic wound pressure dressings in closed laparotomy incisions performed for general and colorectal surgery?
Findings
In this meta-analysis consisting of 9 studies with 1266 patients, the use of negative pressure wound therapy was associated with a significantly lower rate of surgical site infections compared with standard nonpressure dressings (12.4% vs 27.1%).
Meaning
Prophylactic negative pressure dressings appear to be superior to conventional nonpressure dressings in preventing surgical site infections in closed laparotomy incisions in general and colorectal surgery, and their use should be considered.
This meta-analysis evaluates the association of prophylactic negative pressure wound therapy with rates of surgical site infection among patients with closed laparotomy incisions performed for general and colorectal surgery in elective and emergency settings.
Abstract
Importance
Surgical site infections (SSIs) are common after laparotomy wounds and are associated with a significant economic burden. The use of negative pressure wound therapy (NPWT) has recently been broadened to closed surgical incisions.
Objective
To evaluate the association of prophylactic NPWT with SSI rates in closed laparotomy incisions performed for general and colorectal surgery in elective and emergency settings.
Data Sources
The PubMed, Embase, Cochrane Central Register of Controlled Trials, and Google Scholar databases were searched without language restrictions for relevant articles from inception until December 2017. The latest search was performed on December 31, 2017. The bibliographies of retrieved studies were further screened for potential additional studies.
Study Selection
Randomized clinical trials and nonrandomized studies were included. Unpublished reports were excluded, as were studies that examined NPWT (or standard nonpressure) dressings only without a comparator group. Studies that evaluated the use of NPWT in open abdominal incisions were also excluded. Disagreement was resolved by discussion, and if the question remained unsettled, the opinion of the senior author was sought. A total of 198 citations were identified, and 189 were excluded.
Data Extraction and Synthesis
This meta-analysis was conducted according to PRISMA guidelines. Data were independently extracted by 2 authors. A random-effects model was used for statistical analysis.
Main Outcomes and Measures
The primary outcome measure was SSI, and secondary outcomes included seroma and wound dehiscence rates. These outcomes were chosen before data collection.
Results
Nine unique studies (3 randomized trials and 2 prospective and 4 retrospective studies) capturing 1266 unique patients were included. Of these, 1187 patients with 1189 incisions were included in the final analysis (52.3% male among 7 studies reporting data on sex; mean [SD] age, 52 [15] years among 8 studies reporting data on age). Significant clinical and methodologic heterogeneity existed among studies. On random-effects analysis, NPWT was associated with a significantly lower rate of SSI compared with standard dressings (pooled odds ratio [OR], 0.25; 95% CI, 0.12-0.52; P < .001). However, no difference in rates of seroma (pooled OR, 0.38; 95% CI, 0.12-1.23; P = .11) or wound dehiscence (pooled OR, 2.03; 95% CI, 0.61-6.78; P = .25) was found. On sensitivity analysis, focusing solely on colorectal procedures, NPWT significantly reduced SSI rates (pooled OR, 0.16; 95% CI, 0.07-0.36; P < .001).
Conclusions and Relevance
Application of NPWT on closed laparotomy wounds in general and colorectal surgery is associated with reduced SSI rates but similar rates of seroma and wound dehiscence compared with conventional nonpressure dressings.
Introduction
Postoperative wound complications are a common occurrence after open abdominal surgery and include surgical site infections (SSIs), seroma or hematoma formation, and wound dehiscence. Surgical site infections constitute 36% of all health care-associated infections in the United States alone1 and are directly attributable to more than US $1.6 billion in costs and 1 million extra hospital days in affected patients,2 thus representing a substantial health economic burden. Colorectal surgery is associated with the highest rate of SSI (≤45%)3 owing to the inherent contaminated nature of the surgery. The net outcomes of SSI include prolonged hospital stays, delay in adjuvant treatment, potential development of incisional hernias, and ultimately a decrease in patient quality of life.4,5 The cause of SSI is multifactorial, resulting from an interplay between patient-related, environmental, and surgical factors. As such, traditional care bundles aim to target these different components and include the use of preoperative antibiotic prophylaxis and aseptic surgical technique, maintenance of intraoperative normothermia, and preoperative optimization of patient risk factors.6,7 However, these measures have failed to alter the incidence of SSI substantially.6,8 Laparoscopic surgery has been demonstrated to result in a significantly lower incidence of SSI compared with open surgery9,10; however, not all patients are suited for this approach. Therefore, novel preventive measures are needed to abrogate the development of SSI after open surgery.
Negative pressure wound therapy (NPWT) consists of the continuous delivery of negative pressure to the wound bed via a vacuum device, thereby removing excess tissue edema and promoting granulation tissue formation.11 Although initially used solely in open wounds,11 use of NPWT has recently been extended to include closed surgical incisions. Numerous studies in orthopedic12,13 and cardiothoracic14 surgery have demonstrated decreased SSI rates with the use of NPWT in closed incisions. Commercially available NPWT devices include a vacuum-assisted closure device (VAC; KCI) and the more recent disposable incision management system (PREVENA; KCI) and pocket-sized NPWT device (PICO; Smith and Nephew). The PREVENA and PICO devices have been simplified such that they consist of a single-use battery-powered device and an easy-to-apply wound dressing with or without a small portable canister to collect the absorbed fluid. A recent meta-analysis15 showed that NPWT decreased wound infection rates and seroma formation compared with nonpressure, standard wound dressings. However, most of the studies included in the latter study evaluated orthopedic procedures, whereas colorectal procedures were not assessed. Therefore, we aimed to perform a systematic review and meta-analysis to assess the association of prophylactic NPWT in closed laparotomy wounds in general and colorectal surgery, compared with conventional surgical dressings.
Methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.16 We did not publish a prior protocol for this review.
Eligibility Criteria
We searched for all studies that directly compared NPWT with standard dressings for closed laparotomy wounds in general and/or colorectal surgery. Randomized clinical trials (RCTs) and nonrandomized studies were eligible for inclusion. Patients undergoing elective and emergency laparotomies were included. We excluded unpublished reports and studies that examined NPWT (or standard nonpressure) dressings only, without a comparator group. Studies that evaluated the use of NPWT in open abdominal incisions were excluded, as were studies that involved placement of foreign material (eg, mesh, drain) in the subcutaneous space, owing to these factors being confounding variables in the development of postoperative wound complications.
Search Strategy
The online literature was searched using the following combination of medical subject heading terms: “laparotomy incisions” OR “closed laparotomy” AND “negative pressure wound therapy” OR “negative pressure dressings.” Medline, Embase, Cochrane Central Register of Controlled Trials, and Google Scholar were searched without any language restrictions. The search was performed from inception until December 2017, with the last search performed on December 31. The titles and abstracts of citations were individually reviewed by 2 authors (S.M.S. and K.M.), and full texts of suitable studies were retrieved. Disagreement was resolved by discussion, and if the question remained unsettled, the opinion of the senior author was sought. The bibliographies of recovered studies were further assessed for potential additional publications suitable for inclusion. The primary end point for this review was SSI. Secondary end points included seroma formation and wound dehiscence rates.
Data Analysis
The following data were extracted from the included studies on an Excel spreadsheet (Microsoft Corporation): authors’ names, journal, year of publication, sex, mean age, sample size, type of study, type of surgery, inclusion and exclusion criteria, type of NPWT (or standard) dressing used, duration of treatment, SSI rates, seroma formation rates, wound dehiscence rates, and length of follow-up. A random-effects model was used to define all pooled outcome measures, as previously described,17 and the odds ratio (OR) was estimated with its variance and 95% CI. The prevailing heterogeneity between ORs for the comparable outcomes between different studies was calculated using the I2 inconsistency test that depicts the percentage of total variation across studies and reflects heterogeneity rather than chance. The absence of statistical heterogeneity is indicated by a value of 0%, whereas larger values indicate increasing heterogeneity. The methodologic robustness of included studies was determined using the Downs and Black scale,18 a validated tool for assessing randomized and nonrandomized studies. The scale consists of 27 items evaluating study reporting and external and internal validity and power, with a maximum assigned score of 32 (highest assessment). However, we were unable to assess publication bias, because we included fewer than 10 studies in the final analysis. Analyses were performed using RevMan software (version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration).
Results
Study Selection and Characteristics
The initial search criteria captured 198 citations. After application of inclusion and exclusion criteria, 20 full-text articles were assessed for eligibility. Of these, 9 studies19,20,21,22,23,24,25,26,27 were subsequently excluded; reasons for exclusion are outlined in eTable 1 in the Supplement. Two articles (NEPTUNE [Negative Pressure Wound Therapy Use to Decrease Surgical Nosocomial Events in Colorectal Resections]28 and PONIY [Postoperative Negative-Pressure Incision Therapy Following Open Colorectal Surgery]29 trials) were published protocols of RCTs that were currently recruiting patients and were therefore also excluded.
Therefore, 9 studies (3 randomized trials30,31,32 and 2 prospective33,34 and 4 retrospective35,36,37,38 studies [Table]) describing a total of 1266 unique patients were assessed. Of these, 50 patients underwent breast surgery34 and 29 patients underwent delayed primary closure32 and were subsequently excluded from the analysis. As a result, 1187 patients and 1189 incisions were included in the final analysis. A total of 485 incisions constituted the NPWT group and 704 constituted the standard dressing group. A flow diagram of the selection process is shown in eFigure 1 in the Supplement. The study characteristics and their respective inclusion and exclusion criteria are summarized in eTables 2 and 3 in the Supplement. After excluding the studies by Zaidi and El-Masry38 and Blackham et al,36 in which data on sex were not provided, 427 of 817 male (52.3%) and 390 of 817 female (47.7%) patients were represented in the final analysis. Data on mean age of the study cohort was unavailable from Zaidi and El-Masry.38 Overall, the mean (SD) age of the represented study population was 52 (15) years.
Table. Characteristics of Included Studies.
| Source/Country/Study Type | No. of Patients, NPWT/Control Groups | Relevant Outcome (Time Measured) | Wound Categories | Details of NPWTa | Type of Procedure | Antibiotic Therapy | Mechanical Bowel Preparation | Follow-up | Downs and Black Scoreb |
|---|---|---|---|---|---|---|---|---|---|
| Bonds et al,35 2013/United States/ retrospective | 32/222 | SSI | Contaminated/dirty (28% NPWT vs 26% control wounds) | VAC (setting, −75 mm Hg) used for 5 to 7 d with foam dressing (Granufoam; KCI) | Emergency (9% NPWT vs 27% control wounds) and elective (15% NPWT vs 85% control wounds) | Not mentioned | Not mentioned | Not specified | 26 |
| Pellino et al,34 2014/ Italy/prospective | 50/50 (25 Colorectal NPWT and 25 colorectal standard dressings) |
Infectious surgical site events (30 d) and LOS | Not mentioned | PICO (setting, −80 mm Hg) used for 7 d | Not mentioned | Not mentioned | Not mentioned | 90 d | 26 |
| Selvaggi et al,33 2014/Italy/prospective | 25/25 | Seroma, infectious surgical site events (30 d), and LOS | Not mentioned | PICO (setting, −80 mm Hg) used for 7 d | Not mentioned | All patients received intraoperative IV cefotaxime, 1 g, and metronidazole, 500 g, and continued therapy postoperatively as required | Not mentioned | 90 d | 28 |
| Lozano-Balderas et al,32 2017/Mexico/RCT | 25/27 (An additional 29 patients underwent DPC in a 3-arm trial) | SSI (30 d) | Contaminated (48% NPWT vs 33% control wounds) and dirty (52% NPWT vs 67% control) wounds | VAC (setting or duration not stated) used | All emergency | Cephalosporin antibiotic and metronidazole | Not mentioned | 30 d | 31 |
| O’Leary et al,30 2017/Ireland/RCT | 25/25 | SSI (4 and 30 d) and LOS | Clean (21% NPWT vs 24% control wounds), clean contaminated (71% NPWT vs 68% control wounds), and dirty (8.3% NPWT vs 8% wounds) | PICO (setting, −80 mm Hg) for 4 d | Elective and emergency | All patients received 1.2 g IV combined amoxicillin and clavulanate potassium (Augmentin) at induction; 2 further postoperative doses for clean contaminated or contaminated wounds only | No | 30 d | 29 |
| Zaidi and El-Masry,38 2017/Ireland/retrospective | 69/112 | Deep incisional wound infection or dehiscence (30 d) | Clean contaminated and contaminated (no breakdown provided) | PREVENA (setting, −125 mm Hg) for 7 d | Elective (70% NPWT vs 73% control wounds) and emergency (30% NPWT vs 27% control wounds) | Not mentioned | Not mentioned | 30 d | 26 |
| Schurtz et al,37 2018/United States/retrospective | 48/48 | SSI (30 d) | Clean (23% NPWT vs 29% control wounds), clean contaminated (39% NPWT vs 42% control wounds), and contaminated (37% NPWT vs 29% control wounds) | PREVENA (setting, −125 mm Hg) for 4 to 8 d | All emergency laparotomies | All patients received preoperative antibiotics (no details) | No | 30 d | 25 |
| Blackham et al,36 2013/United States/retrospective | 104/87 | SSI (30 d), seroma formation, and wound dehiscence | Clean (4% NPWT vs 29% control wounds), clean contaminated (96% NPWT vs 71% control wounds) | VAC (setting, −125 mm Hg) for 4 d | All elective | Preoperative antibiotics at induction (not continued postoperatively) | Yes | 30 d | 25 |
| Shen et al,31 2017/United States/RCT | 132/133 | SSI (30 d), seroma formation, and wound dehiscence | All were clean contaminated | VAC (setting, −125 mm Hg) for 4 d | All elective | Not explicitly mentioned | Not mentioned | 30 d | 31 |
Abbreviations: DPC, delayed primary closure; IV, intravenous; LOS, length of stay; NPWT, negative pressure wound therapy; RCT, randomized controlled trial; SSI, surgical site infection.
Devices include VAC (KCI), PREVENA (KCI), and PICO (Smith and Nephew).
The scale consists of 27 items evaluating study reporting and external and internal validity and power in randomized and nonrandomized studies, with a maximum assigned score of 32 (best assessment).18
Types of Surgery Assessed
Laparotomies were performed for purely colorectal indications in 3 studies.33,35,38 In addition, general (89%) and colorectal (11%) procedures were examined by Lozano-Balderas et al32; general (14%), colorectal (24%), and gynecologic (60%) procedures, by O’Leary et al30; and general (46%), colorectal (48%), and trauma (6%) laparotomies, by Schurtz et al.37 In the study by Blackham et al,36 indications for laparotomies were subdivided into colorectal (31%), cytoreductive and/or hyperthermic intraperitoneal chemotherapy (51%), and pancreatic (23%) procedures, whereas in the study by Shen et al,31 colorectal procedures constituted 11% of all laparotomies, with the remainder including cytoreductive and/or hyperthermic intraperitoneal chemotherapy (51%), pancreatic (27%), and upper gastrointestinal tract (11%) procedures.
The study by Pellino et al34 included a mixture of colorectal (50%) and breast (50%) procedures. Only the colorectal procedures were included in the quantitative data for the current meta-analysis.
Details of Study Interventions
In the study by Bonds et al,35 the fascia was closed with 1-0 polydioxanone suture. In the NPWT group, the skin was closed with staples before application of the NPWT device (VAC; pressure setting, −75 mm Hg for 5-7 days), whereas it was closed with a subcuticular suture (or stapled) before application of the occlusive dressing in the standard dressing group. No mention was made of antibiotic prophylaxis.
In the studies by Selvaggi et al33 and Pellino et al,34 the wound edges were approximated using nonabsorbable, subcuticular 3-0 polypropylene sutures in both study groups. In the NPWT group, a PICO device (pressure setting, −80 mm Hg for 7 days) was applied, whereas in the control group, basic wound absorbent dressings were used and removed on postoperative day two34 or three.33 All patients received 1 g of intravenous cefotaxime and 500 mg of intravenous metronidazole intraoperatively and continued therapy postoperatively as required.
In the study by Lozano-Balderas et al,32 the fascia was closed with a polyglycolic acid 0 running suture. In the NWPT group, the VAC device was applied but the pressure and duration of the treatment were not specified. In the control group, the skin was closed with 2-0 polypropylene sutures. Dual cephalosporin antibiotic and metronidazole therapy was used in all patients.
In the study by O’Leary et al,30 the fascia was closed with a 1-0 loop polydioxanone suture, and clips were applied to the skin. In the NPWT cohort, a PICO device (pressure setting, −80 mm Hg for 4 days) was used, whereas in the control group, a transparent waterproof dressing was applied. All patients received 1.2 g of intravenous combined amoxicillin and clavulanate potassium (Augmentin) at induction and thereafter 2 postoperative doses for clean contaminated or contaminated wounds only. No mechanical bowel preparation was used.
In the study by Zaidi and El-Masry,38 no information was provided on fascial or skin closure. In the NPWT cohort, a PREVENA device (pressure setting, −125 mm Hg) was applied for 1 week. No details of the dressing used in the control group or of antibiotic prophylaxis were provided.
In the study by Schurtz et al,37 the skin was closed using staples or a running subcuticular suture. In the NPWT group, a PREVENA device (pressure setting −125 mm Hg) was applied for 4 to 8 days; in the control group, an adhesive dressing (Primapore; Smith and Nephew) was applied. All patients received preoperative antibiotics or were already receiving antibiotics.
In the study by Blackham et al,36 mechanical bowel preparation was used in all colonic resections or cytoreductive surgery. Prophylactic preoperative antibiotics were administered to all patients. In the NPWT group, the skin was loosely apposed with staples, and then a strip of nonadhesive dressing (Adaptic; Johnson & Johnson) was applied between the skin and NPWT foam before application of the VAC device (pressure setting, −125 mm Hg for 4 days). In the control group, the skin was closed with subcuticular monofilament sutures (or staples), and the incision was dressed with a sterile surgical dressing.
In the study by Shen et al,31 the fascia was closed with a running loop polydioxanone suture, and clips were applied to the skin. In the NPWT cohort, a strip of nonadhesive dressing was applied between the skin and NPWT foam before application of the device (pressure setting, −125 mm Hg for 4 days). In the control group, an adhesive dressing was used. No information was provided on mechanical bowel preparation or antibiotic prophylaxis, however.
Study End Points
Overall, the rate of SSIs was lower with use of NPWT (60 of 485 incisions [12.4%]) compared with standard dressings (191 of 704 incisions [27.1%]). Most studies used the Centers for Disease Control and Prevention (CDC) criteria39 to define SSI. In the studies by Selvaggi et al33 and Pellino et al,34 wound complications were classified according to CDC criteria39 and the Global ASEPSIS score.40 In the studies by O’Leary et al,30 Schurtz et al,37 and Lozano-Balderas et al,32 SSIs were assessed using the CDC criteria.39
Using the CDC criteria, Blackham et al36 provided explicit definitions of SSI and classified them into superficial or deep wound infections. Organ space infections were excluded by these authors because they are unaffected by NPWT. Similarly, Shen et al31 defined SSI using the CDC criteria. In addition, they defined wound dehiscence as any spontaneous separation of the skin or fascia not associated with an SSI, seroma, or hematoma.
Zaidi and El-Masry38 did not explicitly define SSIs but classified them into deep incisional wound infections or dehiscence. Finally, Bonds et al35 deemed SSIs to be present if any portion of the surgical incision had to be opened during inpatient or outpatient follow-up.
Primary Outcome
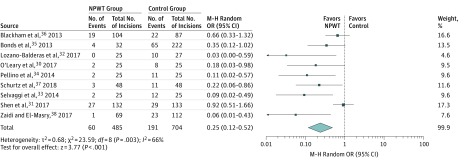
Data on SSIs were available in all 9 studies (1189 incisions).30,31,32,33,34,35,36,37,38 On random-effects analysis, NPWT was associated with a statistically significantly lower rate of SSI compared with standard dressings (pooled OR, 0.25; 95% CI, 0.12-0.52; P < .001) (Figure 1). However, heterogeneity among the trials was statistically significant (τ28 = 0.68; P = .003; I2 = 66%).
Figure 1. Meta-analysis of Surgical Site Infection (SSI) Rates Between Negative Pressure Wound Therapy (NPWT) and Standard Dressings.
Different marker size indicates weight; diamond, pooled OR. M-H indicates Mantel-Haenszel; OR, odds ratio.
Secondary Outcomes
Seroma Formation
Four studies31,33,34,36 provided data on seroma formation (556 incisions). We found no significant difference between NPWT and standard dressings (pooled OR, 0.38; 95% CI, 0.12-1.23; P = .11) (Figure 2). However, heterogeneity among the trials was statistically significant (τ23 = 0.86; P = .05; I2 = 62%).
Figure 2. Meta-analysis of Seroma Rates Between Negative Pressure Wound Therapy (NPWT) and Standard Dressings.
Different marker size indicates weight; diamond, pooled OR. M-H indicates Mantel-Haenszel; OR, odds ratio.
Wound Dehiscence
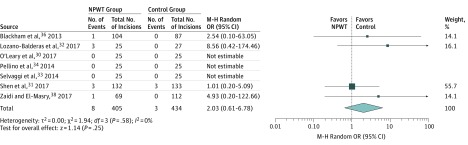
Data on wound dehiscence were available in 7 studies30,31,32,33,34,36,38 (839 incisions). We found no significant difference between NPWT and standard dressings (pooled OR, 2.03; 95% CI, 0.61-6.78; P = .25) (Figure 3). Heterogeneity among the trials was not significant (τ23 = 0.00; P = .58; I2 = 0%).
Figure 3. Meta-analysis of Wound Dehiscence Rates Between Negative Pressure Wound Therapy (NPWT) and Standard Dressings.
Different marker size indicates weight; diamond, pooled OR. M-H indicates Mantel-Haenszel; OR, odds ratio.
Sensitivity Analysis
A sensitivity analysis was performed to examine the role of NPWT in colorectal surgery specifically. Four studies33,34,35,38 provided data for analysis. Data on individual colorectal procedures were sought from other authors31,32,36,37 via correspondence but were not available at the time of statistical analysis.
Colorectal Surgery
Four studies33,34,35,38 provided data for analysis in colorectal surgery specifically (535 incisions). Negative pressure wound therapy was superior to conventional dressings and resulted in fewer SSIs (pooled OR, 0.16; 95% CI, 0.07-0.36; P < .001) (eFigure 2 in the Supplement). Heterogeneity among the trials was not significant (τ23 = 0.11; P = .31; I2 = 15%).
Data for seroma in colorectal surgery were available from 2 studies33,34 only; hence, generating the summative outcome from the available data was inappropriate. Although data were available on wound dehiscence in colorectal surgery from 3 studies,33,34,38 we elected not to generate the summative outcome from the data, because the respective studies reported individually low events rates, and statistical heterogeneity could not be assessed.
Discussion
Our study demonstrates that the use of NPWT in closed laparotomy incisions for general and colorectal surgery is associated with a reduced incidence of SSI, with no difference in seroma or wound dehiscence rates, compared with nonpressure surgical dressings. On sensitivity analysis, focusing purely on colorectal procedures and excluding general surgery procedures, the results still favored NPWT in regard to SSI development. Our findings must be construed with caution, however, because the studies have significant clinical heterogeneity. Most important, different NPWT devices with different pressure settings (range, −75 to −125 mm Hg) and dissimilar duration of application (4-7 days) were used across studies. Although no evidence yet suggests that longer duration of NPWT therapy is superior with regard to wound outcomes, further research is warranted to investigate this possibility. The use of perioperative antibiotic therapy also differed among studies, with some studies continuing therapy postoperatively when required30,33 and others36 only administering a prophylactic dose at induction. Other studies34,35,38 did not explicitly report use of antibiotics. Mechanical bowel preparation was used in 1 study only36 and may also have positively swayed the subsequent SSI rates. Finally, in the study by Bonds et al,35 significantly fewer patients receiving NPWT underwent emergency surgical procedures compared with the control group. Emergency surgery, together with immunosuppression, obesity, malnutrition, and malignant disease are associated with a higher risk of SSI41 and a higher likelihood of stoma formation.
Inclusion and exclusion criteria also varied among studies, with some incorporating contaminated and dirty wounds32,35 and others31,36 excluding these wound categories. We found no statistically significant differences in the distribution of wound categories between NPWT and control groups in 5 studies.30,31,32,35,37 However, in the nonrandomized study by Blackham et al,36 significantly fewer clean wounds and more clean contaminated wounds were included in the NPWT cohort than in the control group because the authors used NPWT in patients who were at high risk for SSI, thus reflecting selection bias.
Wound complications remain common after laparotomy incisions, especially after emergency surgery.42 Various methods of wound closure techniques43 (eg, delayed primary closure) have been described in an attempt to decrease SSI rates. However, healing by secondary intention is labor intensive and costly. Prophylactic NPWT has proved to have a positive association in open incisions but may also confer benefits in closed surgical incisions. Negative pressure wound therapy provides stability to the closed incision by redistributing lateral tension and increasing the amount of force required for wound disruption.44 In addition, porcine models of incisional NPWT have demonstrated enhanced lymphatic clearance from the subcutaneous dead space and an associated reduction in hematoma and seroma formation.45 Although standard occlusive wound dressings effectively separate the incision from the hospital environment, they lack the additional benefits of wound stability and improved lymphatic clearance. Animal models of NPWT have demonstrated an enhanced microvascular blood flow and an improved partial pressure of oxygen in the wound environment.46 Furthermore, the distinct molecular mechanisms through which NPWT improves wound healing are also being unraveled. Local concentrations of the proinflammatory cytokines tumor necrosis factor and interleukin-1β are significantly reduced with NPWT application,47 whereas concentrations of interleukin-8, a proangiogenic factor, as well as vascular endothelial growth factor are increased.48 Negative pressure wound therapy appears to promote a shift to an anti-inflammatory phenotype at the molecular level, culminating in neovascularization, extracellular matrix regeneration, and deposition of granulation tissue.49 The optimal duration of therapy for NPWT as well as the optimal suction pressure on a closed incision has yet to be determined. Different devices harbor varying recommendations regarding duration of application and pressure settings (eg, VAC and PREVENA recommend a pressure setting of −125 mm Hg, whereas that of PICO is −80 mm Hg). Further research is required to investigate these factors.
The major drawback associated with NPWT use is cost. These costs could be justified if NPWT use reduced SSI rates by at least 15%, according to Heard et al.50 With regard to length of hospital stay, the new disposable, user-friendly, pocket-size devices allow patients to be safely discharged home with the device, without the cumbersomeness associated with conventional NPWT canisters. Although not reported as an end point in our study, length of stay was significantly shorter in the NPWT cohort on pooled analysis of data from 4 studies (mean, −0.76 days; 95% CI, −1.16 to −0.37 days; P < .001). Although NPWT application to closed wounds is largely associated with minimal unwanted effects, minor skin irritation, discoloration, and/or ecchymosis may be sometimes encountered. However, these effects resolve with discontinuation of the treatment. Polyurethane foam, which forms part of the VAC and PREVENA systems, may cause excoriation and blistering if applied directly to the skin; however, the NPWT dressings come with a nonadherent layer for application between the foam and the skin to abrogate these adverse effects.51,52
Limitations
Our study is not without limitations. Most studies assessed were nonrandomized, with an inherent risk of bias. Different NPWT devices were used, each with varying recommendations regarding optimal pressure settings and duration of application. However, all of them were included together in this quantitative meta-analysis. Also, we did not perform an economic evaluation to determine the cost-effectiveness of NPWT compared with standard surgical dressings. In addition, we were unable to evaluate elective and emergency surgery outcomes separately owing to insufficient data reported in the studies.
Conclusions
Colorectal surgical procedures endure the highest rates of SSI, reported to be as high as 45%,3,6 despite established prophylactic measures such as wound protectors, maintenance of normoglycemia perioperatively, and appropriate antibiotic selection. Moreover, the presence of a stoma has been shown to be an independent risk factor for postoperative SSI development.53 Therefore, NPWT use in the colorectal surgical population may be beneficial in the setting of a stoma, not only by isolating the wound but also by promoting effective wound healing. Two RCTs28,29 are currently investigating the role of NPWT in elective, open colorectal surgery but have not been published yet. Further research is required to determine the wound category (clean vs clean contaminated vs contaminated vs dirty) in which NPWT has the greatest benefits before recommending its routine use in surgical practice.
eTable 1. Characteristics of Excluded Studies
eTable 2. Inclusion and Exclusion Criteria of Studies
eTable 3. Clinical and Operative Parameters of Patient Cohort in Meta-analysis
eFigure 1. PRISMA Diagram of Studies Included in the Meta-analysis
eFigure 2. Sensitivity Analysis of SSI Rates Between NPWT and Standard Dressings in Colorectal Surgery Only
eReferences.
References
- 1.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):-. doi: 10.1001/jamainternmed.2013.9763 [DOI] [PubMed] [Google Scholar]
- 2.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. doi: 10.1016/j.ajic.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 3.Wick EC, Vogel JD, Church JM, Remzi F, Fazio VW. Surgical site infections in a “high outlier” institution: are colorectal surgeons to blame? Dis Colon Rectum. 2009;52(3):374-379. doi: 10.1007/DCR.0b013e31819a5e45 [DOI] [PubMed] [Google Scholar]
- 4.Gheorghe A, Moran G, Duffy H, Roberts T, Pinkney T, Calvert M. Health utility values associated with surgical site infection: a systematic review. Value Health. 2015;18(8):1126-1137. doi: 10.1016/j.jval.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 5.Murray BW, Cipher DJ, Pham T, Anthony T. The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am J Surg. 2011;202(5):558-560. doi: 10.1016/j.amjsurg.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 6.Anthony T, Murray BW, Sum-Ping JT, et al. Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Arch Surg. 2011;146(3):263-269. doi: 10.1001/archsurg.2010.249 [DOI] [PubMed] [Google Scholar]
- 7.Ploegmakers IB, Olde Damink SW, Breukink SO. Alternatives to antibiotics for prevention of surgical infection. Br J Surg. 2017;104(2):e24-e33. doi: 10.1002/bjs.10426 [DOI] [PubMed] [Google Scholar]
- 8.Pastor C, Artinyan A, Varma MG, Kim E, Gibbs L, Garcia-Aguilar J. An increase in compliance with the Surgical Care Improvement Project measures does not prevent surgical site infection in colorectal surgery. Dis Colon Rectum. 2010;53(1):24-30. doi: 10.1007/DCR.0b013e3181ba782a [DOI] [PubMed] [Google Scholar]
- 9.Aimaq R, Akopian G, Kaufman HS. Surgical site infection rates in laparoscopic versus open colorectal surgery. Am Surg. 2011;77(10):1290-1294. [DOI] [PubMed] [Google Scholar]
- 10.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;(3):CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563-576. doi: 10.1097/00000637-199706000-00002 [DOI] [PubMed] [Google Scholar]
- 12.Reddix RN Jr, Leng XI, Woodall J, Jackson B, Dedmond B, Webb LX. The effect of incisional negative pressure therapy on wound complications after acetabular fracture surgery. J Surg Orthop Adv. 2010;19(2):91-97. [PubMed] [Google Scholar]
- 13.DeCarbo WT, Hyer CF. Negative-pressure wound therapy applied to high-risk surgical incisions. J Foot Ankle Surg. 2010;49(3):299-300. doi: 10.1053/j.jfas.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 14.Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov. 2009;16(2):140-146. doi: 10.1177/1553350609334821 [DOI] [PubMed] [Google Scholar]
- 15.Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477-486. doi: 10.1002/bjs.10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(pt A):139-145. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhen ZJ, Lai EC, Lee QH, Chen HW, Lau WY, Wang FJ. Conventional wound management versus a closed suction irrigation method for infected laparotomy wound: a comparative study. Int J Surg. 2011;9(5):378-381. doi: 10.1016/j.ijsu.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 20.Vargo D. Negative pressure wound therapy in the prevention of wound infection in high risk abdominal wound closures. Am J Surg. 2012;204(6):1021-1023. doi: 10.1016/j.amjsurg.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Mees J, Mardin WA, Senninger N, Bruewer M, Palmes D, Mees ST. Treatment options for postoperatively infected abdominal wall wounds healing by secondary intention. Langenbecks Arch Surg. 2012;397(8):1359-1366. doi: 10.1007/s00423-012-0988-7 [DOI] [PubMed] [Google Scholar]
- 22.Masden D, Goldstein J, Endara M, Xu K, Steinberg J, Attinger C. Negative pressure wound therapy for at-risk surgical closures in patients with multiple comorbidities: a prospective randomized controlled study. Ann Surg. 2012;255(6):1043-1047. doi: 10.1097/SLA.0b013e3182501bae [DOI] [PubMed] [Google Scholar]
- 23.Bertelsen CA, Fabricius R, Kleif J, Kristensen B, Gögenur I. Outcome of negative-pressure wound therapy for open abdomen treatment after nontraumatic lower gastrointestinal surgery: analysis of factors affecting delayed fascial closure in 101 patients. World J Surg. 2014;38(4):774-781. doi: 10.1007/s00268-013-2360-7 [DOI] [PubMed] [Google Scholar]
- 24.Soares KC, Baltodano PA, Hicks CW, et al. Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg. 2015;209(2):324-332. doi: 10.1016/j.amjsurg.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Unda N, Soares KC, Azoury SC, et al. Negative-pressure wound therapy in the management of high-grade ventral hernia repairs. J Gastrointest Surg. 2015;19(11):2054-2061. doi: 10.1007/s11605-015-2894-0 [DOI] [PubMed] [Google Scholar]
- 26.Kugler NW, Carver TW, Paul JS. Negative pressure therapy is effective in abdominal incision closure. J Surg Res. 2016;203(2):491-494. doi: 10.1016/j.jss.2016.04.032 [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Hao F, Yang Y, Shang Q, Guo C. Prophylactic vacuum sealing drainage (VSD) in the prevention of postoperative surgical site infections in pediatric patients with contaminated laparotomy incisions. Medicine (Baltimore). 2017;96(13):e6511. doi: 10.1097/MD.0000000000006511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chadi SA, Vogt KN, Knowles S, et al. Negative pressure wound therapy use to decrease surgical nosocomial events in colorectal resections (NEPTUNE): study protocol for a randomized controlled trial. Trials. 2015;16:322. doi: 10.1186/s13063-015-0817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihaljevic AL, Schirren R, Müller TC, Kehl V, Friess H, Kleeff J. Postoperative negative-pressure incision therapy following open colorectal surgery (PONIY): study protocol for a randomized controlled trial. Trials. 2015;16:471. doi: 10.1186/s13063-015-0995-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Leary DP, Peirce C, Anglim B, et al. Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: the PICO Trial. Ann Surg. 2017;265(6):1082-1086. doi: 10.1097/SLA.0000000000002098 [DOI] [PubMed] [Google Scholar]
- 31.Shen P, Blackham AU, Lewis S, et al. Phase II randomized trial of negative-pressure wound therapy to decrease surgical site infection in patients undergoing laparotomy for gastrointestinal, pancreatic, and peritoneal surface malignancies. J Am Coll Surg. 2017;224(4):726-737. doi: 10.1016/j.jamcollsurg.2016.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozano-Balderas G, Ruiz-Velasco-Santacruz A, Díaz-Elizondo JA, Gómez-Navarro JA, Flores-Villalba E. Surgical site infection rate drops to 0% using a vacuum-assisted closure in contaminated/dirty infected laparotomy wounds. Am Surg. 2017;83(5):512-514. [PubMed] [Google Scholar]
- 33.Selvaggi F, Pellino G, Sciaudone G, et al. New advances in negative pressure wound therapy (NPWT) for surgical wounds of patients affected with Crohn’s disease. Surg Technol Int. 2014;24:83-89. [PubMed] [Google Scholar]
- 34.Pellino G, Sciaudone G, Candilio G, et al. Preventive NPWT over closed incisions in general surgery: does age matter? Int J Surg. 2014;12(suppl 2):S64-S68. doi: 10.1016/j.ijsu.2014.08.378 [DOI] [PubMed] [Google Scholar]
- 35.Bonds AM, Novick TK, Dietert JB, Araghizadeh FY, Olson CH. Incisional negative pressure wound therapy significantly reduces surgical site infection in open colorectal surgery. Dis Colon Rectum. 2013;56(12):1403-1408. doi: 10.1097/DCR.0b013e3182a39959 [DOI] [PubMed] [Google Scholar]
- 36.Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am J Surg. 2013;205(6):647-654. doi: 10.1016/j.amjsurg.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Schurtz E, Differding J, Jacobson E, Maki C, Ahmeti M. Evaluation of negative pressure wound therapy to closed laparotomy incisions in acute care surgery. Am J Surg. 2018;215(1):113-115. doi: 10.1016/j.amjsurg.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 38.Zaidi A, El-Masry S. Closed-incision negative-pressure therapy in high-risk general surgery patients following laparotomy: a retrospective study. Colorectal Dis. 2017;19(3):283-287. doi: 10.1111/codi.13458 [DOI] [PubMed] [Google Scholar]
- 39.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR; Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee . Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999;27(2):97-132. doi: 10.1016/S0196-6553(99)70088-X [DOI] [PubMed] [Google Scholar]
- 40.Wilson AP, Treasure T, Sturridge MF, Grüneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet. 1986;1(8476):311-313. doi: 10.1016/S0140-6736(86)90838-X [DOI] [PubMed] [Google Scholar]
- 41.Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4(1):5. doi: 10.1186/1754-9493-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silvestri M, Dobrinja C, Scomersi S, et al. Modifiable and non-modifiable risk factors for surgical site infection after colorectal surgery: a single-center experience. Surg Today. 2018;48(3):338-345. doi: 10.1007/s00595-017-1590-y [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M, Tanaka K, Masubuchi S, et al. Risk factors for surgical site infection after stoma closure comparison between pursestring wound closure and conventional linear wound closure: propensity score matching analysis. Am J Surg. 2018;215(1):58-61. doi: 10.1016/j.amjsurg.2017.09.031 [DOI] [PubMed] [Google Scholar]
- 44.Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov. 2012;19(1):67-75. doi: 10.1177/1553350611414920 [DOI] [PubMed] [Google Scholar]
- 45.Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011;19(5):588-596. doi: 10.1111/j.1524-475X.2011.00714.x [DOI] [PubMed] [Google Scholar]
- 46.Wackenfors A, Gustafsson R, Sjögren J, Algotsson L, Ingemansson R, Malmsjö M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg. 2005;79(5):1724-1730. doi: 10.1016/j.athoracsur.2004.10.053 [DOI] [PubMed] [Google Scholar]
- 47.Eisenhardt SU, Schmidt Y, Thiele JR, et al. Negative pressure wound therapy reduces the ischaemia/reperfusion-associated inflammatory response in free muscle flaps. J Plast Reconstr Aesthet Surg. 2012;65(5):640-649. doi: 10.1016/j.bjps.2011.11.037 [DOI] [PubMed] [Google Scholar]
- 48.Labler L, Rancan M, Mica L, Härter L, Mihic-Probst D, Keel M. Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma. 2009;66(3):749-757. doi: 10.1097/TA.0b013e318171971a [DOI] [PubMed] [Google Scholar]
- 49.Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg. 2014;101(13):1627-1636. doi: 10.1002/bjs.9636 [DOI] [PubMed] [Google Scholar]
- 50.Heard C, Chaboyer W, Anderson V, Gillespie BM, Whitty JA. Cost-effectiveness analysis alongside a pilot study of prophylactic negative pressure wound therapy. J Tissue Viability. 2017;26(1):79-84. doi: 10.1016/j.jtv.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 51.Gomoll AH, Lin A, Harris MB. Incisional vacuum-assisted closure therapy. J Orthop Trauma. 2006;20(10):705-709. doi: 10.1097/01.bot.0000211159.98239.d2 [DOI] [PubMed] [Google Scholar]
- 52.Matatov T, Reddy KN, Doucet LD, Zhao CX, Zhang WW. Experience with a new negative pressure incision management system in prevention of groin wound infection in vascular surgery patients. J Vasc Surg. 2013;57(3):791-795. doi: 10.1016/j.jvs.2012.09.037 [DOI] [PubMed] [Google Scholar]
- 53.Konishi T, Watanabe T, Kishimoto J, Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg. 2006;244(5):758-763. doi: 10.1097/01.sla.0000219017.78611.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Excluded Studies
eTable 2. Inclusion and Exclusion Criteria of Studies
eTable 3. Clinical and Operative Parameters of Patient Cohort in Meta-analysis
eFigure 1. PRISMA Diagram of Studies Included in the Meta-analysis
eFigure 2. Sensitivity Analysis of SSI Rates Between NPWT and Standard Dressings in Colorectal Surgery Only
eReferences.