Key Points
Question
Does treatment with active vitamin D reduce cardiovascular risk in patients undergoing hemodialysis?
Findings
In this open-label randomized clinical trial that included 976 patients without secondary hyperparathyroidism receiving maintenance hemodialysis, the use of oral alfacalcidol compared with usual care over a median of 4 years resulted in a composite outcome of select cardiovascular events in 21.1% vs 17.9% of participants, respectively, which was not a statistically significant difference.
Meaning
Active vitamin D did not reduce the risk of a composite outcome of cardiovascular events.
Abstract
Importance
Patients with chronic kidney disease have impaired vitamin D activation and elevated cardiovascular risk. Observational studies in patients treated with hemodialysis showed that the use of active vitamin D sterols was associated with lower risk of all-cause mortality, regardless of parathyroid hormone levels.
Objective
To determine whether vitamin D receptor activators reduce cardiovascular events and mortality in patients without secondary hyperparathyroidism undergoing hemodialysis.
Design, Setting, and Participants
Randomized, open-label, blinded end point multicenter study of 1289 patients in 207 dialysis centers in Japan. The study included 976 patients receiving maintenance hemodialysis with serum intact parathyroid hormone levels less than or equal to 180 pg/mL. The first and last participants were enrolled on August 18, 2008, and January 26, 2011, respectively. The final date of follow-up was April 4, 2015.
Interventions
Treatment with 0.5 μg of oral alfacalcidol per day (intervention group; n = 495) vs treatment without vitamin D receptor activators (control group; n = 481).
Main Outcomes and Measures
The primary outcome was a composite measure of fatal and nonfatal cardiovascular events, including myocardial infarctions, hospitalizations for congestive heart failure, stroke, aortic dissection/rupture, amputation of lower limb due to ischemia, and cardiac sudden death; coronary revascularization; and leg artery revascularization during 48 months of follow-up. The secondary outcome was all-cause death.
Results
Among 976 patients who were randomized from 108 dialysis centers, 964 patients were included in the intention-to-treat analysis (median age, 65 years; 386 women [40.0%]), and 944 (97.9%) completed the trial. During follow-up (median, 4.0 years), the primary composite outcome of cardiovascular events occurred in 103 of 488 patients (21.1%) in the intervention group and 85 of 476 patients (17.9%) in the control group (absolute difference, 3.25% [95% CI, −1.75% to 8.24%]; hazard ratio, 1.25 [95% CI, 0.94-1.67]; P = .13). There was no significant difference in the secondary outcome of all-cause mortality between the groups (18.2% vs 16.8%, respectively; hazard ratio, 1.12 [95% CI, 0.83-1.52]; P = .46). Of the 488 participants in the intervention group, 199 (40.8%) experienced serious adverse events that were classified as cardiovascular, 64 (13.1%) experienced adverse events classified as infection, and 22 (4.5%) experienced malignancy-related serious adverse events. Of 476 participants in the control group, 191 (40.1%) experienced cardiovascular-related serious adverse events, 63 (13.2%) experienced infection-related serious adverse events, and 21 (4.4%) experienced malignancy-related adverse events.
Conclusions and Relevance
Among patients without secondary hyperparathyroidism undergoing maintenance hemodialysis, oral alfacalcidol compared with usual care did not reduce the risk of a composite measure of select cardiovascular events. These findings do not support the use of vitamin D receptor activators for patients such as these.
Trial Registration
UMIN-CTR Identifier: UMIN000001194
This randomized, open-label, blinded end point randomized clinical trial examined the effect of oral alfacalcidol, a vitamin D receptor activator, on cardiovascular events in patients with cardiovascular kidney disease receiving hemodialysis.
Introduction
Patients with more advanced chronic kidney disease (CKD) are at a higher risk of cardiovascular disease (CVD), particularly patients requiring hemodialysis.1,2 The additional risk is explained by impairment in traditional and nontraditional risk factors1 associated with CKD. Mineral and bone disorder (MBD) in CKD3 is 1 of the nontraditional risk factors.4 Observational studies5,6 showed that serum phosphate, calcium, and intact parathyroid hormone (PTH) levels were associated with increased mortality risk in patients undergoing hemodialysis.
Impaired activation of vitamin D by the kidneys may be causatively involved in the pathogenesis of CKD-MBD and increased cardiovascular risk in CKD. A review7 of cohort studies revealed that the use of active vitamin D sterols, or vitamin D receptor activators (VDRAs), was associated with a lower risk of all-cause mortality,8 CVD-related mortality,9 and incident CVD10 in patients treated with hemodialysis. In a stratified analysis,8 lower mortality in VDRA users was observed in patients receiving hemodialysis, regardless of serum intact PTH levels. Clinical11 and experimental studies12,13 showed potentially beneficial effects of VDRAs, such as suppression of the renin-angiotensin system; modulation of immune functions; anti-inflammatory effects; antiatherosclerotic properties; inhibition of cardiac hypertrophy; and increase of proteins potentially protective against arterial calcification,14 including fetuin-A and klotho.15
However, the potential benefit of VDRA treatment in patients with CKD receiving hemodialysis has not been examined in randomized trials. Thus, the Japan Dialysis Active Vitamin D (J-DAVID) trial was performed to test the hypothesis that treatment with VDRAs reduces the risk of cardiovascular events and mortality in patients with CKD undergoing hemodialysis.
Methods
Ethical Considerations
This trial was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies by the Ministry of Health, Labor and Welfare, Japan (the original 2003 version, which was modified in 2004 and 2006). The protocol and revisions of this trial were approved by the ethics committee at the Osaka City University Graduate School of Medicine in Japan (approval numbers 1227, 1297, 1385, and 1525) and by the relevant ethics committees or institutional review boards at the study sites. All participants gave written informed consent before the study.
Study Design
This study was a clinical trial with a randomized, open-label, blinded end point design that examined the effect of alfacalcidol vs no VDRAs on cardiovascular events in patients receiving maintenance hemodialysis without secondary hyperparathyroidism over 48 months. The study methods have been published16 and the trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively.
Study Population
The participants were patients aged 20 to 80 years who were receiving maintenance hemodialysis; whose serum calcium was less than or equal to 10.0 mg/dL, phosphate was less than or equal to 6.0 mg/dL, and intact PTH level was less than or equal to 180 pg/mL; and who were not taking any VDRAs at randomization. These laboratory values were derived from the 2006 version of the Clinical Practice Guideline by the Japanese Society for Dialysis Therapy (JSDT),17 in which the recommended target intact PTH level was 60 to 180 pg/mL. Additional inclusion and exclusion criteria are listed in eTable 1 in Supplement 3. Eligible patients were recruited from July 1, 2008, to January 26, 2011, and screened at 207 dialysis centers in Japan.16
Randomization and Masking
Static 1:1 randomization was done using a computer-generated random sequence with a block randomization method stratified by age (<65 years vs ≥65 years), sex, years receiving dialysis (<5 years vs ≥5 years), underlying renal disease (diabetic nephropathy vs other), and history of CVD (yes vs no). Geographic region of dialysis centers was also considered in randomization, because of possible regional diversity in the lifestyle, clinical practice patterns, and risk of CVD in patients at each center. Block size was 4 for the first block and 2 thereafter because of the relatively small number of participants for each stratum. Fax was used for central randomization and communication between dialysis facilities and the data center at Osaka City University. Staff at the data center were not involved in enrollment of participants.
Intervention and Follow-up
The participants in the intervention group were assigned to receive oral alfacalcidol, starting at a dose of 0.5 μg per day, which was derived from a previous cohort study.9 The participants in this group were asked to avoid other VDRAs, but could receive them if it was clinically necessary to adhere to the JSDT Clinical Practice Guidelines.17,18 The participants in the control group were assigned to treatment without any VDRAs. The participants in this group were asked to avoid alfacalcidol and any other VDRA, but they were allowed to receive them if clinically necessary.
All participants were eligible to receive any medications other than VDRAs, including phosphate binders and cinacalcet, for standard medical care as recommended by the JSDT Clinical Practice Guidelines. The 20067 and 201218 versions of these guidelines recommend that the target ranges for serum phosphate and corrected calcium concentrations should be between 3.5 and 6.0 mg/dL and between 8.4 and 10.0 mg/dL, respectively. The target range of intact PTH levels was between 60 and 180 pg/mL in the 2006 version and between 60 and 240 pg/mL in the 2012 version. The target range of intact PTH levels for participants was updated during follow-up based on contemporaneous guidelines. Serum calcium and phosphate levels of the participants were measured twice a month, and intact PTH level was measured once every 3 months at local laboratories.
In the intervention group, hypercalcemia (serum total calcium level ≥10.5 mg/dL) and hyperphosphatemia (phosphate level ≥7.0 mg/dL) were managed with dietary therapy and/or dose adjustment of calcium carbonate, sevelamer hydrochloride, or other medications. If serum calcium or phosphate levels did not improve, dose reduction or temporal cessation of the study drug was allowed. The medication was readministered after confirming the recovery of serum calcium and phosphate levels within the target ranges. The dose of alfacalcidol was allowed to be adjusted within the range of 0.25 and 7.00 μg per week. If patients’ serum calcium and phosphate levels were in the target ranges, but intact PTH level exceeded the recommended limit, a switch from oral alfacalcidol to another oral or intravenous VDRA was allowed. During treatment with intravenous VDRAs, patients’ intact PTH levels were measured once a month. When intact PTH was reduced to a level within the recommended range or lower, a switch back to treatment with oral alfacalcidol was considered. For the intervention group, dropout from the assigned treatment was defined as no treatment with alfacalcidol for more than 12 consecutive weeks.
For patients in the control group, if serum calcium and phosphate levels were in the recommended ranges, but intact PTH levels exceeded the recommended limit, use of oral or intravenous VDRAs was allowed. During treatment with intravenous VDRAs, intact PTH levels were measured once a month. When the intact PTH level was reduced to be within the recommended range or lower, cessation of VDRA treatment was considered. For the control group, dropout from the assigned treatment was defined as treatment with any VDRA for more than 12 consecutive weeks.
Study Outcomes and Adverse Events
The primary outcome was defined as the composite of (1) fatal and nonfatal cardiovascular events, including acute myocardial infarction, congestive heart failure requiring hospitalization, stroke, aortic dissection/rupture, amputation of ischemic limb, and sudden cardiac death; (2) coronary interventions (eg, balloon angioplasty, stenting) or bypass grafting; and (3) lower limb artery interventions (eg, balloon angioplasty, stenting) or bypass grafting. The definitions of the individual cardiovascular events are listed in eTable 2 in Supplement 3. The secondary outcome was all-cause death. These outcomes were analyzed within the fixed period of 48 months. For adverse event assessment, laboratory data and all serious adverse events (SAEs) were reported. SAEs were defined previously16 and were categorized into cardiovascular, infection, malignancy, fall/bone fracture, accident, and other. The number of reported SAEs were summarized for each group. The event evaluation committee prospectively adjudicated the primary and secondary outcomes, as well as SAEs, with allocation groups being masked to the committee.
Sample Size Determination
To detect the difference in the proportion of the occurrence of the primary outcome between the groups with 80% power (β of 0.2) and an α of .05, we initially calculated a sample size of 1600 participants using a formula indicated in eTable 3 in Supplement 3, assuming that (1) 32% of participants in the control group would experience the primary outcome during the 4-year period,19 (2) the risk was reduced by 20% in the intervention group, and (3) 30 participants were lost to follow-up. We took 20% risk reduction as a clinically important difference because in a population without kidney disease, a 20% risk reduction is an accepted level for the prevention of cardiovascular disease.20 However, because of the difficulty in reaching this target sample size and possible overestimation of cardiovascular risk in the control group, we reset the target sample size to 972,16 assuming that (1) 28% of participants in the control group would experience the primary outcome, (2) there would be a risk reduction of 30% in the intervention group, which was based on previous cohort studies of the use of oral VDRAs in patients undergoing hemodialysis,21 and (3) 5% of participants would be lost to follow-up.
Interim Analysis
It was initially planned to perform interim analysis once, upon request of the independent data monitoring committee.16 However, after monitoring the interim data, the committee recommended to continue the study without performing formal interim analysis because the adverse events profile, judged by the numbers of SAEs, was similar between the groups and the total number of participants who experienced the primary outcome was too small.
Statistical Analysis
We defined 3 populations for analysis: a full analysis set, a per-protocol analysis set, and a modified per-protocol analysis set16 (eFigure 1 in Supplement 3). The primary analysis was performed using the time after randomization to the occurrence of the primary outcome with the full analysis set using Kaplan-Meier analysis with a log-rank test. Hazard ratio and 95% CI was calculated with an unadjusted Cox proportional hazards model for the primary composite outcome. The proportionality assumption was tested by log-minus-log plots and Schoenfeld residual tests, and we confirmed the assumption was met. Hazard ratios were also calculated for the individual components of the primary outcome. The key secondary analysis was done using time after randomization to all-cause death with the full analysis set, based on the same methods described above. As additional analyses, the primary and secondary outcomes were analyzed with the per-protocol set and the modified per-protocol set (see eFigure 1 in Supplement 3 for the definitions of the analysis populations) with and without adjustment for age, sex, diabetic nephropathy, duration of hemodialysis, and history of cardiovascular disease. As a post hoc analysis, a mixed-effects model was used to further adjust for regions of the dialysis centers (sites) as a random effect. Two-sided P values <.05 were considered statistically significant. No imputation was done. We used SPSS version 22 (IBM Japan) and R software version 3.5.1 (R Foundation for Statistical Computing) for statistical tests.
Results
Flow of the Participants and Their Baseline Characteristics
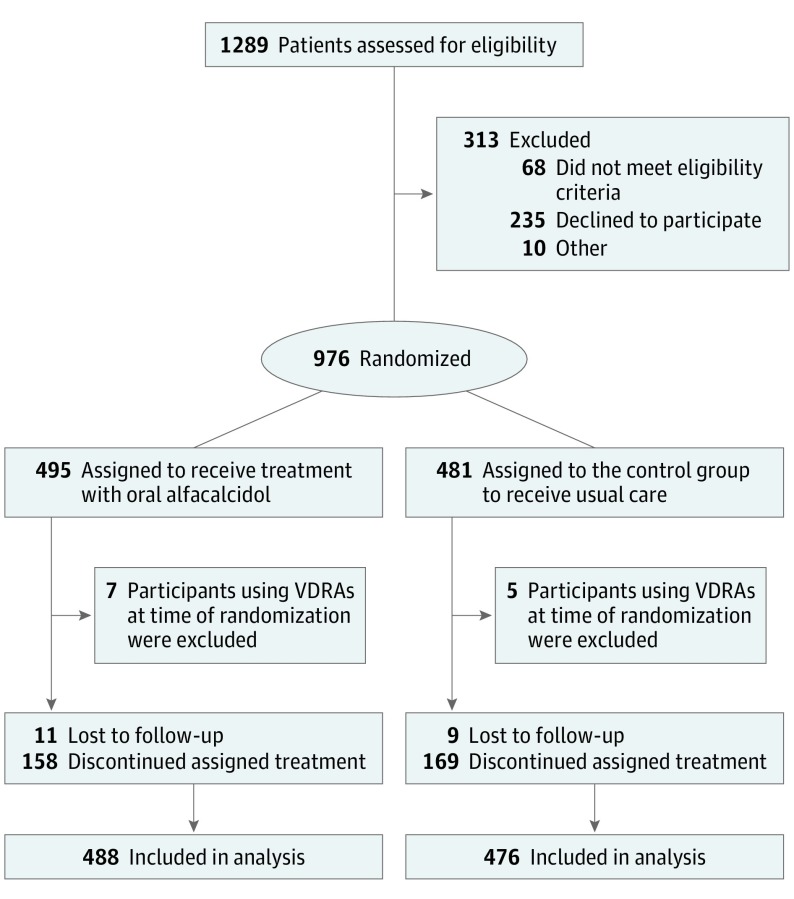
The participant recruitment was open in July 2008, the first patient was enrolled in August 18, 2008, and the last patient was enrolled January 26, 2011. The last day of participant follow-up was April 4, 2015. Eligibility was assessed in 1289 patients, and 976 patients from 108 dialysis centers were enrolled and randomized (Figure 1). Of the 976 participants who were randomized, 7 patients in the intervention group and 5 in the control group were excluded just after randomization because they were using VDRAs at the time of randomization. No follow-up data were available for these participants. Thus, the full analysis set included 488 participants in the intervention group and 476 in the control group. Table 1 shows the baseline characteristics of the participants. There was complete loss to follow-up of 11 and 9 participants in the intervention and control group, respectively, and these participants were censored on the last day of follow-up. The presence or absence of the primary outcome was known in 944 of 964 participants (97.9%) in the full analysis set. Assigned treatment was discontinued in 158 and 169 participants in the intervention group and the control group, respectively, who were censored, as defined, in the per-protocol set and the modified per-protocol set.
Figure 1. Flow of the Participants in a Study of the Effect of Oral Alfacalcidol in Patients Receiving Hemodialysis.
The participants who were lost to follow-up were censored on the last day of follow-up. No imputation was performed. The numbers analyzed are the same for the full analysis set, the per-protocol set, and the modified per-protocol set. These analysis sets differ only in the method of censoring of participants who discontinued their assigned treatment. Such participants were censored at the time of discontinuation in the per-protocol set, whereas participants in the intervention group who switched from oral alfacalcidol to another vitamin D receptor activator (VDRA) were not censored in the modified per-protocol set.
Table 1. Baseline Characteristics of Participants in a Study of the Effect of Oral Alfacalcidol in Patients Receiving Hemodialysis.
| Characteristic | Oral Alfacalcidol Group (n = 488) | Control Group (n = 476) |
|---|---|---|
| Age, median (IQR), y | 65 (58-71) | 65 (58-71) |
| Men, No. (%) | 301 (61.7) | 277 (58.2) |
| Women, No. (%) | 187 (38.3) | 199 (41.8) |
| Duration of hemodialysis, mean (IQR), y | 6 (2-10) | 5 (2-11) |
| Diabetic nephropathy, No. (%) | 207 (42.4) | 204 (42.9) |
| History of cardiovascular disease, No. (%) | 127 (26.0) | 117 (24.6) |
| Blood pressure | ||
| Systolic, No. | 486 | 476 |
| Median (IQR), mm Hg | 145 (130-160) | 148 (134-160) |
| Diastolic, No. | 486 | 475 |
| Median (IQR), mm Hg | 74 (67-82) | 74 (68-83) |
| Pulse rate, bpm | 72 (66-78) | 72 (66-78) |
| Dialysis, median (IQR), h per wk | 12 (12-12) | 12 (12-12) |
| Single pool Kt/Va | ||
| No. | 477 | 465 |
| Median (IQR) | 1.41 (1.23-1.61) | 1.44 (1.20-1.64) |
| Dialysate calcium concentration, No. (%), mEq/L | ||
| 2.5 | 130 (26.6) | 121 (25.4) |
| 3.0 | 333 (68.2) | 331 (69.5) |
| Other | 25 (5.1) | 24 (5.0) |
| Height, cm | ||
| No. | 478 | 468 |
| Median (IQR) | 160 (154-167) | 161 (153-167) |
| Body weight, kg | ||
| No. | 487 | |
| Median (IQR) | 54.5 (48.2-61.5) | 53.9 (47.5-61.9) |
| BMI | ||
| No. | 477 | 468 |
| Median (IQR) | 21.1 (19.0-23.4) | 21.1 (19.1-23.3) |
| Laboratory parameters, median (IQR) | ||
| C-reactive protein, mg/dL | ||
| No. | 427 | 414 |
| Median (IQR) | 0.10 (0.05-0.30) | 0.10 (0.05-0.26) |
| Serum albumin, g/dL | ||
| No. | 487 | 476 |
| Median (IQR) | 3.8 (3.5-3.9) | 3.8 (3.5-4.0) |
| Serum calcium, mg/dL | 8.9 (8.5-9.2) | 8.8 (8.5-9.2) |
| Corrected calcium, mg/dL | 9.1 (8.8-9.5) | 9.1 (8.7-9.5) |
| Phosphate, mg/dL | 4.6 (3.9-5.2) | 4.7 (4.0-5.4) |
| Intact parathyroid hormone, pg/mL | 85.1 (45.2-130.0) | 86.1 (47.0-127.4) |
| Hemogolobin, g/dL | 10.6 (10.0-11.3) | 10.7 (10.1-11.3) |
| Creatinine, mg/dL | 10.6 (8.9-12.4) | 10.4 (8.7-12.1) |
| Blood urea nitrogen, mg/dL | 64 (55-74) | 64 (55-76) |
| Sodium, mEq/L | 140 (138-142) | 140 (138-142) |
| Potassium, mEq/L | 4.8 (4.3-5.3) | 4.8 (4.2-5.3) |
| Chloride, mEq/L | ||
| No. | 486 | 471 |
| Median (IQR) | 103 (101-106) | 103 (101-106) |
| Total cholesterol, mg/dL | ||
| No. | 480 | 464 |
| Median (IQR) | 152 (133-176) | 150 (130-170) |
| Triglycerides, mg/dL | ||
| No. | 449 | 439 |
| Median (IQR) | 97 (68-136) | 96 (70-134) |
| HDLC, mg/dL | ||
| No. | 447 | 434 |
| Median (IQR) | 46 (38-56) | 46 (37-55) |
| AST, IU/L | ||
| No. | 487 | 474 |
| Median (IQR) | 14 (10-18) | 14 (10-17) |
| ALT, IU/L | ||
| No. | 487 | 474 |
| Median (IQR) | 11 (8-14) | 10 (8-14) |
| Hemoglobin A1c,b % | ||
| No. | 186 | 191 |
| Median (IQR) | 6.1 (5.6-6.9) | 6.1 (5.7-7.0) |
| Glycated albumin,b % | ||
| No. | 144 | 159 |
| Median (IQR) | 20.9 (18.3-24.7) | 21.2 (18.9-25.3) |
| Use of medication for MBD in CKD, No. (%) | ||
| Calcium carbonate | 412 (84.4) | 392 (82.4) |
| Sevelamer hydrochloride | 153 (31.4) | 155 (32.6) |
| Lanthanum carbonate | 69 (14.1) | 49 (10.3) |
| Cinacalcet hydrochloride | 27 (5.5) | 29 (6.1) |
| Active vitamin D sterol | 0 (0.0) | 0 (0.0) |
| Use of medication for renal anemia, No. (%) | ||
| Epoetin injection | 209 (42.8) | 212 (44.5) |
| Long-acting ESA | 171 (35.0) | 162 (34.0) |
| Intravenous iron | 112 (23.0) | 118 (24.8) |
| Use of medication for dyslipidemia, No. (%) | ||
| Statin | 77 (15.8) | 81 (17.0) |
| Use of medication for hypertension, No. (%) | ||
| Calcium channel blocker | 246 (50.4) | 262 (55.0) |
| ACEI | 41 (8.4) | 41 (8.6) |
| ARB | 237 (48.6) | 245 (51.5) |
| β-blocker | 109 (22.3) | 123 (25.8) |
| Loop diuretics | 141 (28.9) | 127 (26.7) |
| Use of medication for diabetes mellitus, No. (%) | ||
| Sulfonyl urea | 10 (2.1) | 4 (0.8) |
| Rapid-acting insulin secretagogue | 17 (3.5) | 13 (2.7) |
| α-glucosidase inhibitor | 49 (10.0) | 50 (10.5) |
| Insulin injection | 77 (15.8) | 88 (18.5) |
| Use of antithrombotic agents, No. (%) | ||
| Warfarin | 30 (6.2) | 31 (6.5) |
| Aspirin | 149 (30.5) | 128 (26.9) |
| Ticlopidine | 31 (6.4) | 21 (4.4) |
| Cilostazol | 48 (9.8) | 35 (7.4) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; ESA, erythrocyte stimulating agent; HDLC, high-density lipoprotein cholesterol; IQR, interquartile range; MBD, mineral and bone disorder.
Single pool Kt/V was an indicator of dialysis adequacy calculated by dialyzer clearance of urea (K), dialysis time (t), and volume of distribution of urea (V) using a single pool model.
Reported only for patients with diabetic nephropathy.
Changes in Laboratory Data, Medications, and Adherence
In the control group, there was no apparent change in serum phosphate or corrected calcium levels during follow-up. Intact PTH levels slightly increased over time, but remained within the target range (eFigure 2 in Supplement 3). In contrast, the intervention group showed an initial increase in serum corrected calcium and an initial decrease in intact PTH levels, but these levels returned to the initial levels over time.
There were changes in medications in both groups (eFigure 2 in Supplement 3). Compared with the control group, the intervention group had lower proportions of calcium carbonate users and cinacalcet users, and higher proportions of sevelamer users and lanthanum carbonate users. The proportion of intravenous VDRA users increased over time in both groups, although the proportion was higher in the control group.
The proportion of participants who received the assigned treatment declined over time similarly in the groups (eFigure 3 in Supplement 3). Treatment with alfacalcidol was stopped for 158 of 488 patients (32.4%) in the intervention group, whereas treatment with any VDRA was started for 169 of 476 patients (35.5%) in the control group.
Primary and Secondary Outcomes
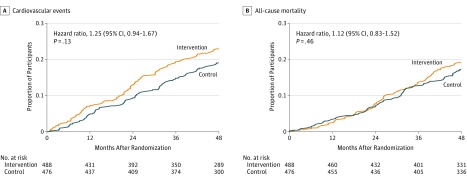
During follow-up (median, 4.0 years; mean, 3.6 years), the primary outcome occurred in 103 patients (21.1%) in the intervention group and 85 patients (17.9%) in the control group (absolute difference, 3.25% [95% CI, −1.75% to 8.24%]; hazard ratio, 1.25 [95% CI, 0.94-1.67]; P = .13). There was no significant difference between the intervention and control group in the secondary outcome of all-cause death (18.2% vs 16.8%, respectively; hazard ratio, 1.12 [95% CI, 0.83-1.52]; P = .46). Figure 2 shows the Kaplan-Meier curves for the primary and secondary outcomes from the intention-to-treat analysis with the full analysis set. Table 2 summarizes the composite and individual cardiovascular events that occurred.
Figure 2. Primary and Secondary Outcomes.
A, For the primary outcome, the median (interquartile range [IQR]) observation time was 4.0 (2.6-4.0) years for the intervention group and 4.0 (3.5-4.0) years for the control group. B, For the secondary outcome, the median (IQR) observation time was 4.0 (4.0-4.0) years for the intervention group and 4.00 (4.0-4.0) years for the control group. The primary outcome (A) included fatal and nonfatal cardiovascular events, coronary revascularization, and leg artery revascularization. The secondary outcome (B) was all-cause mortality.
Table 2. Primary and Secondary Outcomes and Serious Adverse Events in Participants Undergoing Hemodialysis Who Received Alfacalcidol or Standard Care.
| Intervention Group (n = 488) | Control Group (n = 476) | Absolute Difference, % (95% CI) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|
| Composite first cardiovascular events, No. (%) | 103 (21.1) | 85 (17.9) | 3.25 (−1.75 to 8.24) | 1.25 (0.94-1.67) |
| Cardiovascular disease, No. (%) | 67 (13.7) | 56 (11.8) | 1.96 (−2.24 to 6.17) | 1.26 (0.88-1.79) |
| Acute myocardial infarction, No. | 10 | 11 | ||
| Congestive heart failure, No. | 15 | 14 | ||
| Stroke, No. | 28 | 14 | ||
| Aortic dissection/Rupture, No. | 3 | 2 | ||
| Amputation of ischemic limb, No. | 4 | 4 | ||
| Cardiac sudden death, No. | 7 | 11 | ||
| Coronary intervention/bypass, No. (%) | 21 (4.3) | 18 (3.8) | 0.52 (−1.96 to 3.01) | 1.20 (0.64-2.25) |
| Percutaneous intervention, No. | 20 | 11 | ||
| Bypass grafting, No. | 1 | 7 | ||
| Leg artery intervention/bypass, No. (%) | 15 (3.1) | 11 (2.3) | 0.76 (−1.28 to 2.80) | 1.40 (0.64-3.05) |
| Percutaneous intervention, No. | 14 | 11 | ||
| Bypass grafting, No. | 1a | 0 | ||
| All-cause mortality (secondary outcome), No. (%) | 89 (18.2) | 80 (16.8) | −0.25 (−5.14 to 4.34) | 1.12 (0.83-1.52) |
| Serious adverse events, No. (%) | 371 (76.0) | 377 (79.2) | ||
| Cardiovascular, No. | 199 | 191 | ||
| Infection, No. | 64 | 63 | ||
| Malignancy, No. | 22 | 21 | ||
| Falls/Bone fracture, No. | 9 | 12 | ||
| Other,b No. | 77 | 90 |
One patient who received both leg artery bypass grafting and percutaneous intervention at the same time was classified as being treated with bypass grafting in this table.
Including respiratory, hepatobiliary, gastrointestinal, urogenital, dermal, ophthalmologic, and orthopedic adverse events and accidents.
Adverse Events
As shown in Table 2, the number of SAEs was similar between the groups. The number of cardiovascular SAEs was higher than the number of occurrences of the primary outcome because some participants had more than 2 cardiovascular SAEs. The predefined laboratory abnormalities are summarized in eTable 4 in Supplement 3, and show that corrected serum calcium greater than 10.0 mg/dL and phosphate greater than 6.0 mg/dL were more common in the intervention group than the control group, and intact PTH greater than 240 pg/mL was less common, particularly in the first year of follow-up.
Additional Analyses
The adjusted hazard ratios for the primary outcome were 1.32 (95% CI, 0.96-1.82; P = .09) and 1.34 (95% CI, 0.97-1.83; P = .07) when the per-protocol set and the modified per-protocol set, respectively, were analyzed. The adjusted results of the preplanned and post hoc analyses are shown in Table 3.
Table 3. Primary and Secondary Outcomes in Participants Undergoing Hemodialysis Who Received Alfacalcidol or Standard Carea.
| Outcome and Analysis Method | No. (%) | Unadjusted | Adjusted 1b | Adjusted 2c | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention Group (n = 488) | Control Group (n = 476) | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Cardiovascular Events | ||||||||
| Full analysis set | 103 (21.1) | 85 (17.9) | 1.25 (0.94-1.67) | .13 | 1.26 (0.95-1.68) | .11 | 1.29 (0.96-1.72) | .09 |
| Per-protocol set | 87 (17.8) | 68 (14.3) | 1.32 (0.96-1.81) | .09 | 1.32 (0.96-1.82) | .09 | 1.35 (0.98-1.86) | .07 |
| Modified per-protocol set | 89 (18.2) | 68 (14.3) | 1.33 (0.97-1.82) | .08 | 1.34 (0.97-1.83) | .07 | 1.36 (0.99-1.87) | .06 |
| All-Cause Mortality | ||||||||
| Full analysis set | 89 (18.2) | 80 (16.8) | 1.12 (0.83-1.52) | .46 | 1.12 (0.83-1.52) | .46 | 1.13 (0.83-1.53) | .43 |
| Per-protocol set | 66 (13.5) | 67 (14.1) | 0.98 (0.70-1.38) | .91 | 0.97 (0.69-1.36) | .86 | 0.97 (0.69-1.36) | .85 |
| Modified per-protocol set | 67 (13.7) | 67 (14.1) | 0.98 (0.70-1.37) | .89 | 0.97 (0.69-1.36) | .86 | 0.97 (0.69-1.36) | .85 |
The primary intention-to-treat analysis was done for the primary outcome with the full analysis set. Additional analysis was performed with the per-protocol set and the modified per-protocol set. The per-protocol set consists of all eligible participants who were randomized, but participants were censored at the time of discontinuation of the assigned treatment. The modified per-protocol set differs from the per-protocol set in the respect that participants in the intervention group who switched from alfacalcidol to another oral/intravenous vitamin D receptor activators were not censored.
Adjusted for age, sex, dialysis duration, underlying diabetic nephropathy, and history of cardiovascular disease.
Adjusted with a mixed-effects model (post hoc) with regions (sites) as a random effect in addition to age, sex, dialysis duration, underlying diabetic nephropathy, and history of cardiovascular disease as fixed effects.
Discussion
In patients without secondary hyperparathyroidism receiving maintenance hemodialysis, oral alfacalcidol compared with usual care did not reduce the risk of a composite measure of select cardiovascular events.
The PRIMO22 and OPERA23 studies showed no significant effect of oral paricalcitol on left ventricular mass index in patients with CKD. Three 2017 studies that examined the effects of calcitriol and nutritional vitamin D sterols on vascular functions, measured by arterial stiffness24 or flow-mediated dilatation,25,26 reported inconsistent results. The current trial failed to show the cardiovascular benefit of VDRAs in patients with CKD receiving hemodialysis.
Despite the lack of evidence by randomized trials, many nephrologists consider the administration of VDRAs to be mandatory for patients undergoing hemodialysis, based on a number of observational studies that showed associations between VDRA use and lower risk of all-cause mortality8 and cardiovascular outcomes9,10,21 in participants receiving hemodialysis. A trial with VDRAs was deemed not to be feasible by many nephrologists because half of the included patients would not be treated with VDRAs during the trial. This issue was addressed in the current trial by selecting patients with intact PTH levels below the maximum limit of the target range recommended by the JSDT, for whom VDRAs were not necessarily required.
The hazard ratio of 1.25 for the primary outcome derived from intention-to-treat analysis was not statistically significant. However, the hazard ratio was higher when analyzed using the per-protocol and modified per-protocol sets with preplanned or post hoc adjustment. The results of the additional analyses should be interpreted cautiously, but they may be a signal of the potential harm rather than benefit of VDRAs in patients without elevated serum intact PTH levels. VDRAs increase serum FGF23, which induces left ventricular hypertrophy27 and is associated with congestive heart failure28 and mortality.29 In the current trial, the occurrence of congestive heart failure and cardiac sudden death was similar between the groups. The occurrence of coronary events was also similar. The occurrence of stroke was twice as high in the intervention group than the control group (28 vs 14). Although this observation may be coincidental, the results raise the possibility that MBD in CKD and/or methods used to manage it could influence the cerebrovascular system.
The results of this trial were different than the results of previous observational studies. There are possible explanations for this discrepancy. First, parathyroid function or bone turnover may modify the cardiovascular effects of VDRAs. Typically, VDRAs are prescribed to patients with hyperparathyroidism and high bone turnover, whereas alfacalcidol was given to patients with serum intact PTH levels less than or equal to 180 pg/mL in the current trial. VDRAs may only benefit patients with secondary hyperparathyroidism by suppressing phosphate/calcium mobilization from bone. Second, the cardiovascular effects of VDRAs may vary depending on the degree of calcium load. In this trial, more than 68% of the participants received dialysis against 3.0 mEq/L calcium, and more than 80% of the participants were treated with calcium carbonate at baseline. Third, observational studies could be confounded by some important but unmeasured variables for which statistical adjustment cannot be made. Gene polymorphisms affect bone turnover and response to calcium and vitamin D supplementation.30 Patients not using VDRAs in cohort studies may be the patients who could not continue VDRAs in these studies because of hypercalcemic response.
Limitations
This study has several limitations. First, the statistical analysis may have been underpowered for the primary and secondary outcomes. Based on the actual number of patients that experienced the primary outcome in the control group, the power to detect a 20% risk reduction was calculated to be approximately 24%. The null findings are not definitive. Second, because of the unblinded design, there are some potential biases, such as unequal cointerventions and assessment of outcomes in favor of the hypothesis. Although adjudication of outcomes was done by a blinded committee, reporting bias by investigators cannot be excluded. However, such biases are not likely to affect the results, because the results were against the study hypothesis. Third, there was considerable contamination by dropout and readministration of VDRA use, which could reduce statistical power and affect the results. However, the additional analysis with the per-protocol set and modified per-protocol set did not support the cardiovascular benefit of treatment with VDRAs. Fourth, information on serum 25-hydroxyvitamin D level or consumption of native vitamin D supplements are not available. Fifth, the results cannot be generalized to patients with secondary hyperparathyroidism or to non-Japanese populations, particularly not to US patients undergoing hemodialysis, who have much higher levels of intact PTH than the participants of this trial. Sixth, no information was recorded on why the participants discontinued their treatment. Although the reasons are likely to be laboratory abnormalities, such as high serum calcium in the intervention group and high intact PTH levels in the control group, no data are available in case report forms.
Conclusions
Among patients without secondary hyperparathyroidism undergoing maintenance hemodialysis, oral alfacalcidol compared with usual care did not reduce the risk of a composite measure of select cardiovascular events. These findings do not support the use of vitamin D receptor activators for patients such as these.
Trial Protocol
Statistical Analysis Plan
eTable 1. Eligibility criteria
eTable 2. Definitions of cardiovascular events
eTable 3. Sample size calculation
eTable 4. Predefined laboratory abnormalities
eTable 5. Blood pressure measurements during follow-up
eTable 6. List of the J-DAVID Investigators
eFigure 1. Definitions of three populations for analysis
eFigure 2. Changes in laboratory data and medications
eFigure 3. Adherence to the assigned treatments
eFigure 4. Study organization of J-DAVID trial
Data Sharing Statement
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050-1065. doi: 10.1161/01.HYP.0000102971.85504.7c [DOI] [PubMed] [Google Scholar]
- 2.Nakai S, Masakane I, Akiba T, et al. Overview of regular dialysis treatment in Japan as of 31 December 2006. Ther Apher Dial. 2008;12(6):428-456. doi: 10.1111/j.1744-9987.2008.00634.x [DOI] [PubMed] [Google Scholar]
- 3.Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945-1953. doi: 10.1038/sj.ki.5000414 [DOI] [PubMed] [Google Scholar]
- 4.Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388(10041):276-284. doi: 10.1016/S0140-6736(16)30508-6 [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208-2218. doi: 10.1097/01.ASN.0000133041.27682.A2 [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi M, Fukagawa M, Fujii N, et al. Serum phosphate and calcium should be primarily and consistently controlled in prevalent hemodialysis patients. Ther Apher Dial. 2013;17(2):221-228. doi: 10.1111/1744-9987.12030 [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73(12):1355-1363. doi: 10.1038/ki.2008.35 [DOI] [PubMed] [Google Scholar]
- 8.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115-1125. doi: 10.1681/ASN.2004070573 [DOI] [PubMed] [Google Scholar]
- 9.Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19(1):179-184. doi: 10.1093/ndt/gfg513 [DOI] [PubMed] [Google Scholar]
- 10.Shoji T, Marubayashi S, Shigematsu T, et al. Use of vitamin D receptor activator, incident cardiovascular disease and death in a cohort of hemodialysis patients. Ther Apher Dial. 2015;19(3):235-244. doi: 10.1111/1744-9987.12274 [DOI] [PubMed] [Google Scholar]
- 11.Tabata T, Suzuki R, Kikunami K, et al. The effect of 1 alpha-hydroxyvitamin D3 on cell-mediated immunity in hemodialyzed patients. J Clin Endocrinol Metab. 1986;63(5):1218-1221. doi: 10.1210/jcem-63-5-1218 [DOI] [PubMed] [Google Scholar]
- 12.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229-238. doi: 10.1172/JCI0215219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodyak N, Ayus JC, Achinger S, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104(43):16810-16815. doi: 10.1073/pnas.0611202104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virdi AS, Cook LJ, Oreffo RO, Triffitt JT. Modulation of bone morphogenetic protein-2 and bone morphogenetic protein-4 gene expression in osteoblastic cell lines. Cell Mol Biol (Noisy-le-grand). 1998;44(8):1237-1246. [PubMed] [Google Scholar]
- 15.Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243-2255. doi: 10.1161/CIRCULATIONAHA.111.053405 [DOI] [PubMed] [Google Scholar]
- 16.Shoji T, Inaba M, Nishizawa Y. Vitamin D receptor activator and prevention of cardiovascular events in hemodialysis patients—rationale and design of the Japan Dialysis Active Vitamin D (J-DAVID) trial. Renal Replacement Ther. 2016;2:19. doi: 10.1186/s41100-016-0029-z [DOI] [Google Scholar]
- 17.Guideline Working Group, Japanese Society for Dialysis Therapy . Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12(6):514-525. doi: 10.1111/j.1744-9987.2008.00648.x [DOI] [PubMed] [Google Scholar]
- 18.Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247-288. doi: 10.1111/1744-9987.12058 [DOI] [PubMed] [Google Scholar]
- 19.Shoji T, Masakane I, Watanabe Y, et al. Elevated non-high-density lipoprotein cholesterol (non-HDL-C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(5):1112-1120. doi: 10.2215/CJN.09961110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan BM, Li J, White K, et al. 2013 ACC/AHA Cholesterol Guideline and Implications for Healthy People 2020 Cardiovascular Disease Prevention Goals. J Am Heart Assoc. 2016;5(8):e003558. doi: 10.1161/JAHA.116.003558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naves-Díaz M, Alvarez-Hernández D, Passlick-Deetjen J, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74(8):1070-1078. doi: 10.1038/ki.2008.343 [DOI] [PubMed] [Google Scholar]
- 22.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674-684. doi: 10.1001/jama.2012.120 [DOI] [PubMed] [Google Scholar]
- 23.Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014;25(1):175-186. doi: 10.1681/ASN.2013010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin A, Tang M, Perry T, et al. Randomized controlled trial for the effect of vitamin D supplementation on vascular stiffness in CKD. Clin J Am Soc Nephrol. 2017;12(9):1447-1460. doi: 10.2215/CJN.10791016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendrick J, Andrews E, You Z, et al. Cholecalciferol, calcitriol, and vascular function in CKD: a randomized, double-blind trial. Clin J Am Soc Nephrol. 2017;12(9):1438-1446. doi: 10.2215/CJN.01870217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar V, Yadav AK, Lal A, et al. A randomized trial of vitamin D supplementation on vascular function in CKD. J Am Soc Nephrol. 2017;28(10):3100-3108. doi: 10.1681/ASN.2017010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393-4408. doi: 10.1172/JCI46122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349-360. doi: 10.1681/ASN.2013050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584-592. doi: 10.1056/NEJMoa0706130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaffney-Stomberg E, Lutz LJ, Shcherbina A, et al. Association between single gene polymorphisms and bone biomarkers and response to calcium and vitamin D supplementation in young adults undergoing military training. J Bone Miner Res. 2017;32(3):498-507. doi: 10.1002/jbmr.3008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Eligibility criteria
eTable 2. Definitions of cardiovascular events
eTable 3. Sample size calculation
eTable 4. Predefined laboratory abnormalities
eTable 5. Blood pressure measurements during follow-up
eTable 6. List of the J-DAVID Investigators
eFigure 1. Definitions of three populations for analysis
eFigure 2. Changes in laboratory data and medications
eFigure 3. Adherence to the assigned treatments
eFigure 4. Study organization of J-DAVID trial
Data Sharing Statement