This review of electrocorticographic recordings and clinical meta-data assesses seizure control in 12 patients with focal epilepsy who were treated with closed-loop invasive brain stimulation.
Key Points
Question
What is the association of closed-loop invasive brain stimulation with seizure control in patients with epilepsy?
Finding
In this cohort study of 11 patients with focal epilepsy, seizure reduction was not associated with the direct effects of acute responsive stimulation events. Indirect effects on seizure electrophysiology, which occurred remotely to individual stimulation events, were associated with improved seizure control.
Meaning
Therapeutic outcomes of closed-loop stimulation appear to emerge from modulation of the seizure network over time rather than from the acute interruption of individual seizure events.
Abstract
Importance
A bidirectional brain-computer interface that performs neurostimulation has been shown to improve seizure control in patients with refractory epilepsy, but the therapeutic mechanism is unknown.
Objective
To investigate whether electrographic effects of responsive neurostimulation (RNS), identified in electrocorticographic (ECOG) recordings from the device, are associated with patient outcomes.
Design, Setting, and Participants
Retrospective review of ECOG recordings and accompanying clinical meta-data from 11 consecutive patients with focal epilepsy who were implanted with a neurostimulation system between January 28, 2015, and June 6, 2017, with 22 to 112 weeks of follow-up. Recorded ECOG data were obtained from the manufacturer; additional system-generated meta-data, including recording and detection settings, were collected directly from the manufacturer’s management system using an in-house, custom-built platform. Electrographic seizure patterns were identified in RNS recordings and evaluated in the time-frequency domain, which was locked to the onset of the seizure pattern.
Main Outcomes and Measures
Patterns of electrophysiological modulation were identified and then classified according to their latency of onset in relation to triggered stimulation events. Seizure control after RNS implantation was assessed by 3 main variables: mean frequency of seizure occurrence, estimated mean severity of seizures, and mean duration of seizures. Overall seizure outcomes were evaluated by the extended Personal Impact of Epilepsy Scale questionnaires, a patient-reported outcome measure of 3 domains (seizure characteristics, medication adverse effects, and quality of life), with a range of possible scores from 0 to 300 in which lower scores indicate worse status, and the Engel scale, which comprises 4 classes (I-IV) in which lower numbers indicate greater improvement.
Results
Electrocorticographic data from 11 patients (8 female; mean [range] age, 35 [19-65] years; mean [range] duration of epilepsy, 19 [5-37] years) were analyzed. Two main categories of electrophysiological signatures of stimulation-induced modulation of the seizure network were discovered: direct and indirect effects. Direct effects included ictal inhibition and early frequency modulation but were not associated with improved clinical outcomes (odds ratio [OR], 0.67; 95% CI, 0.06-7.35; P > .99). Only indirect effects—those occurring remote from triggered stimulation—were associated with improved clinical outcomes (OR, infinity; 95% CI, –infinity to infinity; P = .02). These indirect effects included spontaneous ictal inhibition, frequency modulation, fragmentation, and ictal duration modulation.
Conclusions and Relevance
These findings suggest that RNS effectiveness may be explained by long-term, stimulation-induced modulation of seizure network activity rather than by direct effects on each detected seizure.
Introduction
Modulation of ongoing seizure activity by applying electrical current directly to the cortex was reported by Penfield and Jasper1 as early as 1954. Sixty years later, the RNS System (NeuroPace, Inc), a closed-loop responsive neurostimulator, was developed to automatically analyze electrocortical potentials to detect seizures and rapidly deliver electrical stimulation, with the goal of suppressing seizure activity.2,3 Cortical electrical stimulation in an open-loop acute, subacute, or combination mode of operation has been shown to suppress electrographic epileptiform discharges2,4,5,6,7 as well as to reduce seizure frequency.4,8,9,10,11,12,13,14,15 Class 1 evidence supports the efficacy of responsive neurostimulation (RNS) in seizure reduction, with 44% seizure reduction at 1 year after implantation and 53% at 2 years,16 and open-label continuation studies show a range of 48% to 66% between the third and sixth years after implantation.17 At 6 years, a median 70% of patients with both mesial temporal and neocortical seizure onset experienced significant reduction in seizure frequency; 26% to 29% exhibited a postimplantation seizure-free period of at least 6 months, and approximately 15% experienced 1 year or more without seizures.18,19
Although the RNS System provides improved seizure control and quality of life, its mechanism of action is unknown and its overall efficacy remains suboptimal. Historically, the primary hypothesis for the mechanism of action of RNS has been the direct inhibition of ongoing ictal activity by triggered electrical stimulation.2,20,21,22,23 We tested the hypothesis that clinical effectiveness arises from successful detection-triggered electrical stimulation and subsequent direct termination of seizure activity.
Methods
Patients
All patients met International League Against Epilepsy criteria for focal epilepsy24,25 and underwent diagnostic intracranial recording, followed by multidisciplinary recommendation for closed-loop neurostimulation therapy using the RNS System. All patients implanted with the RNS System between January 28, 2015, and June 6, 2017, at our center were included in this study. Each participant gave written informed consent under a University of Pittsburgh Institutional Review Board–approved protocol.
Data Acquisition
The device was set to passive recording for the first postoperative month, during which no stimulation was delivered (baseline epoch). Recording and stimulation settings subsequently were activated and then periodically modified based on data review and clinical evaluations. The time interval during which settings remain unchanged is referred to as a programming epoch. Device-recorded data were obtained from the manufacturer as 90-second duration, 4-channel bipolar electrocorticography (ECOG) that was bandpass filtered online at 4 to 125 Hz before sampling at 250 Hz. Additional meta-data, including detection and stimulation settings, were collected directly from the Patient Data Management System (NeuroPace, Inc) using an in-house, custom-built platform for biophysically rational analysis of individualized neural stimulation data (BRAINStim).26
Data Processing
Electrographic seizure patterns (ESPs) were visually identified by an experienced epilepsy neurophysiologist (V.K.). The ESP onset was defined as the point after which the ECOG recording background was no longer interictal and was followed by a paroxysmal discharge of seizure features and developing morphologic features. Electrographic seizure patterns were categorized as having (1) clearly recorded onset after a preceding interictal background of a minimum of 5 seconds that did not receive stimulation owing to belonging to the baseline period, or to detection failure, or to reaching the daily stimulation limit; (2) clearly recorded onset after a preceding interictal background of a minimum of 5 seconds that received at least 1 stimulation pulse after the identified onset; (3) clearly recorded onset after a preceding interictal background of a minimum of 5 seconds that received at least 1 stimulation pulse before or during the onset (eFigure in the Supplement); and (4) no recorded onset owing to regular overwriting of internal memory storage. Only the first 2 groups of recorded ESPs were used for evaluation of modulatory stimulation effects separated into files of 65 seconds (starting 5 seconds before the identified onset, ending 60 seconds after onset). Spectral analysis was performed for each sample by using the fast Fourier transform of a 128-point window and a 125-point overlap for frequencies from 0.05 to 60.00 Hz at a step of 0.05 Hz. For each patient, ESPs were grouped by baseline and recording epochs, and group means from individual, 3-dimensional time-frequency power tables were created (Figure 1).
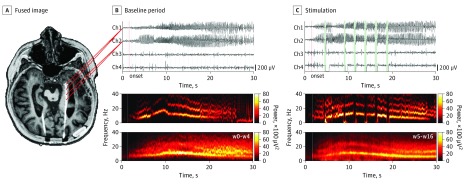
Figure 1. Closed-Loop Responsive Neurostimulation (RNS) Implantation and Data Processing Method.
A, Preoperative magnetic resonance image and postimplantation computed tomography fused image (EpiNav software27) aligned in the axial plane across the trajectory of the implanted RNS lead in the left hippocampus of patient 6. B, The 2 distal anterior hippocampal contacts provide bipolar channel 1, and the 2 proximal posterior hippocampal contacts form bipolar channel 2 (top), which record a unilateral electrographic seizure pattern (ESP) in the left hippocampus during the baseline period. For channel 2, a time-frequency plot shows the spectral evolution of the ESP aligned to onset (red vertical line). C, During stimulation, the amplifier is disconnected (time intervals in green), thereby generating a rectangular pulse artifact in the time domain (top) that is often followed by a considerable amplifier saturation direct current shift. In the frequency domain, stimulation appears as a low-frequency artifact accompanied by broadband cancellation (middle) that is often followed by a wideband artifact corresponding to amplifier saturation. Ch indicates channel; w, week of stimulation.
Significant changes in either the duration or spectral content of ESPs were defined as exceeding 25% of the mean duration of the respective averaged baseline pattern or frequency. Interruptions in the temporal progression of ESPs were scored as discontinuities—namely, as fragmentations—if there was a return to normal background levels for 0.5 to 3.0 seconds; interruptions of less than 0.5 seconds were not scored as discontinuities/fragmentations, whereas those exceeding 3.0 seconds were scored as separate electrographic events. Seizure fragmentations of this temporal resolution (0.5-3.0 seconds) were described as coarse. Increases in the time interval between consecutive seizure spike discharges were scored as fragmentations if their duration exceeded the respective mean baseline interval by a minimum of 100%. Seizure fragmentations of this temporal resolution (100 milliseconds to 1 second) were described as fine. Modulation effects then were categorized as either direct, in which the recorded events manifested systematic changes in time, frequency, or both either during stimulation or in the immediate poststimulation interval (<5 seconds), or indirect, in which the recorded events manifested changes in time, frequency, or both either before or long after the stimulation volley (>10 seconds) or could not be attributed to direct stimulation (eg, not time-locked to the stimulation).
Statistical Analysis
Clinical outcomes were derived by extended Personal Impact of Epilepsy Scale questionnaires,28 administered by one of us (N.D.S.) to all patients. The questionnaire is a simple, brief, patient-reported outcome measure developed at Stanford University to profile the overall impact of seizures, medication adverse effects, and overall quality of life for people with epilepsy. The questions measure 3 domains: characteristics of seizures,9 medication adverse effects,7 and overall quality of life.9 The minimum score is 0 and the maximum score is 300, with lower scores reflecting worse status. Scores in our study ranged from 90 to 271. The Personal Impact of Epilepsy Scale questionnaires were supplemented with 3 variables of interest subjectively describing seizure manifestation: (1) mean monthly seizure frequency before and after RNS implantation; (2) estimated mean severity of seizures on a scale of 1 to 5 (1 indicates not severe; 5, very severe); and (3) mean duration of seizures (minutes). Engel class was used to identify response. Engel class I indicated free of disabling seizures; Engel class II, rare disabling seizures; Engel class III, worthwhile improvement; and Engel class IV, no worthwhile improvement. Patients were grouped as either responders (Engel class I-III) or nonresponders (Engel class IV) based on the scores of the 3 seizure manifestation variables.29 Two binary variables were computed to represent the presence or absence of direct and indirect stimulation-induced modulation effects. The probability of achieving responder status given the presence of either effect type was calculated using 2 Fisher exact tests as well as the probability of improving either of the 3 seizure manifestation variables. MATLAB 2017a (The MathWorks, Inc) was used for the statistical calculations. Statistical significance was determined with Fisher exact tests using the fishertest function. All tests of significance were 2-tailed with 2-sided P values.
Results
Identification of ESPs
One patient was excluded from analysis because the patient was free of seizures after implantation (stimulation never initiated). Electrocorticographic data from 11 patients were analyzed (eTable 1 in the Supplement). Eight of the patients were women, the mean (range) age was 35 (19-65) years, and the mean (range) duration of epilepsy was 19 (5-37) years. The mean (SD) time after implantation to activation of responsive stimulation was 46.6 (19.6) days. A total of 14 634 ECOG files were visually reviewed, corresponding to 170 months of recordings spanning a 34-month total study period; 5148 ESPs were identified.
The spectral features of ESPs per programming epoch, during which the stimulation settings remained constant, were identified. For every patient, we visually evaluated all detected events in time and frequency spectrum domains. We discovered 2 main categories of neuronal electrical stimulation effects: direct effects, whereby the recorded events manifested systematic time and/or frequency changes in the immediate poststimulation interval, and indirect effects, whereby the recorded events manifested changes in time, frequency, or both before or long after stimulation or could not be attributed to direct stimulation.
Inhibition of ESPs
We first characterized the modulation of ESPs that resulted from individual stimulation events. In 4 patients, ESPs were observed in which progression was terminated in the immediate poststimulation period less than 5 seconds after the application of the first stimulation pulse, after which ECOG returned to the interictal background level (Figure 2A). This effect, which we termed direct inhibition, is consistent with that expected from earlier literature.2 Direct ESP inhibition emerged at a mean (SD) of 14 (11.7) weeks after stimulation was activated.
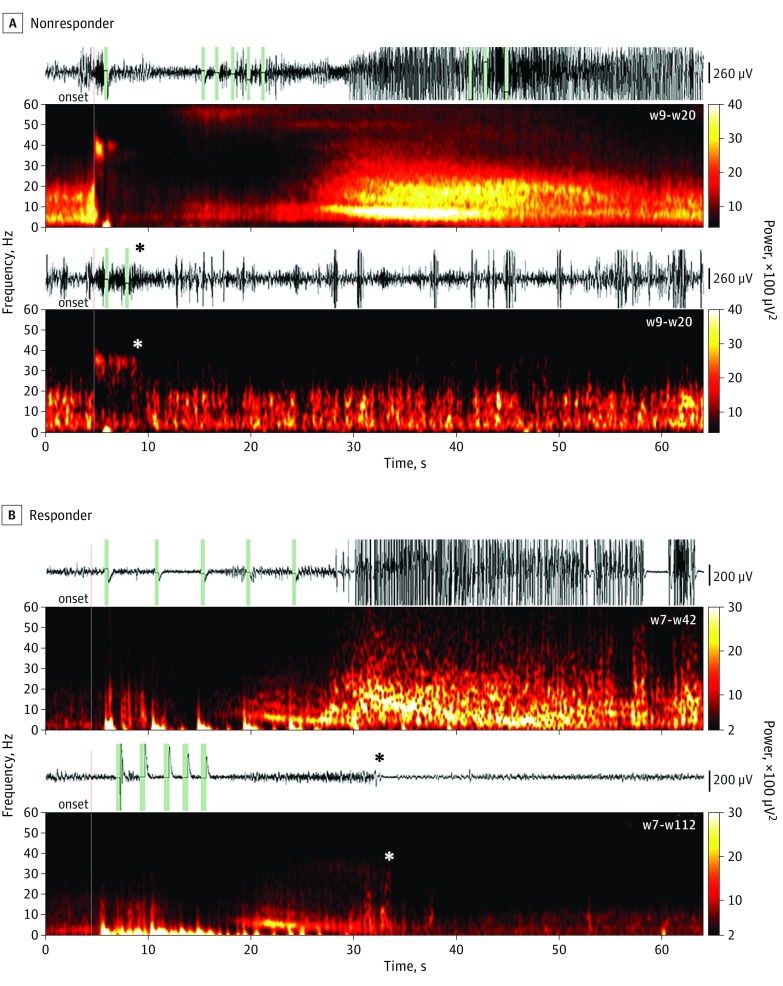
Figure 2. Direct Inhibition and Indirect Spontaneous Attenuation of Electrographic Seizure Patterns (ESPs).
A, Stimulated ESP examples for a nonresponder (patient 3). Most ESPs evolved despite stimulation (top), marked by an abrupt suppression of the normal background band and the appearance of a distinctive, high-beta, 30- to 40-Hz oscillation; some were inhibited shortly after their onset (bottom; asterisk marks the time point of the respective effect). B, Patient 11 (responder), whose typical ESP starts with a diffuse electro-decrement followed by the development of a theta-range (4-8 Hz) rhythm evolving into high–amplitude/power paroxysmal wide-band delta- to beta-range (2-30 Hz) rhythms overlaid with higher gamma (>30 Hz) frequencies (top). From weeks 7 to 112, a distinct number of ESPs were observed in which the development of the ongoing activity was spontaneously interrupted (bottom; asterisk) and the electrocorticography returned to normal background levels. Note that attenuation occurs more than 27 seconds after the first stimulation pulse and the bulk of stimulation ends almost 11 seconds before this spontaneous inhibition. Vertical lines represent ESP onsets (red) and stimulation events (green). w Indicates week of stimulation.
Transient Modulation of Frequency Content of ESPs
We identified changes in the frequency content of ESPs that resulted from individual stimulation events. In 3 patients, we observed modulation of the spectral constituents of ESPs that occurred shortly (mean, <5 seconds) after the application of the first stimulation pulse or during the stimulation interval (Figure 3A). This direct frequency modulation effect was variable and consisted of both attenuation of the baseline frequencies and the genesis of novel oscillations at higher-than-baseline frequencies over time. Direct frequency modulation effects emerged at a mean (SD) of 12 (10.5) weeks after activation of responsive stimulation.
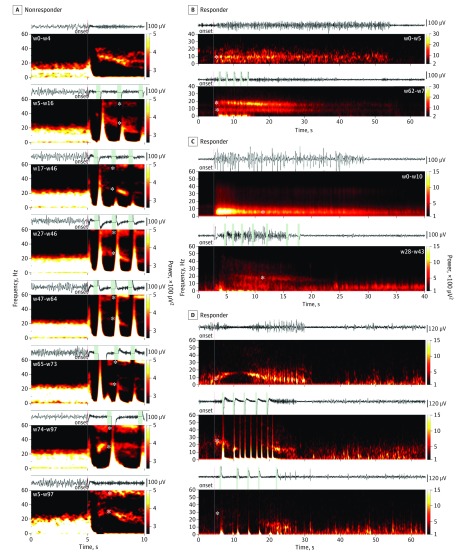
Figure 3. Direct and Indirect Frequency Modulation of Electrographic Seizure Patterns (ESPs).
A, Nonresponder’s ESP shows the 10 seconds centered on seizure pattern onset. A high- to low-beta (from 40 to 20 Hz) frequency band is systematically present at onset during baseline. During consecutive programming epochs, a progressive peristimulus attenuation of the initially dominant beta oscillation is seen, accompanied by progression of a novel gamma (55-60 Hz) oscillation (asterisks, weeks [w] 5-97). Both frequency bands appear concurrently in ESPs that were missed by stimulation throughout the stimulation periods. B, Responder’s ESP shows a baseline alpha-range (asterisk, 9-10 Hz) rhythm being replaced by a double band of independent theta and beta frequencies (asterisks, 6-10 Hz and 13-20 Hz, respectively). C, Responder’s ESP shows a semicontinuous progression of discharges in the delta to alpha range (asterisk, 2-10 Hz) and a newly appearing distinct subgroup of ESPs featuring a wide-band high-frequency distribution of epileptic oscillation (asterisk with range, 4-50 Hz). D, Responder’s electrographic seizures shows an arclike pattern in the delta to low-beta range (asterisk, 7-17 Hz) undergoing progressive changes in their onset spectral content during responsive neurostimulation treatment (asterisk, 20-36 Hz), evolving to diffuse spectrum low-power discharges (asterisk, 10-40 Hz). Vertical lines represent ESP onsets (red) and stimulation events (green).
Spontaneous Attenuation of ESPs
Next, we characterized indirect effects in which recorded events manifested evidence of modulation that could not be attributed to a specific stimulation event. In 1 patient, we observed seizure patterns with progression that was spontaneously discontinued in the absence of a direct stimulation event during periods of baseline activity, defined as more than 27 seconds after the application of the nearest initial stimulation pulse and more than 11 seconds after the end of subsequent triggered stimulation pulses (Figure 1B). We termed this effect indirect attenuation. The onset of this spontaneous indirect effect was first observed during the 9th week of stimulation and reappeared regularly up to the time of the last follow-up (112th stimulation week).
Frequency Modulation of ESPs
We identified changes in the spectral constituents of ESPs that were not related to individual stimulation events (ie, indirect frequency modulation), which emerged over time in 4 patients. Frequency modulation was observed in ESPs that did not receive triggered stimulation, suggesting the presence of an underlying effect from chronic stimulation (Figure 3A). A narrow alpha-band (9-10 Hz) ESP (Figure 3B) transformed into a double-band pattern of concurrently evolving theta (6-10 Hz) and beta (13-20 Hz) frequencies (Figure 3B). In 1 example, an ESP characterized by a semicontinuous progression of discharges in the delta-to-alpha range (2-10 Hz; Figure 3C) progressively developed into a distinct wide-band (4-50 Hz) ESP independent of specific stimulation pulses. In another example, an arclike pattern developed in the delta–to–low-beta range (2-18 Hz; Figure 3D). Over subsequent stimulation periods (1-24 weeks), these patterns underwent progressive changes in their spectral content (10-40 Hz; Figure 3D). Overall, indirect frequency modulation effects emerged at a mean (SD) of 7 (5.2) weeks after activation of responsive stimulation.
Stimulation and the Refractory Interval Between Ictal Spikes
In 1 patient, we observed indirect fine fragmentation of the ESP, in which the refractory interval between consecutive seizure spike discharges increased (mean [SD] baseline interval, 202.1 [15.7] milliseconds; mean [SD] posteffect interval, 798.4 [48.1] milliseconds) (Figure 4A) independent of the timing of stimulation onset. Although increased interdischarge intervals were observed sporadically during baseline and previous programming epochs, they were only established as the dominant ESP after the 60th week of stimulation.
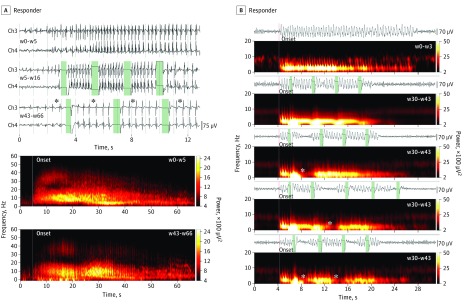
Figure 4. Indirect Fine and Coarse Fragmentation of Electrographic Seizure Patterns (ESPs).
A, ESPs in a responder (patient 6) that became finely fragmented with a systematic and significant increase in interdischarge interval from a previous baseline mean of approximately 200 milliseconds (top, baseline weeks [w] 0-5 and first programming epoch weeks 5-16) to a posteffect interval of approximately 800 milliseconds (top) (asterisk; weeks 43-66). The corresponding spectrogram (bottom) assumes a comblike pattern compared with that of the unmodulated baseline ESP (middle). B, ESPs from a responder (patient 10) that became coarsely fragmented are characterized by pronounced discontinuities in their development during which the electrocorticogram (ECOG) returned to the background levels (asterisks; rows 3-5 of the ECOG/spectrogram pairs) with respect to both baseline (top; weeks 0-3) and unmodulated seizure patterns (second row). The fragmented epochs were not time locked to the electric stimulus and could appear more than once during the epileptic ictal discharge (asterisks; bottom row). Vertical lines represent ESP onsets (red) or stimulation events (green). Ch indicates channel.
Fragmentation of Stimulated ESPs
In 2 patients, we observed indirect coarse fragmentation of the ESP, in which the continuity of an ongoing discharge was interrupted by segments of normal background activity (Figure 4B). These seizure fragments occurred in random intervals from the onset of any given stimulation and with variable duration. The mean (SD) onset of the fragmentation effect was 13 (7.7) weeks after stimulation activation.
Modulation of Mean ESP Duration
We observed significant bidirectional changes in the mean duration of the ESP that occurred in the absence of direct stimulation events in 5 patients (Figure 3C). In 4 of the patients, a mean (SD) 50.25% (12.9%) reduction in total ESP duration was observed. The fifth patient progressively reached a mean (SD) 132% (44.3%) increase in ESP duration. Although stimulation-induced direct inhibition reduces the duration of the ictal pattern, the indirect reduction in ESP duration is a different phenomenon because the offset of the ESP is not related to the offset of any of the applied stimuli. The mean (SD) onset of indirect effects that altered ictal duration was 31 (24.3) weeks.
Nature of Modulation Effects
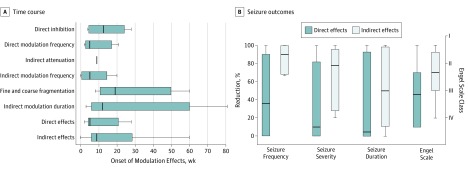
The mean (SD) onset of modulation effects was 11.5 (10.5) stimulation weeks for direct effects and 24.0 (20.8) weeks for indirect effects, suggesting that network plasticity is required for both types of changes (Figure 5A). All effects were highly consistent within each patient and programming epoch. Because stimulation settings often change across the programming epoch, the frequency of an effect also may change (eTable 2 in the Supplement). The particular settings responsible for these variances is the subject of ongoing in-depth analysis, although variance estimation is biased by the incomplete nature of the ECOG recordings on the first-generation device (see Limitations below).
Figure 5. Electrographic Seizure Pattern Effects: Time Course and Association With Seizure Outcomes.
A, Onset of modulation effects in weeks after activation of the stimulation treatment. B, Percentages of the reductions in seizure frequency, severity, and duration compared with preimplantation baseline readings (left y-axis) and Engel scale scores (right y-axis) for patients having only direct effects and for those having indirect effects. The Engel scale consists of 4 classes (I-IV) in which lower numbers indicate greater improvement; class III or better defines a clinical responder. Internal line indicates median; box ends, first and third quartile; and limit lines, minimum and maximum values.
Association of Indirect Modulation Effects With Clinical Outcome
To determine whether direct modulation effects, indirect modulation effects, or both were associated with the reduction of seizures, Fisher exact tests were performed to separately measure the probability of observing either type of effect and achieving responder status (Engel class III or better). The odds ratio (OR) for indirect modulation effects was infinity (95% CI, –infinity to infinity; P = .02), demonstrating an association between the presence of 1 or more indirect modulation effects and good clinical response. The OR for direct effects was not significant (0.67; 95% CI, 0.06-7.35; P > .99) (Figure 5B). Fisher exact tests then were performed to measure the probability of observing a direct or indirect modulation effect and achieving reduction in seizure frequency, severity, or duration. The OR for indirect modulation effects was significant for each of these outcomes (seizure occurrence frequency: OR, infinity; 95% CI, –infinity to infinity; P = .005; seizure severity: OR, infinity; 95% CI, –infinity to infinity; P = .007; and seizure duration: OR, 28.0; 95% CI, 1.35-580.60; P = .03), whereas the OR for direct modulation effects was not significant for any outcome (seizure frequency: OR, 0.67; 95% CI, 0.07-6.41; P > .99; seizure severity: OR, 0.0; 95% CI, –infinity to infinity; P = .10; and seizure duration: OR, 0.25; 95% CI, 0.02-2.76; P = .56).
Discussion
We investigated the validity of the direct inhibition hypothesis in 11 patients implanted with RNS. We discovered 2 categories of stimulus-related modulation effects: (1) direct inhibition of the ESP in accordance with traditional hypotheses of the RNS mechanism of action and (2) direct frequency modulation, a novel finding of direct poststimulus changes in the spectral content (spectral) signature of the ESP that includes attenuation of prominent baseline bands, which may rebound in time, as well as the genesis of a completely new ESP. Direct stimulation effects were not associated with clinical outcomes. We discovered, however, that indirect modulation effects, which occurred independent of specific stimulation events, were associated with clinical outcomes.
The direct effects of electrical stimulation during ongoing seizure activity have been widely described. Penfield and Jasper1 were the first to observe and report stimulation-induced inhibitory effects of electrical stimulation on active epileptogenic neural tissue; this finding was later also verified by stimulation of hippocampal slices in vitro.30,31 Electrical stimulation also has been reported to have a significant inhibitory effect on interictal and ictal cortical activity in patients with epilepsy.2,4,5,6,7,8,9,10,11,12,13,14,15 The RNS System is a valuable surgical option for patients whose epilepsy is refractory to both antiepileptic drugs and traditional epilepsy surgery,3 with data supporting its superiority to medical management.16,18,19 However, apart from the few original publications,2,20,32 little is known about how closed-loop stimulation affects the time course of the detected ESP and related mechanisms of action for reducing seizure severity and frequency. The previously assumed mechanism is a direct one, by which the application of an electrical pulse close to the origin of the ESP interrupts its evolution and returns the ECOG background to its interictal state.2,21,22 Direct inhibition of ongoing seizure activity could occur through transient stimulation-induced activation of local postsynaptic potentials, creating extracellular fields opposing those created by the epileptogenic excitatory postsynaptic potentials. In this model, stimulation could reduce the ability of the excitatory neuronal population to synchronize, thereby acting as a neuronal desynchronizer.31
Our discovery of types of modulation that cannot be explained by acute responses to individual stimulation events suggests an alternate hypothesis. All indirect modulation patterns did not appear during the stimulation period and were not affected by individual stimulation events. One explanation for these findings is that stimulation establishes extracellular electrical field barriers between functionally interconnected epileptogenic populations, thereby isolating excitatory neuronal pools. These neuronal pools become separated and independent of the core epileptogenic pool over time, resulting in lower amplitude and power of baseline oscillations (progressive attenuation) owing to the reduced number of participating neuronal pools and the appearance of higher frequency oscillations owing to multiple spatially scattered populations unable to achieve high levels of synchronization.33,34 In general, frequency modulation effects indicate that stimulation may drive parts of the underlying epileptogenic network to synchronize at alternative frequencies. On the other hand, a progressive failure of excitatory neuronal populations to achieve sufficiently high levels of synchronization could account for the observed seizure attenuation affect. Likewise, fine and coarse fragmented ESPs can be viewed respectively as manifestations of consecutive and terminal synchronization failures during the development of epileptic excitatory postsynaptic potentials. Alternately, stimulation could drive the topographical tightening of connections that accelerate seizure termination.35
Electrical stimulation over long periods of time may progressively disrupt the connectivity of the epileptogenic network and reduce the core synchronized population, rendering the clinical manifestation of seizures less severe or subclinical.36,37 Such an effect would correspond to responsiveness to RNS16,18,19 and is supported by the positive association of indirect modulation effects with improved postimplantation outcomes in this cohort. Despite previous reports of effects of acute and subacute chronic stimulation on both ECOG content and seizure control10,11,12,13,14 with background normalization over time and improved seizure control,4,9,15 our results suggest that both direct seizure inhibition and frequency modulation may have no appreciable association with outcome. The cohort size, however, did not allow us to study whether there may be either synergistic or antagonistic interactions between the 2 classes of modulation.
Limitations
Patient noncompliance with routine data uploads, combined with limited onboard data storage, resulted in a small subset of ECOGs being preserved relative to the continuous neural signal analyzed online by the device. We found recently that events reported by the device are biased and incomplete, but these limitations can be partially overcome by manual review and by extrapolating the results, which correspond to patient-reported seizure frequency.26 Evaluation of direct inhibitory modulation on clinical rather than electrographic events, however, remains limited without the confirmed presence of clinical seizures during modulated electrographic events. We acknowledge that changes in antiepileptic medication and other important variables cannot be controlled for in a study with this sample size, which includes patients with different types of epilepsy.
Conclusions
These results are the first, to our knowledge, to demonstrate electrophysiological signatures of therapeutic responses to closed-loop brain stimulation for epilepsy. The fact that indirect modulation effects were associated with improved seizure control rather than the effects of direct stimulation being associated with triggered seizures indicates that neuroplasticity may be required for a therapeutic response and constitutes a paradigm shift in thinking about neuromodulation for epilepsy. It may be possible to improve the therapeutic speed and efficacy of closed-loop brain stimulation by identifying the specific stimulation scenarios that produce these modulatory effects.
eFigure. Electrographic Seizure Patterns Relative to the Onset of the First RNS Stimulation Pulse
eTable 1. Patients, Closed-Loop Stimulation Effects, and Outcomes
eTable 2. Temporal Evolution of Direct and Indirect Modulation Effects
References
- 1.Penfield W, Jasper H. Electrocorticography In: Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little Brown; 1954. [Google Scholar]
- 2.Kossoff EH, Ritzl EK, Politsky JM, et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45(12):1560-1567. doi: 10.1111/j.0013-9580.2004.26104.x [DOI] [PubMed] [Google Scholar]
- 3.Stacey WC, Litt B. Technology insight: neuroengineering and epilepsy-designing devices for seizure control. Nat Clin Pract Neurol. 2008;4(4):190-201. doi: 10.1038/ncpneuro0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velasco M, Velasco F, Velasco AL, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. 2000;41(2):158-169. doi: 10.1111/j.1528-1157.2000.tb00135.x [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto J, Ikeda A, Satow T, et al. Low-frequency electric cortical stimulation has an inhibitory effect on epileptic focus in mesial temporal lobe epilepsy. Epilepsia. 2002;43(5):491-495. doi: 10.1046/j.1528-1157.2002.29001.x [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita M, Ikeda A, Matsumoto R, et al. Electric stimulation on human cortex suppresses fast cortical activity and epileptic spikes. Epilepsia. 2004;45(7):787-791. doi: 10.1111/j.0013-9580.2004.60203.x [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita M, Ikeda A, Matsuhashi M, et al. Electric cortical stimulation suppresses epileptic and background activities in neocortical epilepsy and mesial temporal lobe epilepsy. Clin Neurophysiol. 2005;116(6):1291-1299. doi: 10.1016/j.clinph.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto J, Ikeda A, Kinoshita M, et al. Low-frequency electric cortical stimulation decreases interictal and ictal activity in human epilepsy. Seizure. 2006;15(7):520-527. doi: 10.1016/j.seizure.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Elisevich K, Jenrow K, Schuh L, Smith B. Long-term electrical stimulation-induced inhibition of partial epilepsy: case report. J Neurosurg. 2006;105(6):894-897. doi: 10.3171/jns.2006.105.6.894 [DOI] [PubMed] [Google Scholar]
- 10.Velasco AL, Velasco F, Velasco M, María Núñez J, Trejo D, García I. Neuromodulation of epileptic foci in patients with non-lesional refractory motor epilepsy. Int J Neural Syst. 2009;19(3):139-147. doi: 10.1142/S0129065709001914 [DOI] [PubMed] [Google Scholar]
- 11.Child ND, Stead M, Wirrell EC, et al. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia. 2014;55(3):e18-e21. doi: 10.1111/epi.12525 [DOI] [PubMed] [Google Scholar]
- 12.Lundstrom BN, Van Gompel J, Britton J, et al. Chronic subthreshold cortical stimulation to treat focal epilepsy. JAMA Neurol. 2016;73(11):1370-1372. doi: 10.1001/jamaneurol.2016.2857 [DOI] [PubMed] [Google Scholar]
- 13.Lundstrom BN, Worrell GA, Stead M, Van Gompel JJ. Chronic subthreshold cortical stimulation: a therapeutic and potentially restorative therapy for focal epilepsy. Expert Rev Neurother. 2017;17(7):661-666. doi: 10.1080/14737175.2017.1331129 [DOI] [PubMed] [Google Scholar]
- 14.Valentin A, Ughratdar I, Venkatachalam G, et al. Sustained seizure control in a child with drug resistant epilepsy after subacute cortical electrical stimulation (SCES). Brain Stimul. 2016;9(2):307-309. doi: 10.1016/j.brs.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Kerezoudis P, Grewal SS, Stead M, et al. Chronic subthreshold cortical stimulation for adult drug-resistant focal epilepsy: safety, feasibility, and technique. J Neurosurg. 2018;129(2):533-543. doi: 10.3171/2017.5.JNS163134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System pivotal trial. Epilepsia. 2014;55(3):432-441. doi: 10.1111/epi.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergey GK, Morrell MJ, Mizrahi EM, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810-817. doi: 10.1212/WNL.0000000000001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994-1004. doi: 10.1111/epi.13740 [DOI] [PubMed] [Google Scholar]
- 19.Jobst BC, Kapur R, Barkley GL, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58(6):1005-1014. doi: 10.1111/epi.13739 [DOI] [PubMed] [Google Scholar]
- 20.Lesser RP, Kim SH, Beyderman L, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999;53(9):2073-2081. doi: 10.1212/WNL.53.9.2073 [DOI] [PubMed] [Google Scholar]
- 21.Skarpaas TL, Morrell MJ. Intracranial stimulation therapy for epilepsy. Neurotherapeutics. 2009;6(2):238-243. doi: 10.1016/j.nurt.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrell MJ, Halpern C. Responsive direct brain stimulation for epilepsy. Neurosurg Clin N Am. 2016;27(1):111-121. doi: 10.1016/j.nec.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 23.Thomas GP, Jobst BC. Critical review of the responsive neurostimulator system for epilepsy. Med Devices (Auckl). 2015;8:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. doi: 10.1111/j.1528-1167.2010.02522.x [DOI] [PubMed] [Google Scholar]
- 25.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):522-530. doi: 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 26.Sisterson ND, Wosny TA, Kokkinos V, Constantino A, Richardson RM. Closed-loop brain stimulation for drug-resistant epilepsy: towards an evidence-based approach to personalized medicine. Neurotherapeutics. 2019;16(1):119-127. doi: 10.1007/s13311-018-00682-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowell M, Rodionov R, Zombori G, et al. Utility of 3D multimodality imaging in the implantation of intracranial electrodes in epilepsy. Epilepsia. 2015;56(3):403-413. doi: 10.1111/epi.12924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher RS, Nune G, Roberts SE, Cramer JA. The Personal Impact of Epilepsy Scale (PIES). Epilepsy Behav. 2015;42:140-146. doi: 10.1016/j.yebeh.2014.09.060 [DOI] [PubMed] [Google Scholar]
- 29.Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures In: Engel J, Jr, ed. Surgical Treatment of the Epilepsies. New York, NY: Raven Press; 1993:609-621. [Google Scholar]
- 30.Jefferys JGR. Influence of electric fields on the excitability of granule cells in guinea-pig hippocampal slices. J Physiol. 1981;319:143-152. doi: 10.1113/jphysiol.1981.sp013897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durand D. Electrical stimulation can inhibit synchronized neuronal activity. Brain Res. 1986;382(1):139-144. doi: 10.1016/0006-8993(86)90121-6 [DOI] [PubMed] [Google Scholar]
- 32.Osorio I, Frei MG, Sunderam S, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57(2):258-268. doi: 10.1002/ana.20377 [DOI] [PubMed] [Google Scholar]
- 33.Bragin A, Wilson CL, Engel J Jr. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41(suppl 6):S144-S152. doi: 10.1111/j.1528-1157.2000.tb01573.x [DOI] [PubMed] [Google Scholar]
- 34.Schevon CA, Ng SK, Cappell J, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25(6):321-330. doi: 10.1097/WNP.0b013e31818e8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khambhati AN, Davis KA, Oommen BS, et al. Dynamic network drivers of seizure generation, propagation and termination in human neocortical epilepsy. PLoS Comput Biol. 2015;11(12):e1004608. doi: 10.1371/journal.pcbi.1004608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zangaladze A, Nei M, Liporace JD, Sperling MR. Characteristics and clinical significance of subclinical seizures. Epilepsia. 2008;49(12):2016-2021. doi: 10.1111/j.1528-1167.2008.01672.x [DOI] [PubMed] [Google Scholar]
- 37.Singh S, Sandy S, Wiebe S. Ictal onset on intracranial EEG: do we know it when we see it? state of the evidence. Epilepsia. 2015;56(10):1629-1638. doi: 10.1111/epi.13120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Electrographic Seizure Patterns Relative to the Onset of the First RNS Stimulation Pulse
eTable 1. Patients, Closed-Loop Stimulation Effects, and Outcomes
eTable 2. Temporal Evolution of Direct and Indirect Modulation Effects