This randomized noninferiority clinical trial compares the effectiveness of guided internet-based cognitive behavioral therapy vs face-to-face clinical care among patients receiving medical treatment for tinnitus.
Key Points
Question
Is undertaking an internet-based cognitive behavioral therapy program as effective as undergoing individualized face-to-face clinical care in reducing distress from tinnitus?
Findings
In this randomized, multicenter, noninferiority clinical trial of 92 adults, internet-based cognitive behavioral therapy for tinnitus led to outcomes similar to those of individualized face-to-face clinical care for tinnitus.
Meaning
Internet-based cognitive behavioral therapy has shown potential as an evidence-based intervention that could increase access to managing tinnitus care.
Abstract
Importance
Accessible clinical care is not always available to individuals with distressing tinnitus. Internet-based cognitive behavioral therapy has the potential to increase access to evidence-based services that manage tinnitus. Research comparing the effectiveness of this internet-based intervention with face-to-face care is required.
Objective
To evaluate whether an internet-based cognitive behavioral therapy intervention is at least as effective as established individualized face-to-face clinical care in reducing tinnitus distress and tinnitus-related difficulties.
Design, Setting, and Participants
A randomized, multicenter, 2-arm parallel group, noninferiority trial with 2-month follow-up was performed between October 4, 2016, and July 14, 2017. Invited to participate were 374 adults based in the United Kingdom who had been referred to their local tinnitus clinics because of bothersome tinnitus. The experimental group received the internet-based intervention online, and the active control group underwent the usual face-to-face tinnitus care at 1 of 3 UK-based National Health Service hospitals. Participants were randomly assigned (1:1) to either intervention using variable permuted block sizes of 4 and 6. Of 92 participants who were randomized (46 each in the experimental and control groups), 88 participants completed the assessment immediately after intervention and 74 participants completed the follow-up assessment.
Interventions
Participants were randomized to receive either 8 weeks of guided internet-based cognitive behavioral therapy or a mean of 2 to 3 individualized face-to-face appointments in a tinnitus clinic.
Main Outcomes and Measures
The primary outcome was a change in tinnitus distress (assessed by the Tinnitus Functional Index). Secondary assessment measures were included for insomnia, anxiety, depression, hearing disability, hyperacusis, cognitive failures, and satisfaction with life.
Results
Of 92 patients overall, 55 (60%) were men with a mean (SD) age of 52.96 (12.07) years and mean (SD) tinnitus duration of 6.54 (9.25) years. The between-group difference in the Tinnitus Functional Index scores after intervention were 5.18 (95% CI, –4.17 to 14.53) at the initial assessment and 5.52 (95% CI, –4.60 to 15.61) at follow-up; both differences were within the noninferiority margin of 13 points for the lower 95% CI. For the secondary outcomes, only outcomes for insomnia fell outside the noninferiority margin, both after intervention and at follow-up, favoring internet-based cognitive behavioral therapy.
Conclusions and Relevance
This is the first trial, to our knowledge, to compare an internet-based intervention with standard individualized face-to-face care for tinnitus. It revealed that both interventions are equally effective for reducing tinnitus distress and most tinnitus-related difficulties.
Trial Registration
ClinicalTrials.gov identifier: NCT02665975
Introduction
Tinnitus, described as the conscious perception of unwanted sounds in the absence of a corresponding external acoustic stimulus,1 is a prevalent complaint and one of the most distressing audiologic symptoms.2 Because no cure has been identified, managing tinnitus remains challenging and costly. The estimated health care cost of tinnitus is $660 per patient per year in the United States,3 with an annual health care cost of £750 million (US $965 million) and resulting societal cost of £2.7 billion (US $3.5 billion) per year in the United Kingdom.4 Tinnitus clinics are not always readily accessible because of service and geographic constraints.5,6 Moreover, although various tinnitus management approaches exist, evidence for their efficacy is scarce.7 To date, cognitive behavioral therapy (CBT), has the most evidence of efficacy in reducing tinnitus distress.8 Despite positive outcomes, there is limited accessibility to CBT for tinnitus, largely because of a shortage of suitably trained clinicians.5 To improve access to evidence-based tinnitus care, an internet-based cognitive behavioral therapy intervention (iCBT) for tinnitus was pioneered in Sweden.9 An iCBT intervention aimed at a UK population was adapted10 from previous versions of the Swedish program. Both feasibility11 and efficacy12 of the UK version of iCBT have been indicated. It is, however, not known how outcomes for tinnitus using iCBT compare with those of the individualized face-to-face (F2F) care that is typically provided in the United Kingdom. Previous study comparisons with iCBT used group-based CBT (GCBT) as the active control group.13,14,15
The primary aim of this study was to evaluate whether iCBT for managing tinnitus is at least as effective as established F2F care in reducing tinnitus severity. The secondary objective was to compare the effects of these interventions for tinnitus-related difficulties, such as insomnia, depression, and anxiety. An additional objective was to assess the stability of the results 2 months after undertaking the intervention. The study hypothesis was that iCBT is not inferior to F2F care for managing tinnitus.
Methods
Trial Design and Participants
A randomized, multicenter, 2-arm parallel group, noninferiority trial with a sequential adaptive design and 2-month follow-up was performed between October 4, 2016, and July 14, 2017, to compare the clinical effectiveness of iCBT with the usual F2F tinnitus care. The recruitment and treatment sites for the control group were 3 hospitals in eastern England: Norfolk and Norwich University Hospitals National Health Service Foundation Trust (Norwich), Milton Keynes University Hospital National Health Service Foundation Trust (Milton Keynes), and Hinchingbrooke Health Care National Health Service Trust (Huntingdon). Eligibility criteria included age of 18 years or older, regular computer and internet access, no report of any major medical or psychiatric disorder, and not undergoing any tinnitus treatment. Participants were examined clinically (hearing test, ear examination, and case history of symptoms) and had been referred to the local tinnitus clinic by an audiologist and/or an ear, nose, and throat specialist. Because this was an effectiveness trial, the study was not advertised. Nurses and ear, nose, and throat specialists shared details of the study with patients who met the inclusion criteria. Ethical approval was granted by the East of England–Cambridge South Research Ethics Committee and Health Research Authority. Individuals who wanted to participate provided informed consent online. The study protocol is detailed in the Supplement; no changes were made to the protocol after the trial commenced.
Interventions
The guided iCBT and F2F intervention groups received information about managing tinnitus from an audiology professional. Participants were provided with hearing aids or combination devices regardless of group allocation.
Guided iCBT Intervention
The iCBT intervention content was based on a CBT self-help program originally developed in the Swedish language9 and adapted into an 8-week, interactive e-learning version consisting of 16 recommended modules and 5 optional modules for a UK population.10,16 To monitor progress and provide feedback on completed worksheets, a minimum of 10 minutes of asynchronous audiologist guidance using an encrypted 2-way messaging system was provided.
F2F Intervention
The F2F group received tinnitus information counseling which was generally used for the management of tinnitus in the United Kingdom. The initial appointment (60 minutes) was used to provide explanations about tinnitus and some basic management strategies. Patients received additional strategies for tinnitus management, including sleep hygiene, relaxation strategies, and negative thought analysis, during follow-up appointments.
Randomization and Masking
Participants were randomly assigned (1:1) by an independent researcher to either treatment arm using a randomization sequence generated by computer algorithm and variable randomly permuted block sizes of 4 and 6. To prevent a delay in providing the interventions, an adaptive design was used to sequentially allocate groups of participants as they were recruited. Participants were stratified using the Tinnitus Functional Index (TFI) for tinnitus severity (score, ≤50, indicating lower severity, or >50, indicating greater severity).17 A masked design in this context was not feasible. Allocation to the treatment arms was known to the participants and the clinicians. To minimize bias, the data analysis was masked in terms of group allocation.
Outcomes
Data were collected online at baseline (T0), immediately after undertaking the (T1), and at 2-month follow-up (T2). A demographic questionnaire was used to establish health-related and tinnitus-specific information.
Primary Assessment Measure
The primary outcome was a change in tinnitus distress between the groups. The TFI17 was selected to measure tinnitus distress because of its validation for assessing intervention responsiveness. In addition, the Tinnitus Handicap Index18 was administered for comparison because this is the most common tinnitus assessment measure used within clinics globally.19 Both questionnaires consist of 25 items, scored on a scale of 0 to 100, with the lower scores indicating less distress.
Secondary Assessment Measures
The following secondary measures were incorporated to assess commonly reported tinnitus-related difficulties: (1) the Insomnia Severity Index20 assessed the presence of insomnia; (2) the Generalized Anxiety Disorder–721 assessed symptoms of generalized anxiety disorder; (3) the Patient Health Questionnaire–922 indicated symptoms of depression; (4) the Hearing Handicap Inventory for Adults–Screening version23 assessed difficulty in hearing; (5) the Hyperacusis Questionnaire24assessed the presence of reduced tolerance to everyday sounds; (6) the Cognitive Failures Questionnaire25 assessed cognitive functions; and (7) the Satisfaction with Life Scales26 assessed life satisfaction. In addition, participants were monitored weekly using the Tinnitus Handicap Inventory–Screening version.27
Statistical Analysis
The CONSORT guidelines for noninferiority randomized clinical trials were followed.28 Statistical analyses were performed using SPSS, version 23.0 (SPSS Inc).
Sample Size
Sample size calculations were performed using the SampSize app29 for noninferiority parallel groups. Power was 90%; α was 0.025; and the estimated SD was 17 points, as indicated by the preceding efficacy trial.12 The noninferiority margin was set to 13 points, as indicated during the validation of the TFI17 to be a clinically significant change in scores. Thus, 39 participants were required for each arm. Each intervention arm was assigned 46 participants to allow for possible dropouts, which were estimated to be between 10% to 20% by the previous effectiveness trials.30,31
Group Comparisons
Both intention-to-treat and per-protocol results were analyzed. Participants were categorized as per protocol if they completed the assessment measures after intervention at the time under investigation (T1 or T2). Because there were no differences in the results, the per-protocol results were reported, in accordance with current guidelines for noninferiority trials.28
Compared with F2F care for tinnitus distress, noninferiority of iCBT was established if the lower limit of the 2-sided, 95% CI for the mean TFI difference between these 2 interventions was smaller than the noninferiority margin of 13 points. For the secondary assessment measures, noninferiority was established if a marginal between-group effect size of Cohen d < 0.20 was found.
Mixed 2 × 3 analyses of variance were carried out for repeated measures with the between-subject factor of group (iCBT and F2F) and within-subject factor of time (T0, T1, and T2) for each assessment measure. A Greenhouse-Geisser correction for nonsphericity was applied. For significant group by time interactions, the main effects were followed up by Bonferroni-corrected post hoc testing. Effect sizes after intervention and follow-up were calculated, with Cohen d = 0.20-0.49 representing small effect sizes; Cohen d = 0.5-0.79, medium effect sizes; and Cohen d 0.80, large effect sizes.32 A subanalysis was performed by comparing effect sizes in each group with and without amplification to determine the effect of amplification.
The Reliable Change Index33 was used to determine clinical significance. The criteria were calculated to be a 21-point difference score, using the means (SDs) at baseline, means after intervention, and the test-retest reliability coefficient of 0.8 for the TFI.17
Monitoring Intervention Effects Between T0 and T1
A mixed 2 × 8 analysis of variance for repeated measures was used to compare the weekly Tinnitus Handicap Inventory–Screening scores with the within-subject factor of time (weeks 1–8) and between-subject factor of each group (iCBT and F2F). The main effects were followed up by Bonferroni-corrected post hoc testing.
Results
Participant Characteristics and Attrition
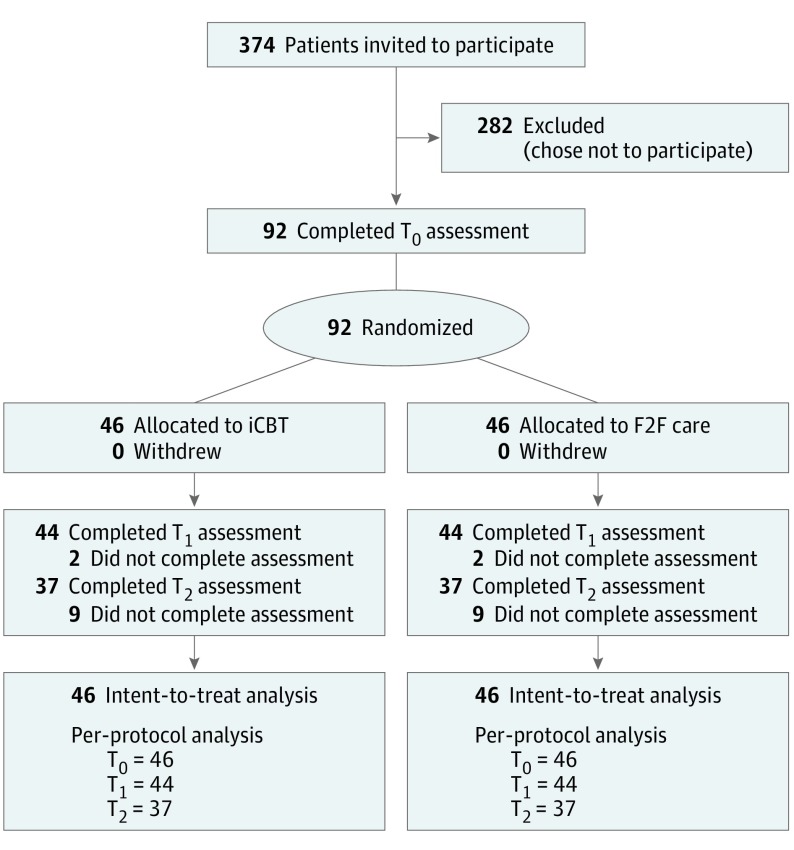
Among 374 adults who had been referred to their local tinnitus clinics because of bothersome tinnitus, the baseline assessment measures were completed by 92 participants who met the eligibility criteria (Figure 1). Of the 92 patients, 55 (60%) were men with a mean (SD) age of 52.96 (12.07) years and mean (SD) tinnitus duration of 6.54 (9.25) years. Hearing aids were fitted before or during the trial to 38 of 92 participants (41%), with 19 participants from each group. The groups were well matched; there were no clinically meaningful imbalances between the groups at baseline (Table 1 and Table 2). No participants withdrew participation during the study, and no adverse events were reported. Assessment measures were completed by 88 of 92 participants (96%) at T1, and by 74 of 92 participants (80%) at T2, with no group differences.
Figure 1. CONSORT Study Profile.
F2F indicates face-to-face intervention; iCBT, internet-based cognitive behavioral therapy; T0, time before intervention; T1, time after intervention; and T2, time at 2-month follow-up.
Table 1. Participant Characteristics.
| Characteristic | Intervention | Overall (N = 92) | |
|---|---|---|---|
| iCBT (n = 46) | F2F (n = 46) | ||
| Sex, No. (%) | |||
| Male | 29 (63) | 26 (57) | 55 (60) |
| Female | 17 (37) | 20 (43) | 37 (40) |
| Age, mean (SD) [range], y | 50.65 (12.19) [26-79] | 55.26 (11.62) [29-76] | 52.96 (12.07) [26-79] |
| Tinnitus duration, mean (SD) [range], y | 5.23 (9.01) [0.4-40] | 7.85 (9.62) [0.4-50] | 6.54 (9.25) [0.4-50] |
| Hearing aids, No. (%) | |||
| Not using | 27 (59) | 27 (59) | 54 (59) |
| Using | 19 (41) | 19 (41) | 38 (41) |
Abbreviations: F2F, face-to-face; iCBT, internet-based cognitive behavioral therapy.
Table 2. Group Comparisons Before and After Intervention and at 2-Month Follow-Up.
| Measure, Group | Intervention, Mean (SD) | Group Comparison, P Valuea | Between-Group Effect Sizesb | Within-Group Effect Sizes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before, T0 | After, T1 | 2-mo Follow-up, T2 | Time by Group Interaction | Within-Group Time Effect | Between-Group Effect | T1 Cohen d (95% CI) | T2 Cohen d (95% CI) | T0-T1 Cohen d (95% CI) | T0-T2 Cohen d (95% CI) | |
| TFI | ||||||||||
| iCBT | 55.01 (21.58) | 27.88 (20.84) | 22.85 (19.26) | .36 | <.001a | .072 | 0.30 (–0.12 to 0.72) | 0.45 (–0.01 to 0.91) | 1.28 (0.81 to 1.72) | 1.56 (1.06 to 2.04) |
| F2F | 56.57 (20.61) | 34.88 (24.91) | 32.51 (23.28) | 0.95 (0.51 to 1.38) | 1.10 (0.63 to 1.56) | |||||
| THI | ||||||||||
| iCBT | 44.57 (23.40) | 22.33 (19.63) | 17.78 (14.77) | .38 | <.001a | .022a | 0.32 (–0.11 to 0.73) | 0.33 (–0.13 to 0.79) | 1.08 (0.63 to 1.51) | 1.28 (0.80 to 1.74) |
| F2F | 47.13 (20.31) | 28.74 (20.07) | 27.11 (21.62) | 0.96 (0.55 to 1.38) | 1.05 (0.58 to 1.50) | |||||
| ISI | ||||||||||
| iCBT | 11.43 (6.36) | 6.71 (6.20) | 5.69 (4.64) | .33 | <.001a | .002a | 0.46 (0.03 to 0.88) | 0.74 (0.26 to 1.20) | 0.75 (0.32 to 1.17) | 1.01 (0.55 to 1.46) |
| F2F | 13.65 (6.62) | 9.55 (6.15) | 10.03 (6.88) | 0.65 (0.21 to 1.06) | 0.54 (0.09 to 0.97) | |||||
| GAD-7 | ||||||||||
| iCBT | 6.43 (5.64) | 3.45 (3.66) | 3.33 (3.21) | .56 | <.001a | .67 | –0.06 (–0.36 to 0.48) | –0.03 (–0.49 to 0.42) | 0.62 (0.20 to 1.04) | 0.66 (0.21 to 1.09) |
| F2F | 6.78 (5.54) | 3.33 (3.78) | 3.42 (3.60) | 0.72 (0.29 to 1.14) | 0.70 (0.25 to 1.14) | |||||
| PHQ-9 | ||||||||||
| iCBT | 6.50 (5.48) | 3.67 (3.64) | 2.78 (3.02) | .55 | <.001a | .042 | –0.03 (–0.42 to 0.49) | 0.57 (0.10 to 1.03) | 0.61 (0.18 to 1.02) | 0.82 (0.36 to 1.26) |
| F2F | 7.98 (6.05) | 4.19 (4.08) | 4.97 (4.54) | 0.73 (0.30 to 1.15) | 0.55 (0.11 to 0.99) | |||||
| HHIA-S | ||||||||||
| iCBT | 11.74 (10.66) | 10.10 (10.82) | 9.11 (11.59) | .98 | .006a | .23 | –0.19 (–0.23 to 0.61) | 0.27 (–0.19 to 0.73) | 0.15 (−0.26 to 0.57) | 0.24 (−0.20 to 0.67) |
| F2F | 14.30 (11.58) | 12.14 (10.71) | 12.00 (9.56) | 0.19 (−0.22 to 0.61) | 0.21 (−0.22 to 0.65) | |||||
| HQ | ||||||||||
| iCBT | 15.65 (9.06) | 12.24 (7.61) | 12.51 (9.01) | .94 | <.001a | .68 | 0.16 (–0.26 to 0.57) | –0.05 (–0.40 to 0.51) | 0.41 (−0.01 to 0.82) | 0.35 (−0.09 to 0.78) |
| F2F | 16.54 (7.42) | 13.40 (7.29) | 12.94 (7.51) | 0.43 (0.01 to 0.84) | 0.48 (0.04 to 0.92) | |||||
| CFQ | ||||||||||
| iCBT | 34.93 (14.38) | 30.83 (12.14) | 30.06 (12.89) | .91 | .009a | .32 | 0.29 (–0.23 to 0.61) | –0.18 (–0.28 to 0.64) | 0.31 (−0.11 to 0.72) | 0.35 (−0.08 to 0.79) |
| F2F | 39.65 (19.31) | 35.56 (19.23) | 33.06 (19.24) | 0.21 (−0.20 to 0.62) | 0.34 (−0.10 to 0.77) | |||||
| SWLS | ||||||||||
| iCBT | 18.70 (5.73) | 20.10 (4.96) | 21.00 (5.05) | .44 | .04a | .61 | 0.01 (–0.41 to 0.43) | 0.10 (–0.36 to 0.56) | 0.26 (−0.16 to 0.67) | 0.43 (0.00 to 0.84) |
| F2F | 19.48 (5.54) | 20.05 (5.61) | 20.50 (4.95) | 0.10 (−0.31 to 0.51) | 0.19 (−0.24 to 0.62) | |||||
Abbreviations: CFQ, Cognitive Failures Questionnaire; F2F, face-to-face intervention; GAD-7, Generalized Anxiety Disorder–7; HHIA-S, Hearing Handicap Inventory for Adults–Screening version; HQ, Hyperacusis Questionnaire; iCBT, internet-based cognitive behavioral therapy intervention; ISI, Insomnia Severity Index; PHQ-9, Patient Health Questionnaire–9; SWLS, Satisfaction With Life Scales; TFI, Tinnitus Functional Index; THI, Tinnitus Handicap Index.
Indicates statistical significance at P < .05.
For the difference in scores between baseline and T1 or T2.
Effectiveness of iCBT vs F2F for Tinnitus Distress
The within-group effect sizes of the iCBT and F2F groups for both tinnitus assessment measures (TFI and Tinnitus Handicap Index) were large at T1 and T2 (Table 2).
For the iCBT group, the mean (SD) TFI scores at T1 were 27.13 (21.21) points lower than baseline. The mean (SD) TFI scores at T2 were 32.16 (20.45) points lower than baseline.
For the F2F group, the mean (SD) TFI scores at T1 were 21.69 (22.86) points lower and, at T2, were 24.06 (21.98) points lower compared with baseline.
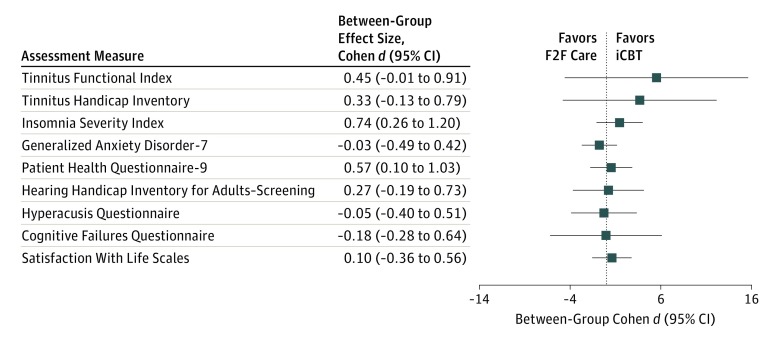
The magnitude of the between-group difference was 5.18 points (95% CI, –4.17 to 14.53) at T1 and 5.52 points at T2 (95% CI, –4.60 to 15.61), favoring the iCBT group. The between-group difference (T0–T1 and T0–T2) in TFI scores fell within the noninferiority margin of 13 points for the lower 95% CI of both per-protocol and intention-to-treat analyses. Similar results were obtained for the Tinnitus Handicap Index (Figure 2).
Figure 2. Mean Between-Group Difference in Scores Between Baseline and Follow-up for Each Assessment Measure.
F2F indicates face-to-face intervention; iCBT, internet-based cognitive behavioral therapy intervention.
There were no significant differences in the range of difference scores before and after intervention between the 2 groups (F1,9 = 0.008, P = .93). A clinically significant improvement was achieved by 25 of 44 participants (57%) in the iCBT group and 18 of 44 (41%) in the F2F group at T1 and by 20 of 37 (54%) in the iCBT group and 17 of 37 (46%) in the F2F group at T2. At T1, 23 of 44 (52%) from the iCBT group and 15 of 44 (34%) from the F2F group had a clinically significant improvement and TFI scores below the level of requiring intervention (score <25). There were no significant differences in tinnitus distress after intervention when comparing only those using or not using hearing aids (F2,57 = 1.20, P = .23).
Monitoring Intervention Effects Between T0 and T1
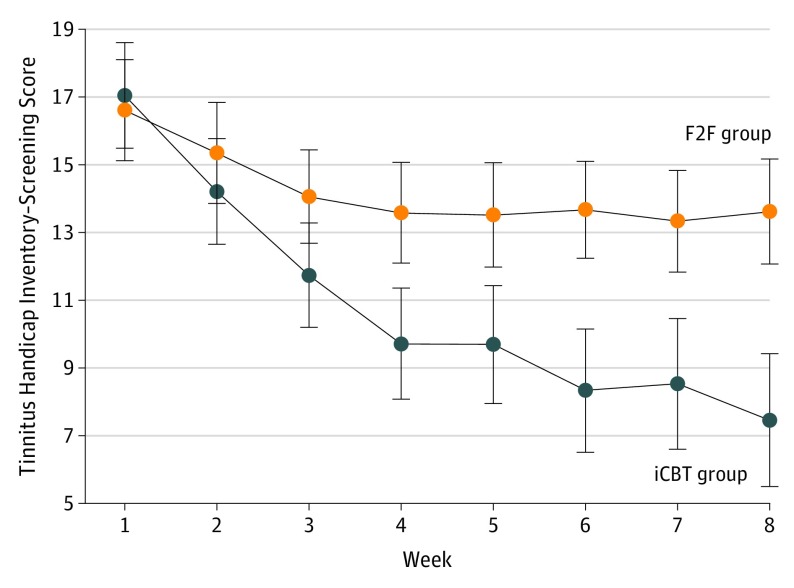
The iCBT group had greater weekly reductions in tinnitus distress (Figure 3), as evidenced by the significant between-group effects (time by group interaction: F7,524 = 2.80, P = .04) and effect size (Cohen d = 0.57). Follow-up analysis indicated that tinnitus distress was significantly lower in the iCBT group from weeks 4 to 8, compared with the F2F group.
Figure 3. Weekly Tinnitus Handicap Inventory–Screening Scores for Each Group Across the First 8-Week Intervention Period Before and After Intervention.
Error bars represent the SE of the mean. F2F indicates face-to-face intervention; iCBT indicates internet cognitive behavioral therapy.
Effectiveness of iCBT vs F2F for Tinnitus-Related Difficulties
The Cohen d within-group effect sizes (Table 2) for the Insomnia Severity Index were medium to large for both groups. They were medium for the General Anxiety Disorder–7 and Patient Health Questionnaire–9 (except at T2 for the iCBT group, where a large difference occurred). They were small for the other assessment measures. The T1 between-group effect sizes for the secondary assessment measures were within the noninferiority margin (Cohen d 0.20) for anxiety, depression, hearing disability, hyperacusis, and life satisfaction (Table 2). They were outside this margin, favoring the iCBT group for insomnia and cognitive failures. At T2 between-group effect sizes were outside this margin for insomnia, hearing handicap, and depression, again favoring the iCBT group.
Treatment Adherence and Clinician Resources
Participants in the F2F group received a mean (SD) of 2.28 (1.10) appointments (mean treatment duration of 137 minutes) with a maximum of 5 appointments. Seven individuals did not attend their appointment. Those in the iCBT group read a mean (SD) of 13 (8) modules of the 21 modules, and 17 of 46 participants (37%) completed all the modules. Users sent a mean (SD) of 7 (10) messages, and the audiologist sent a mean (SD) of 20 (11) messages per iCBT participant (corresponding to 64 minutes contact time per participant during the intervention period). When time spent was divided by the mean TFI score change (iCBT, 64/27.13 = 2.36; F2F, 137/21.69 = 6.32), iCBT was 2.68 times as time-effective as F2F when only taking the audiologist’s time into account.
Discussion
Effectiveness of iCBT vs F2F Care for Tinnitus
To our knowledge, this is the first randomized clinical trial to compare the effectiveness of iCBT for tinnitus with that of standard F2F clinical care in a clinical population. The results indicate that the interventions are equally effective and within the boundaries of noninferiority for reducing tinnitus distress. The present trial is unique because it compared iCBT with individualized F2F clinical care instead of GCBT, which was used in previous efficacy studies. Those previous studies found no significant group differences between iCBT and GCBT.13,15
During the monitoring of groups weekly for the first 8 weeks of the active treatment phase, tinnitus distress in the iCBT group was rated significantly lower than that of the F2F group from week 4 onward. This was possibly because of the differences in the intensive weekly input for the iCBT group compared with longer follow-up periods for the F2F group.
There were 2 previous nonrandomized iCBT studies for tinnitus effectiveness at the Uppsala Clinic in Sweden. The within-group effect sizes were smaller than those in the present study (Cohen d = 0.5630 and Cohen d = 0.58).31 In the present study, a clinically significant improvement was achieved by 57% at T1 and 54% at T2 for the iCBT group compared with 41% at T1 and 46% at T2 for the F2F group. This is higher than the 27%13 and 38%31 reaching clinical significance in some previous studies. Differences in the ways of calculating clinical significance (50% reduction in scores vs using Reliable Change Index criteria) may have contributed to these discrepancies.
Secondary intervention effects for both groups were largest for insomnia, followed by anxiety and depression. The combined results after intervention and 2-month follow-up indicated that the interventions are equally effective within the boundaries of noninferiority for tinnitus-related difficulties except for insomnia, which favored the iCBT group. In a preceding efficacy study by Beukes et al,12 intervention effects were also greatest for insomnia. This result is of interest because previous meta-analyses8,34 and a Cochrane review,35 which were largely based on F2F interventions, failed to show the effectiveness of CBT for sleep problems in a population of patients with tinnitus. In the previous iCBT nonrandomized effectiveness trials,30,31 significant before and after intervention within-group differences for insomnia, anxiety, and depression were found. Further work is required to identify how interventions for tinnitus can improve the results for tinnitus-related problems.
Both groups indicated stability of results at 2-month follow-up for tinnitus distress and the secondary assessment measures. Stability of results have been reported for longer follow-up periods of 6 months15 and 1 year,13 when comparing iCBT with GCBT for tinnitus distress. Further studies with longer follow-up periods are required to establish the long-term effects of these interventions.
Intervention Adherence and Clinician Resources
Completion rates of assessment measures (96% at T1 and 80% at T2) were equal in both groups regardless of allocation. No demographic or clinical differences were identified between participants who completed assessment measures and those who did not complete these measures in the present study. This finding differed from that of Kaldo et al,31 who found that younger participants were more likely to drop out of the study. Studies with larger sample sizes are required to further investigate these effects.
When assessing the resources required, iCBT was 2.68 times more time-effective than individualized F2F care when taking only clinician time into account (assuming equality of grading by the audiology professionals involved). Kaldo et al13 reported that, compared with iCBT, the therapist time was twice as long for the GCBT sessions. These sessions included 7 participants per group attending 120-minute group sessions. Therefore, in terms of staff time, iCBT was 1.7 times more time-effective compared with GCBT. In contrast, Jasper and colleagues15 found no difference in therapist time because more participants (10 participants) were included in each GCBT group, with shorter 90-minute sessions, whereas there was more therapist time for the iCBT group, with a mean of 14 minutes per week.
The present study focused on clinical effectiveness. More work is required to determine cost-effectiveness because this information is required by stakeholders.4,36 A lexicon of assessment and outcome measures for telemental health has been developed as a resource for the evaluation of these services.37 Evaluation metrics include treatment utilization, travel costs, stigma, anxiety, waiting times, training, and motivational readiness. Future research can use these domains to standardize approaches, to determine cost-effectiveness, and to provide a more comprehensive comparison of services.
Limitations
This trial had many challenges, such as difficulty recruiting a sufficient number of participants. After possibly following a long pathway before being able to obtain audiology and ear, nose, and throat services, some patients may have wanted to continue this pathway and not participate in a research study. Implementing more effective recruitment strategies will be required for future effectiveness trials. The low ratio of people participating in the study in comparison with those who were invited was a potential source of bias. In addition, the nonuniform nature of the clinical care received from the various study centers may have contributed to the variability. Interpretation of the data are limited to participants with similar demographic and clinical profiles, and further generalizability of the results to other populations is not possible without further systematic replication in other settings. Moreover, some of the outcome measures selected may not have been optimal for a population with tinnitus. Although the General Anxiety Disorder–7 can identify generalized anxiety disorder, other anxiety symptoms more specific to a population with tinnitus may be missed.
Conclusions
This study revealed that iCBT and F2F interventions are equally effective for reducing tinnitus distress and most tinnitus-related difficulties. Although further work is required to differentiate which patients are best suited for iCBT or F2F interventions and whether including low-intensity interventions would be cost-effective and clinically effective, this study adds to the evidence of effectiveness of iCBT for management of tinnitus.
Trial Protocol
References
- 1.Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600-1607. doi: 10.1016/S0140-6736(13)60142-7 [DOI] [PubMed] [Google Scholar]
- 2.Cima RF, Vlaeyen JW, Maes IH, Joore MA, Anteunis LJ. Tinnitus interferes with daily life activities: a psychometric examination of the Tinnitus Disability Index. Ear Hear. 2011;32(5):623-633. doi: 10.1097/AUD.0b013e31820dd411 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein E, Ho CX, Hanna R, et al. Cost of care for subjective tinnitus in relation to patient satisfaction. Otolaryngol Head Neck Surg. 2015;152(3):518-523. doi: 10.1177/0194599814566179 [DOI] [PubMed] [Google Scholar]
- 4.Stockdale D, McFerran D, Brazier P, et al. An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Serv Res. 2017;17(1):577. doi: 10.1186/s12913-017-2527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoare DJ, Broomhead E, Stockdale D, Kennedy V. Equity and person-centredness in provision of tinnitus services in UK National Health Service audiology departments. Eur J Pers Cent Healthc. 2015;3(3):318-326. doi: 10.5750/ejpch.v3i3.984 [DOI] [Google Scholar]
- 6.Gander PE, Hoare DJ, Collins L, Smith S, Hall DA. Tinnitus referral pathways within the National Health Service in England: a survey of their perceived effectiveness among audiology staff. BMC Health Serv Res. 2011;11:162. doi: 10.1186/1472-6963-11-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope. 2011;121(7):1555-1564. doi: 10.1002/lary.21825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesser H, Weise C, Westin VZ, Andersson G. A systematic review and meta-analysis of randomized controlled trials of cognitive-behavioral therapy for tinnitus distress. Clin Psychol Rev. 2011;31(4):545-553. doi: 10.1016/j.cpr.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 9.Andersson G, Strömgren T, Ström L, Lyttkens L. Randomized controlled trial of internet-based cognitive behavior therapy for distress associated with tinnitus. Psychosom Med. 2002;64(5):810-816. [DOI] [PubMed] [Google Scholar]
- 10.Beukes EW, Vlaescu G, Manchaiah V, et al. Development and technical functionality of an internet-based intervention for tinnitus in the UK. Internet Interv. 2016;6:6-15. doi: 10.1016/j.invent.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beukes EW, Allen PM, Manchaiah V, Baguley DM, Andersson G. Internet-based intervention for tinnitus: outcome of a single-group open trial. J Am Acad Audiol. 2017;28(4):340-351. doi: 10.3766/jaaa.16055 [DOI] [PubMed] [Google Scholar]
- 12.Beukes EW, Baguley DM, Allen PM, Manchaiah V, Andersson G. Audiologist-guided internet-based cognitive behavior therapy for adults with tinnitus in the United Kingdom: a randomized controlled trial. Ear Hear. 2018.39(3):423-433. doi: 10.1097/AUD.0000000000000505 [DOI] [PubMed] [Google Scholar]
- 13.Kaldo V, Levin S, Widarsson J, Buhrman M, Larsen HC, Andersson G. Internet versus group cognitive-behavioral treatment of distress associated with tinnitus: a randomized controlled trial. Behav Ther. 2008;39(4):348-359. doi: 10.1016/j.beth.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 14.Nyenhuis N, Zastrutzki S, Jäger B, Kröner-Herwig B. An internet-based cognitive-behavioural training for acute tinnitus: secondary analysis of acceptance in terms of satisfaction, trial attrition and non-usage attrition. Cogn Behav Ther. 2013;42(2):139-145. doi: 10.1080/16506073.2012.724081 [DOI] [PubMed] [Google Scholar]
- 15.Jasper K, Weise C, Conrad I, Andersson G, Hiller W, Kleinstäuber M. Internet-based guided self-help versus group cognitive behavioral therapy for chronic tinnitus: a randomized controlled trial. Psychother Psychosom. 2014;83(4):234-246. doi: 10.1159/000360705 [DOI] [PubMed] [Google Scholar]
- 16.Vlaescu G, Carlbring P, Lunner T, Andersson G. An e-platform for rehabilitation of persons with hearing problems. Am J Audiol. 2015;24(3):271-275. doi: 10.1044/2015_AJA-14-0083 [DOI] [PubMed] [Google Scholar]
- 17.Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33(2):153-176. doi: 10.1097/AUD.0b013e31822f67c0 [DOI] [PubMed] [Google Scholar]
- 18.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148. doi: 10.1001/archotol.1996.01890140029007 [DOI] [PubMed] [Google Scholar]
- 19.Hall DA, Haider H, Szczepek AJ, et al. Systematic review of outcome domains and instruments used in clinical trials of tinnitus treatments in adults. Trials. 2016;17(1):270. doi: 10.1186/s13063-016-1399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Kroenke K, Williams JB; Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 23.Newman CW, Weinstein BE, Jacobson GP, Hug GA. Test-retest reliability of the hearing handicap inventory for adults. Ear Hear. 1991;12(5):355-357. doi: 10.1097/00003446-199110000-00009 [DOI] [PubMed] [Google Scholar]
- 24.Khalfa S, Dubal S, Veuillet E, Perez-Diaz F, Jouvent R, Collet L. Psychometric normalization of a hyperacusis questionnaire. ORL J Otorhinolaryngol Relat Spec. 2002;64(6):436-442. doi: 10.1159/000067570 [DOI] [PubMed] [Google Scholar]
- 25.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(pt 1):1-16. doi: 10.1111/j.2044-8260.1982.tb01421.x [DOI] [PubMed] [Google Scholar]
- 26.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71-75. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 27.Newman CW, Sandridge SA, Bolek L. Development and psychometric adequacy of the screening version of the tinnitus handicap inventory. Otol Neurotol. 2008;29(3):276-281. doi: 10.1097/MAO.0b013e31816569c4 [DOI] [PubMed] [Google Scholar]
- 28.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 29.Flight L, Julious SA. Practical guide to sample size calculations: an introduction. Pharm Stat. 2016;15(1):68-74. doi: 10.1002/pst.1709 [DOI] [PubMed] [Google Scholar]
- 30.Kaldo-Sandström V, Larsen HC, Andersson G. Internet-based cognitive-behavioral self-help treatment of tinnitus: clinical effectiveness and predictors of outcome. Am J Audiol. 2004;13(2):185-192. doi: 10.1044/1059-0889(2004/023) [DOI] [PubMed] [Google Scholar]
- 31.Kaldo V, Haak T, Buhrman M, Alfonsson S, Larsen HC, Andersson G. Internet-based cognitive behaviour therapy for tinnitus patients delivered in a regular clinical setting: outcome and analysis of treatment dropout. Cogn Behav Ther. 2013;42(2):146-158. doi: 10.1080/16506073.2013.769622 [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 33.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12-19. doi: 10.1037/0022-006X.59.1.12 [DOI] [PubMed] [Google Scholar]
- 34.Andersson G, Lyttkens L. A meta-analytic review of psychological treatments for tinnitus. Br J Audiol. 1999;33(4):201-210. doi: 10.3109/03005369909090101 [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010;(9):CD005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cima R, Joore M, Maes I, et al. Cost-effectiveness of multidisciplinary management of tinnitus at a specialized tinnitus centre. BMC Health Serv Res. 2009;9:29. doi: 10.1186/1472-6963-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shore JH, Mishkind MC, Bernard J, et al. A lexicon of assessment and outcome measures for telemental health. Telemed J E Health. 2014;20(3):282-292. doi: 10.1089/tmj.2013.0357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol