Key Points
Question
Do pediatric patients with oropharyngeal dysphagia who are treated with proton pump inhibitors (PPI) have increased hospitalizations compared with those who are not treated with PPI?
Findings
In this retrospective cohort study of 293 children under 2 years with aspiration/penetration on videofluoroscopic swallow study, patients treated with PPI had significantly higher hospitalization rates and admission nights, with an incident rate ratio of 1.77 compared with those not treated with PPI, even after adjustment for comorbidities.
Meaning
Children with aspiration who are treated with PPI have increased risk of hospitalization, supporting growing concern about the risks of PPI use in children.
This retrospective cohort study of 293 children with oropharyngeal dysphagia examines hospitalization risk for those treated with proton pump inhibitors compared with those who were not.
Abstract
Importance
Proton pump inhibitors (PPI) are commonly prescribed to children with oropharyngeal dysphagia and resultant aspiration based on the assumption that these patients are at greater risk for reflux-related lung disease. There is little data to support this approach and the potential risk for increased infections in children treated with PPI may outweigh any potential benefit.
Objective
The aim of this study was to determine if there is an association between hospitalization risk in pediatric patients with oropharyngeal dysphagia and treatment with PPI.
Design, Setting, and Participants
We performed a retrospective cohort study to compare the frequency and length of hospitalizations for children who had abnormal results on videofluoroscopic swallow studies that were performed between January 1, 2015, and December 31, 2015, and who were or were not treated with PPI, with follow-up through December 31, 2016. Records were reviewed for children who presented for care at Boston Children’s Hospital, a tertiary referral center. Participants included 293 children 2 years and younger with evidence of aspiration or penetration on videofluoroscopic swallow study.
Exposures
Groups were compared based on their exposure to PPI treatment.
Main Outcomes and Measures
The primary outcomes were hospital admission rate and hospital admission nights and these were measured as incident rates. Multivariable analyses were performed to determine predictors of hospitalization risk after adjusting for comorbidities. Kaplan-Meier curves were created to determine the association of PPI prescribing with time until first hospitalization.
Results
A total of 293 patients with a mean (SD) age of 8.8 (0.4) months and a mean (SD) follow-up time of 18.15 (0.20) months were included in the analysis. Patients treated with PPI had higher admission rates (Incidence rate ratio [IRR], 1.77; 95% CI, 1.16-2.68) and admission nights (IRR, 2.51; 95% CI, 1.36-4.62) even after adjustment for comorbidities. Patients with enteral tubes who were prescribed PPIs were at the highest risk for admission (hazard ratio [HR], 2.31; 95% CI, 1.24-4.31).
Conclusions and Relevance
Children with aspiration who are treated with PPI have increased risk of hospitalization compared with untreated patients. These results support growing concern about the risks of PPI use in children.
Introduction
There is growing concern in the medical community about the risks of proton pump inhibitor (PPI) use.1,2,3 These commonly prescribed acid-suppressing medications have been associated with adverse effects including increased risk of both pulmonary and gastrointestinal infections in adults and children.4,5,6,7,8,9,10,11 Acid suppression causes alterations in the gastric, oropharyngeal, and lung microbiome and patients treated with PPI are at increased risk for pneumonia, upper respiratory tract infections, gastrointestinal infections, and even sepsis.4,5,6,7,10,12,13,14,15,16,17
Despite these ongoing concerns, clinicians including pediatricians, pediatric gastroenterologists, pediatric pulmonologists, and otolaryngologists continue to prescribe these medications to young children with dysphagia. In particular, acid suppressing medications are still commonly used for empirical therapy in pediatric patients with oropharyngeal dysphagia and aspiration because of the frequent symptom overlap (including coughing, feeding difficulties, and vomiting) between reflux and oropharyngeal dysphagia in young children.18,19 Although many clinicians are now more cautious about prescribing acid suppression, prescribing rates are still high, though with the creation of aerodigestive centers, more discussions about the need for these medications are occurring.20,21,22,23 Some clinicians also specifically use PPIs in patients with aspiration based on the assumption that if children cannot protect their airway they might be at increased risk for acid-related lung injury. While these medications are therefore often prescribed to theoretically reduce pulmonary and gastrointestinal complications of gastroesophageal reflux events, there is little data on their efficacy in reducing these morbidities.
Although little is known about the risk of PPI treatment in aspirating children, in studies of adult stroke patients with dysphagia, acid suppression has been associated with a 2-times increased relative risk of pneumonia, even after adjustment for other comorbidities.24,25,26 In addition, a single randomized placebo-controlled trial of PPI vs the prokinetic medication mosapride in adults with oropharyngeal dysphagia and/or aspiration suggested that PPIs might increase the risk of pneumonia.27 Based on adult data and our clinical experience that PPIs do not improve respiratory symptoms in children, we hypothesized that PPI use in children with oropharyngeal dysphagia would be associated with increased hospitalizations and admission nights.
Methods
We reviewed the records of children who were (1) aged 2 years or younger, and (2) with evidence of aspiration and/or penetration on an initial videofluoroscopic swallow study (VFSS), performed at Boston Children’s Hospital between January 2015 and December 2015. Records were reviewed by complete manual medical chart review to determine comorbidities, PPI treatment status, and type and length of hospitalizations at Boston Children’s Hospital in the period following each patient’s swallow study.
The study was approved by the institutional review board at Boston Children’s Hospital (IRB-P00023746). Written informed consent was waived owing to the retrospective nature of the study.
Children younger than 2 were chosen because PPIs are prescribed for this population at higher rates than any other pediatric age group and this group has the highest rate of oropharyngeal dysphagia of any pediatric age group.18,28,29,30,31 Patient PPI treatment status was defined as exposed or unexposed based on medical record review of both prescriptions and physician notes indicating that the patient was prescribed and reported taking a PPI within 12 months of initial VFSS. Patients placed on PPI after their VFSS received their first prescription within an average of 1 month after their VFSS and were therefore included in the analysis because most of their follow-up time occurred after their PPI exposure.
Hospitalizations were categorized into 3 groups: total hospitalizations, urgent pulmonary (ie, tachypnea, wheezing, respiratory distress, pneumonia, desaturations), and urgent gastrointestinal hospitalizations (ie, vomiting, feeding intolerance, diarrhea, poor growth) based on primary discharge diagnosis. Elective hospitalizations (ie, for scheduled procedures) were excluded from the pulmonary and gastrointestinal categories but were included in the total hospitalizations. The number and length of hospitalizations were counted for a minimum of 12 months following the initial VFSS to span all 4 seasons. Because the exact duration of swallowing dysfunction was unknown but the date of initial swallow study was known, the choice of 1 year follow-up was made based on the understanding that swallowing dysfunction in infants typically can last up to 2 years.31
The VFSS results were considered abnormal if there was evidence of aspiration or penetration seen for any texture. Penetration was considered abnormal based on our clinical experience that these patients have similar outcomes to patients with frank aspiration.32,33,34 The study used VFSS results because this test is considered the gold standard for the diagnosis of oropharyngeal dysphagia and aspiration; clinical feeding evaluations were not included owing to their known insensitivity in diagnosing aspiration in the pediatric population.35,36 Only initial VFSS results were used in the analysis. Medical records were also reviewed to determine comorbidities (enteral tube status including presence of gastrostomy or nasogastric tube, cardiac, neurologic, metabolic comorbidities, prematurity).
The primary aim of the study was to determine if there was increased hospitalization risk in patients with abnormal VFSS who were treated with PPI compared with those not treated with PPI. Participant characteristics are presented as frequency (percent) if categorical, mean (standard error [SE]) if continuous, or median (interquartile range [IQR]) if highly skewed. A comparison was made of participants ever on PPI with those never on PPI by sex, age, history of feeding tube, comorbidities, and presenting symptoms based on clinical notes including speech-language pathologist reports. To insure the robustness of the results, we also performed a regression analysis adjusting for propensity scores using an inverse probability of treatment weight (IPTW), which has been shown to perform equivalently to propensity score matching.37,38,39
Negative binomial regression was used to compare admission rates, with an offset defined as the natural logarithm of the number of days from first VFSS until the end of the observation period. For participants who had less than a year of follow-up, we included a follow-up period equal to their age at first VFSS to minimize bias. The effect of enteral tube status on PPI use was investigated with an interaction term and main effects explored only when the interaction was not statistically significant. The presence of comorbidities was controlled by including dichotomous main effects in the model for each. We additionally incorporated dysphagia severity (based on consistency of liquids required to correct dysphagia and adjustment for aspiration vs penetration) and neurologic disability (epilepsy, developmental delay, cerebral palsy, hydrocephalus, Chiari malformation, or neurologic syndrome) into our propensity score model. To evaluate for seasonal variation in admission rates, we compared admission number for each calendar month between the PPI exposed and the PPI unexposed groups using χ2 testing.
The number of days from first VFSS until first hospital admission, stratified by PPI treatment status and enteral tube status, was described using Kaplan-Meier plots. The first VFSS date was chosen, not because this was the start of the aspiration, but rather because most PPI prescriptions were written within 2 months of this date. The impact of PPI and enteral tube use on number of days from first VFSS until first hospitalization was evaluated by proportional hazards regression, with PPI and enteral tube status included in the model as time-varying covariates to accommodate changes in status over time.40 Enteral tube presence was chosen because of our prior study showing that tube placement increases risk of hospitalization in aspirating patients.41 Results are presented as effect sizes and 95% CI around the difference in the effect size metric. Statistical analysis was conducted with SAS statistical software (version 9.4, SAS).
Results
Patient Characteristics
We evaluated 293 participants with a mean (SD) age of 8.8 (0.4) months at the time of their initial VFSS and a mean (SD) follow-up length of 18.15 (0.20) months. In the cohort overall, 45% (132 of 293) of patients had at least 1 admission during the study period with a mean (SD) of 0.97 (0.09) total admissions and 4.02 (0.85) total nights. On swallow evaluation, 53% (156 of 293) had aspiration and 47% (137 of 293) had isolated laryngeal penetration on VFSS. Overall, 59% (92 of 156) aspirated thin liquids, 37% (58 of 156) aspirated nectar consistency, 1% (2 of 156) aspirated honey consistency, and 3% (4 of 156) aspirated all textures. These proportions did not vary in a clinically meaningful way between the subjects treated or not treated with PPI. Patient characteristics, comorbidities, and symptoms prior to VFSS are shown in Table 1. Of note, 69% (203 of 293) of the patients did not have an underlying neurologic diagnosis. Of the 31% (90 of 293) of patients that did, 20% (18 of 90) had epilepsy, 76% (68 of 90) had developmental delay, and 30% (27 of 90) had cerebral palsy.
Table 1. Patient Characteristics (N = 293).
| Characteristic | No. (%) |
|---|---|
| Demographics | |
| Male sex | 177 (60.4) |
| Premature birth | 100 (34.1) |
| Gestational age if premature [range 22-36], median (IQR), wk | 33 (28-36) |
| Age at first VFSS [range 0.2-33.8], median (IQR), mo | 7.0 (3.0-13.4) |
| Comorbidities | |
| Any comorbidities | 210 (71.7) |
| If comorbidities, No. [range 1-7], median (IQR) | 2 (2-3) |
| Neurologic | 90 (30.7) |
| GI | 50 (17.1) |
| Pulmonary | 46 (15.7) |
| Metabolic/genetic | 37 (12.7) |
| Cardiac | 33 (11.3) |
| Immunologic | 2 (0.7) |
| Symptoms | |
| Coughing | 173 (59.0) |
| Choking or gagging | 112 (38.2) |
| Noisy breathing | 81 (27.7) |
| Vomiting | 72 (24.6) |
| Poor feeding | 63 (21.5) |
| Congestion | 58 (19.8) |
| Spells | 53 (18.1) |
| Increased work of breathing | 38 (13.0) |
| Pneumonia | 34 (11.6) |
| Slow feeding | 16 (5.5) |
| Oxygen requirement | 16 (5.5) |
| Eyes watering | 3 (1.0) |
Abbreviations: IQR, interquartile range; VFSS, videofluoroscopic swallow study.
PPI Treatment Status
In a comparison of PPI treatment status in the cohort, 49% (144 of 293) were never treated with PPI and 51% (149 of 293) were prescribed and took a PPI during the period of review. Of the patients treated with PPI, 73% (109 of 149) were taking PPI before their VFSS at a median of 31 (IQR, 18.25-80.75) days prior to VFSS and 27% (40 of 149) were taking PPI after their VFSS at a median of 38.5 (IQR, 13-79.5) days following VFSS. Only 11% (16 of 149) were taken off PPI after their swallow study.
Patients taking PPI were prescribed their PPI for a mean (SD) 7.11 (0.38) months. Mean (SD) treatment dose was 1.67 (0.05) mg/kg per day and 75% (112 of 149) of participants received their PPI twice daily, whereas 25% (37 of 149) were on daily dosing. Most (92%, 137 of 149) participants were treated with omeprazole; the rest were treated with lansoprazole (7%, 10 of 149) or pantoprazole (1%, 2 of 149).
Potentially Confounding Covariates
In a comparison of potentially confounding covariates, there were no meaningful differences in demographic characteristics or prevalence of comorbidities between the groups, as shown in Table 2. Patients treated with PPI were more likely to have symptoms after meals and both vomiting and slow feeding (characterized by taking an extended period of time to finish feeding) as presenting symptoms prior to VFSS. There was no clinically meaningful relationship between any of the comorbidities, including each neurologic comorbidity, and risk of PPI usage.
Table 2. Potentially Confounding Covariates in 293 Participantsa.
| Variable | No. (%) | Effect Size (95% CI) | |
|---|---|---|---|
| Ever PPI (n = 149) | Never PPI (n = 144) | ||
| Demographics | |||
| Male sex | 87 (58.4) | 90 (62.5) | 0.84 (0.53 to 1.35) |
| Premature | 51 (34.2) | 49 (34.0) | 1.01 (0.62 to 1.64) |
| Age at first VFSS, mean (SE), mo | 8.1 (0.5) | 9.2 (0.6) | 0.16 (−0.07 to 0.39) |
| Comorbidities | |||
| Feeding tube | 49 (32.9) | 33 (22.9) | 1.65 (0.98 to 2.77) |
| Neurologic | 39 (26.2) | 51 (35.4) | 0.65 (0.39 to 1.07) |
| Cardiac | 19 (12.8) | 14 (9.7) | 1.36 (0.65 to 2.82) |
| Metabolic | 18 (12.2) | 19 (13.2) | 0.91 (0.46 to 1.82) |
| Symptoms | |||
| Symptoms during meals | 114 (76.5) | 106 (73.6) | 1.17 (0.69 to 1.98) |
| Symptoms after meals | 56 (37.6) | 27 (18.8) | 2.61 (1.53 to 4.45) |
| Vomiting | 48 (32.2) | 24 (16.7) | 2.38 (1.36 to 4.15) |
| Coughing | 84 (56.4) | 89 (61.8) | 0.80 (0.50 to 1.27) |
| Congestion | 32 (21.5) | 26 (18.1) | 1.24 (0.70 to 2.21) |
| Eyes watering | 2 (1.3) | 1 (0.7) | 1.95 (0.17 to 21.69) |
| Noisy breathing | 40 (26.9) | 41 (28.5) | 0.92 (0.55 to 1.54) |
| Increased work of breathing | 19 (12.8) | 19 (13.2) | 0.96 (0.49 to 1.90) |
| Choking or gagging | 61 (40.9) | 51 (35.4) | 1.26 (0.79 to 2.03) |
| Slow feeding | 14 (9.4) | 2 (1.4) | 7.36 (1.64 to 33.01) |
| Poor feeding | 35 (23.5) | 28 (19.4) | 1.27 (0.73 to 2.23) |
| Oxygen requirement | 5 (3.4) | 11 (7.6) | 0.42 (0.14 to 1.24) |
| Spells | 27 (18.1) | 26 (18.1) | 1.00 (0.55 to 1.82) |
| Pneumonia | 20 (13.4) | 14 (9.7) | 1.44 (0.70 to 2.97) |
Abbreviations: PPI, proton pump inhibitor; SE, standard error; VFSS, videofluoroscopic swallow study.
Data are expressed as No. (percentage) or mean (SE) with effect size expressed as odds ratio or Cohen’s d, respectively. Demographic variables and comorbidities were balanced between the two groups with no significant differences.
Hospitalization Risk
In a comparison of hospitalization risk, patients treated with PPI had nearly a 2-fold increase in total hospitalizations at our institution, even after adjustment for comorbidities, enteral tube status, and propensity weights including severity of dysphagia and neurologic diagnosis, with an incident rate ratio (IRR) of 1.77 (95% CI, 1.16-2.68), compared with those not treated with PPI (Table 3). Patients treated with PPI also had a 2- to 3-fold increase in hospital admission nights, even after adjustment, compared with those who were not treated with PPI, with an IRR of 2.51 (95% CI, 1.36-4.62) (Table 3). Reasons for pulmonary admission included tachypnea, wheezing, respiratory distress, pneumonia, and desaturations. Reasons for gastrointestinal admission included vomiting, feeding intolerance, diarrhea, and poor growth. Overall, 12% (36 of 293) of pulmonary admissions had viral panels sent and of those 19% (7 of 36) had positive results, with findings including RSV, parainfluenza, EBV, and adenovirus.
Table 3. Association of PPI Use With Number of Hospital Admissions and Hospital Admission Nights, Based on Negative Binomial Regressiona.
| Variable | PPI Use | IRR (95% CI) | |
|---|---|---|---|
| Yes (n = 149) | No (n = 144) | ||
| Hospital Admissions | |||
| Total admissions | |||
| Unadjusted | 1.18 (0.89-1.55) | 0.62 (0.45-0.86) | 1.89 (1.24-2.89) |
| Adjusted for enteral tube status | 0.99 (0.76-1.29) | 0.64 (0.47-0.87) | 1.54 (1.03-2.31) |
| Adjusted for enteral tube status + Comorbidities | 0.96 (0.73-1.25) | 0.61 (0.45-0.84) | 1.56 (1.04-2.35) |
| Adjusted for propensity weights | 1.10 (0.84-1.44) | 0.62 (0.45-0.86) | 1.77 (1.16-2.68) |
| Pulmonary admissions | |||
| Unadjusted | 0.38 (0.23-0.65) | 0.17 (0.09-0.31) | 2.31 (1.02-5.20) |
| Adjusted for enteral tube status | 0.33 (0.20-0.55) | 0.17 (0.09-0.32) | 1.90 (0.85-4.23) |
| Adjusted for enteral tube status + Comorbidities | 0.32 (0.19-0.53) | 0.18 (0.09-0.33) | 1.81 (0.80-4.07) |
| Adjusted for propensity weights | 0.36 (0.22-0.59) | 0.17 (0.09-0.31) | 2.13 (0.97-4.70) |
| Gastrointestinal admissions | |||
| Unadjusted | 0.26 (0.15-0.47) | 0.12 (0.06-0.25) | 2.24 (0.87-5.72) |
| Adjusted for enteral tube status | 0.09 (0.04-0.19) | 0.06 (0.03-0.14) | 1.49 (0.63-3.50) |
| Adjusted for enteral tube status + Comorbidities | 0.09 (0.04-0.19) | 0.06 (0.02-0.14) | 1.50 (0.62-3.65) |
| Adjusted for propensity weights | 0.23 (0.14-0.40) | 0.11 (0.06-0.23) | 2.07 (0.85-5.02) |
| Hospital Admission Nights | |||
| Total inpatient nights | |||
| Unadjusted | 6.16 (4.02-9.44) | 2.49 (1.55-4.01) | 2.47 (1.31-4.68) |
| Adjusted for enteral tube status | 2.88 (1.98-4.19) | 2.02 (1.35-3.02) | 1.43 (0.82-2.49) |
| Adjusted for enteral tube status + comorbidities | 2.62 (1.80-3.80) | 1.59 (1.06-2.40) | 1.64 (0.93-2.89) |
| Adjusted for propensity weights | 5.48 (3.64-8.27) | 2.19 (1.39-3.44) | 2.51 (1.36-4.62) |
| Pulmonary inpatient nights | |||
| Unadjusted | 3.36 (1.48-7.61) | 0.69 (0.28-1.72) | 4.85 (1.43-16.49) |
| Adjusted for enteral tube status | 1.13 (0.49-2.59) | 0.94 (0.38-2.30) | 1.21 (0.33-4.47) |
| Adjusted for enteral tube status + Comorbidities | 0.93 (0.44-1.97) | 0.57 (0.24-1.35) | 1.64 (0.49-5.45) |
| Adjusted for propensity weights | 3.10 (1.41-6.79) | 0.63 (0.27-1.49) | 4.92 (1.53-15.80) |
| Gastrointestinal inpatient nights | |||
| Unadjusted | 1.16 (0.45-2.96) | 1.18 (0.43-3.24) | 0.98 (0.25-3.92) |
| Adjusted for enteral tube status | 0.65 (0.29-1.43) | 0.14 (0.05-0.41) | 4.76 (1.09-20.72) |
| Adjusted for enteral tube status + Comorbidities | 0.40 (0.18-0.91) | 0.07 (0.02-0.24) | 5.89 (1.37-25.38) |
| Adjusted for propensity weights | 0.88 (0.36-2.12) | 1.00 (0.39-2.61) | 0.87 (0.24-3.22) |
Abbreviations: IRR, incident rate ratio; PPI, proton pump inhibitor.
Patients treated with PPI had more hospitalizations and more hospital admission nights, even after adjustment for comorbidities. All models based on negative binomial regression with adjusted model controlling for the presence of neurologic, cardiac, and metabolic comorbidities. Inverse probability of treatment propensity weights adjusted for all comorbidities and presenting symptoms shown in Table 2.
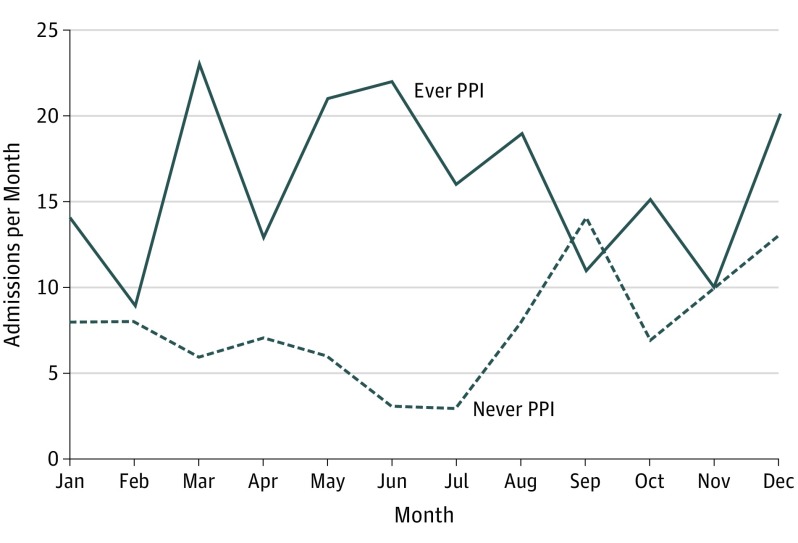
In a comparison of PPI dosing, there was a larger number of admissions for daily compared to twice daily dosing of any PPI (0.87 vs. 0.71; difference 0.16, 95% CI −0.287 to 0.596). The confidence interval is wide and the finding is imprecise, thus no definitive conclusion regarding relationship between PPI dosing and number of admissions can be made. In a comparison of seasonal variation in admission rates, we found that admission rates were higher across the months in patients treated with PPI, as shown in Figure 1.
Figure 1. Admission Month Comparison for Patients Treated With Proton Pump Inhibitors (PPI) and Patients Never Treated With PPI.
Total hospital admissions were higher across the months of the year in patients treated with PPI (solid line) compared with patients never treated with PPI (dashed line).
Survival Analysis
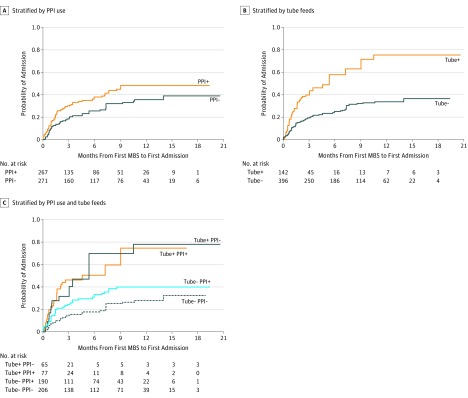
Survival analysis revealed shorter time from oropharyngeal dysphagia diagnosis to first hospital admission for patients exposed to PPI vs those not exposed with propensity weights (HR, 1.25; 95% CI, 0.92-1.68) as well as for those who were tube fed vs those not (HR, 1.87; 95% CI, 1.33-2.65). With reference to patients not exposed to PPI nor tube fed, the interaction of these effects was greatest for patients exposed to PPI and tube fed (HR, 2.31; 95% CI, 1.24-4.31), followed by those not exposed to PPI but tube fed (HR, 1.92; 95% CI, 1.10-3.36) and finally, those exposed to PPI and not tube fed (HR, 0.90; 95% CI, 0.83-1.95) (Figure 2).
Figure 2. Patients With Enteral Tubes Exposed to PPI and Time to First Admission.
Kaplan-Meier curves stratified by PPI use (A), tube feeds (B), and their combined effects (C), after applying inverse probability of treatment weights. There was a shorter time from oropharyngeal dysphagia diagnosis to first hospital admission for patients exposed to PPI vs those not exposed with propensity weights (hazard ratio [HR], 1.25; 95% CI, 0.92-1.68) as well as for those who were tube fed vs those not (HR, 1.87; 95% CI, 1.33-2.65). With reference to patients not exposed to PPI nor tube fed, the interaction of these effects was greatest for those exposed to PPI and tube fed (HR, 2.31; 95% CI, 1.24-4.31), followed by those not exposed to PPI but tube fed (HR, 1.92; 95% CI, 1.10-3.36) and finally, those exposed to PPI and not tube fed (HR, 0.90; 95% CI, 0.83-1.95). All proportional hazards models included time-varying covariates for PPI use and tube feeds, with Bonferroni adjustment for 95% CIs involving interaction effects.
Discussion
To our knowledge, this is the first study to examine the hospitalization risk associated with PPI use in pediatric patients with oropharyngeal dysphagia and aspiration. Our results suggest that PPI use in children with abnormal swallow function is associated with almost double the rate of hospitalization when compared with patients who were not treated with PPIs. This significantly increased risk remained even after adjustment for potentially confounding covariates using multiple approaches including propensity weights.
Young children with oropharyngeal dysphagia and aspiration are commonly placed on PPI for several possible reasons. One of the most common reasons that PPIs are prescribed in this age group is that feeding difficulties and other symptoms are often felt to be reflux-related when, in fact, they are more likely owing to oropharyngeal dysphagia. Symptoms may include coughing, choking, wheezing, gagging, food refusal, and arching. In this study, PPIs were prescribed in 109 (73%) of 149 patients a median of 31 days prior to obtaining the VFSS, likely for empirical therapy of possible reflux before a diagnosis of oropharyngeal dysphagia was made. Apart from confusion over symptoms with misattribution of aspiration symptoms for reflux, PPIs are also prescribed in aspirating patients based on the theory that if acidic gastric contents are aspirated, the damage to the lungs is greater than if nonacidic contents are aspirated, an assumption that has not been supported in the literature. Many studies have also shown that reflux in infants is primarily nonacidic, so there would be no benefit to additional acid suppression.42,43 Despite this, children with aspiration are still frequently prescribed PPI and a recent pediatric study from Svystun et al23 showed that 70% of dysphagia patients were either started or continued taking PPI after diagnosis of dysphagia. In our study, 93 (84%) of 109 participants remained on PPI after VFSS.
We categorized PPI use into exposed or unexposed because prior research has suggested that it might be the exposure to PPI and not necessarily long-term use that can lead to adverse effects, possibly owing to changes in the microbiome.5 Almost three-quarters of the patients in our study were prescribed PPI prior to their swallow study and all hospitalizations counted in our primary outcome were those that occurred in the time period that followed. In addition, patients who were placed on PPI after their swallow study received their first prescription within an average of 1 month after their VFSS and were therefore included in the analysis because most of their follow-up time occurred after PPI exposure. Regardless of when prescribed acid suppression, participants in the present study were prescribed their PPI for more than 7 months on average and therefore were likely to have had clinically significant exposure time, especially given prior findings that infectious risk associated with PPI use typically occurs in the first 1 to 3 months of treatment.5,11,13
Importantly, in this cohort, there were almost equal proportions of children with oropharyngeal dysphagia who were treated or not treated with PPI. One potential concern about our retrospective study design would be potential for confounding by indication. We used multiple approaches to attempt to control for this limitation. In our evaluation of potential confounding covariates, we did not find any difference in comorbidities to suggest that the patients treated with PPI were sicker or carried more comorbidities that would have predisposed them to more hospitalizations, but we did find that patients treated with PPI were more likely to have symptoms after meals, vomiting, and slow feeding as presenting symptoms prior to VFSS; 1 potential explanation for these differences might be that these infants were given the clinical diagnosis of reflux based on these clinical symptoms and as a result empirically placed on acid suppression when, in fact, their problem was aspiration. Of note, none of the patients had pH or impedance studies for objective assessment of reflux but our prior work has shown that gastroesophageal reflux alone is not associated with increased hospitalizations in children with aspiration.44
To further control for differences between the groups of patients exposed or not exposed to PPI, we used regression to adjust for comorbidities and propensity weights to address concerns about possible confounding by indication. Each model showed similar results, with increased hospitalization risk in those patients exposed to PPI, suggesting a robust association between PPI use and increased hospitalizations and hospital nights. The results of this study therefore potentially have important implications for all young children with oropharyngeal dysphagia and particularly those with symptoms commonly attributed to reflux who might be more likely to receive empirical PPI treatment.
Despite historical and more recent mounting evidence for the myriad risks of PPI use in children and guidance from professional organizations that these medications be used with caution, PPIs continue to be frequently prescribed.1,2,3,4,5,6,7,8,9,10,16,17,45 A review of pediatric prescribing practices from 2014 revealed that PPIs were prescribed for almost 3% of infants in the hospital and 1.6% in the outpatient setting.18 Multiple recent studies have shown that PPIs continue to be frequently prescribed after NICU discharge and particularly in medically complex children, with rates rising 7-fold from 1997 to 2009, and 75% of infants ever treated with PPI remained on PPI at discharge.19,22,46 In other infant case series, feeding difficulties have been associated with starting PPI in the outpatient setting with an odds ratio of 2.05 (95% CI, 1.24-3.39) and up to 23% of infants are prescribed PPI specifically for feeding intolerance.19,47 A population-based study of prescribing practices in New Zealand found that 4.6% of infants were prescribed a PPI before 1 year; that proportion doubled over the study time period and very few patients had formal reflux testing.21 Our study shows that this prescribing is associated with poorer outcomes, particularly in young children at high risk for swallow dysfunction.
An additional important consideration in the treatment of pediatric patients with aspiration is the use of enteral tubes. We previously showed that children with aspiration who are fed by enteral tubes are at higher risk for hospitalizations than orally fed aspirating children.41 This current study takes these results further by showing that the combination of enteral feeding plus PPI use results in the highest hospitalization risk, suggesting that the pros and cons of PPI prescribing should be carefully weighed in patients with enteral tubes. Our findings are consistent with adult studies in patients with oropharyngeal dysphagia and aspiration resulting from stroke.24,25,26 In a randomized clinical trial of adults with oropharyngeal dysphagia and gastrostomy tubes, Takatori et al27 also found an increased risk of pneumonia in those randomized to PPI treatment with lansoprazole vs placebo compared with those randomized to the prokinetic mosapride.
Limitations
There are several limitations to our study. We used a retrospective approach and although this entails the possibility of bias from unmeasured confounders, this is the same approach that has been used in many of the landmark adult studies in this field.25,26,48,49,50,51 Although no statistical approach can completely eliminate this limitation, we used multiple models in an attempt to decrease this potential. Another possible critique is that more medically complex patients are prescribed PPIs so therefore they are getting hospitalized at higher rates because of their comorbidities rather than as a result of PPI-related complications. However, we not only found no differences in the frequency of the comorbidities between patients who were and were not prescribed PPIs but we also performed a multivariable analysis using propensity scores and showed a persistent effect. Another consideration is that we used hospitalizations at our institution as a primary outcome. Although we recognize that this may not account for other types of morbidity (eg, escalation of medications, missed work days, quality of life) and necessarily excludes hospitalizations at other institutions, we do feel that this is a valid outcome because these medications are frequently started to prevent symptom exacerbations that can lead to hospitalization and furthermore these hospitalizations are costly for patients and society.52,53 Although the participants in this study may have had hospitalizations at other institutions, many are cared for by multiple clinicians in our institution and tend to be primarily admitted to our hospital. Another limitation is that swallow function in this age group frequently improves with time. We were only able to use initial VFSS results in the current study because not enough patients had follow-up studies owing to variability in the clinical approach to this patient population. We therefore feel our results support the growing adult literature that PPIs may increase morbidity and should only be prescribed thoughtfully and with a confirmed diagnosis of acid-related complications.
Conclusions
Use of PPI was associated with significantly increased hospitalization risk in children with oropharyngeal dysphagia. These results support growing concern about potential risks of PPIs and suggest the need to reevaluate the use of pharmacologic acid suppression in children with aspiration.
References
- 1.Stark CM, Nylund CM. Side effects and complications of proton pump inhibitors: a pediatric perspective. J Pediatr. 2016;168:16-22. doi: 10.1016/j.jpeds.2015.08.064 [DOI] [PubMed] [Google Scholar]
- 2.Eusebi LH, Rabitti S, Artesiani ML, et al. . Proton pump inhibitors: Risks of long-term use. J Gastroenterol Hepatol. 2017;32(7):1295-1302. doi: 10.1111/jgh.13737 [DOI] [PubMed] [Google Scholar]
- 3.Freedberg DE, Kim LS, Yang YX. The Risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi: 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 4.Brown KE, Knoderer CA, Nichols KR, Crumby AS. Acid-suppressing agents and risk for clostridium difficile infection in pediatric patients. Clin Pediatr (Phila). 2015;54(11):1102-1106. doi: 10.1177/0009922815569201 [DOI] [PubMed] [Google Scholar]
- 5.Freedberg DE, Lamousé-Smith ES, Lightdale JR, Jin Z, Yang YX, Abrams JA. Use of acid suppression medication is associated with risk for c. difficile infection in infants and children: a population-based study. Clin Infect Dis. 2015;61(6):912-917. doi: 10.1093/cid/civ432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canani RB, Cirillo P, Roggero P, et al. ; Working Group on Intestinal Infections of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP) . Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5):e817-e820. doi: 10.1542/peds.2005-1655 [DOI] [PubMed] [Google Scholar]
- 7.Rosen R, Hu L, Amirault J, Khatwa U, Ward DV, Onderdonk A. 16S community profiling identifies proton pump inhibitor related differences in gastric, lung, and oropharyngeal microflora. J Pediatr. 2015;166(4):917-923. doi: 10.1016/j.jpeds.2014.12.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imhann F, Vich Vila A, Bonder MJ, et al. . The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes. 2017;8(4):351-358. doi: 10.1080/19490976.2017.1284732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedberg DE, Toussaint NC, Chen SP, et al. . Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149(4):883-5.e9. doi: 10.1053/j.gastro.2015.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen R, Amirault J, Liu H, et al. . Changes in gastric and lung microflora with acid suppression: acid suppression and bacterial growth. JAMA Pediatr. 2014;168(10):932-937. doi: 10.1001/jamapediatrics.2014.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14(12):697-710. doi: 10.1038/nrgastro.2017.117 [DOI] [PubMed] [Google Scholar]
- 12.Rossi PD, Bilotta C, Consonni D, et al. ; REPOSI Investigators . Predictors of clinical events occurring during hospital stay among elderly patients admitted to medical wards in Italy. Eur J Intern Med. 2016;32:38-42. doi: 10.1016/j.ejim.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 13.Naito Y, Kashiwagi K, Takagi T, Andoh A, Inoue R. Intestinal dysbiosis secondary to proton-pump inhibitor use. Digestion. 2018;97(2):195-204. doi: 10.1159/000481813 [DOI] [PubMed] [Google Scholar]
- 14.Manzoni P, García Sánchez R, Meyer M, et al. ; Italian Task Force for the Study, and Prevention of Neonatal Fungal Infections and the Italian Society of Neonatology . Exposure to gastric acid inhibitors increases the risk of infection in preterm very low birth weight infants but concomitant administration of lactoferrin counteracts this effect. J Pediatr. 2018;193:62-67.e1. doi: 10.1016/j.jpeds.2017.09.080 [DOI] [PubMed] [Google Scholar]
- 15.Graham PL III, Begg MD, Larson E, Della-Latta P, Allen A, Saiman L. Risk factors for late onset gram-negative sepsis in low birth weight infants hospitalized in the neonatal intensive care unit. Pediatr Infect Dis J. 2006;25(2):113-117. doi: 10.1097/01.inf.0000199310.52875.10 [DOI] [PubMed] [Google Scholar]
- 16.Ephgrave KS, Kleiman-Wexler R, Pfaller M, et al. . Effects of sucralfate vs antacids on gastric pathogens: results of a double-blind clinical trial. Arch Surg. 1998;133(3):251-257. doi: 10.1001/archsurg.133.3.251 [DOI] [PubMed] [Google Scholar]
- 17.Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154(4):514-520.e4. doi: 10.1016/j.jpeds.2008.09.054 [DOI] [PubMed] [Google Scholar]
- 18.Illueca M, Alemayehu B, Shoetan N, Yang H. Proton pump inhibitor prescribing patterns in newborns and infants. J Pediatr Pharmacol Ther. 2014;19(4):283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Agostino JA, Passarella M, Martin AE, Lorch SA. Use of gastroesophageal reflux medications in premature infants after NICU discharge. Pediatrics. 2016;138(6):e20161977. doi: 10.1542/peds.2016-1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boesch RP, Balakrishnan K, Acra S, et al. . Structure and functions of pediatric aerodigestive programs: a consensus statement. Pediatrics. 2018;e20171701. doi: 10.1542/peds.2017-1701 [DOI] [PubMed] [Google Scholar]
- 21.Blank ML, Parkin L. National study of off-label proton pump inhibitor use among New Zealand infants in the first year of life (2005-2012). J Pediatr Gastroenterol Nutr. 2017;65(2):179-184. doi: 10.1097/MPG.0000000000001596 [DOI] [PubMed] [Google Scholar]
- 22.Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. neonatal histamine-2 receptor antagonist and proton pump inhibitor treatment at United States children’s hospitals. J Pediatr. 2016;174:63-70.e3, e63. doi: 10.1016/j.jpeds.2016.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svystun O, Johannsen W, Persad R, Turner JM, Majaesic C, El-Hakim H. Dysphagia in healthy children: characteristics and management of a consecutive cohort at a tertiary centre. Int J Pediatr Otorhinolaryngol. 2017;99:54-59. doi: 10.1016/j.ijporl.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 24.Marciniak C, Korutz AW, Lin E, Roth E, Welty L, Lovell L. Examination of selected clinical factors and medication use as risk factors for pneumonia during stroke rehabilitation: a case-control study. Am J Phys Med Rehabil. 2009;88(1):30-38. doi: 10.1097/PHM.0b013e3181909b73 [DOI] [PubMed] [Google Scholar]
- 25.Herzig SJ, Doughty C, Lahoti S, et al. . Acid-suppressive medication use in acute stroke and hospital-acquired pneumonia. Ann Neurol. 2014;76(5):712-718. doi: 10.1002/ana.24262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai N, Nakamizo T, Ihara H, et al. . Histamine H2-blocker and proton pump inhibitor use and the risk of pneumonia in acute stroke: a retrospective analysis on susceptible patients. PLoS One. 2017;12(1):e0169300. doi: 10.1371/journal.pone.0169300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takatori K, Yoshida R, Horai A, et al. . Therapeutic effects of mosapride citrate and lansoprazole for prevention of aspiration pneumonia in patients receiving gastrostomy feeding. J Gastroenterol. 2013;48(10):1105-1110. doi: 10.1007/s00535-012-0725-6 [DOI] [PubMed] [Google Scholar]
- 28.Weir KA, McMahon S, Taylor S, Chang AB. Oropharyngeal aspiration and silent aspiration in children. Chest. 2011;140(3):589-597. doi: 10.1378/chest.10-1618 [DOI] [PubMed] [Google Scholar]
- 29.Davis NL, Liu A, Rhein L. Feeding immaturity in preterm neonates: risk factors for oropharyngeal aspiration and timing of maturation. J Pediatr Gastroenterol Nutr. 2013;57(6):735-740. doi: 10.1097/MPG.0b013e3182a9392d [DOI] [PubMed] [Google Scholar]
- 30.Lefton-Greif MA, Carroll JL, Loughlin GM. Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatr Pulmonol. 2006;41(11):1040-1048. doi: 10.1002/ppul.20488 [DOI] [PubMed] [Google Scholar]
- 31.Adil E, Al Shemari H, Kacprowicz A, et al. . Evaluation and management of chronic aspiration in children with normal upper airway anatomy. JAMA Otolaryngol Head Neck Surg. 2015;141(11):1006-1011. doi: 10.1001/jamaoto.2015.2266 [DOI] [PubMed] [Google Scholar]
- 32.Gurberg J, Birnbaum R, Daniel SJ. Laryngeal penetration on videofluoroscopic swallowing study is associated with increased pneumonia in children. Int J Pediatr Otorhinolaryngol. 2015;79(11):1827-1830. doi: 10.1016/j.ijporl.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 33.Serel Arslan S, Demir N, Karaduman AA. Both pharyngeal and esophageal phases of swallowing are associated with recurrent pneumonia in pediatric patients. Clin Respir J. 2018;12(2):767-771. doi: 10.1111/crj.12592 [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Chang YS, Yoo HS, et al. . Swallowing dysfunction in very low birth weight infants with oral feeding desaturation. World J Pediatr. 2011;7(4):337-343. doi: 10.1007/s12519-011-0281-9 [DOI] [PubMed] [Google Scholar]
- 35.Weir K, McMahon S, Barry L, Masters IB, Chang AB. Clinical signs and symptoms of oropharyngeal aspiration and dysphagia in children. Eur Respir J. 2009;33(3):604-611. doi: 10.1183/09031936.00090308 [DOI] [PubMed] [Google Scholar]
- 36.Duncan DR, Amirault J, Mitchell PD, Larson K, Rosen RL. Oropharyngeal dysphagia is strongly correlated with apparent life-threatening events. J Pediatr Gastroenterol Nutr. 2017;65(2):168-172. doi: 10.1097/MPG.0000000000001439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29(6):661-677. doi: 10.1177/0272989X09341755 [DOI] [PubMed] [Google Scholar]
- 38.Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29(20):2137-2148. doi: 10.1002/sim.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937-2960. doi: 10.1002/sim.1903 [DOI] [PubMed] [Google Scholar]
- 40.Cox D. Regression models and life tables. J Roy Stat Soc. 1972;Series B(20):187-220. [Google Scholar]
- 41.McSweeney ME, Kerr J, Amirault J, Mitchell PD, Larson K, Rosen R. Oral feeding reduces hospitalizations compared with gastrostomy feeding in infants and children who aspirate. J Pediatr. 2016;170:79-84. doi: 10.1016/j.jpeds.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen R, Mitchell PD, Amirault J, Amin M, Watters K, Rahbar R. The edematous and erythematous airway does not denote pathologic gastroesophageal reflux. J Pediatr. 2017;183:127-131. doi: 10.1016/j.jpeds.2016.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garza JM, Nylund CM, Kaul A. Time to stop blaming gastroesophageal reflux. Clin Pediatr (Phila). 2011;50(12):1110-1115. doi: 10.1177/0009922811412585 [DOI] [PubMed] [Google Scholar]
- 44.Duncan DR, Amirault J, Johnston N, Mitchell P, Larson K, Rosen RL. Gastroesophageal reflux burden, even in children that aspirate, does not increase pediatric hospitalization. J Pediatr Gastroenterol Nutr. 2016;63(2):210-217. doi: 10.1097/MPG.0000000000001092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. ; North American Society for Pediatric Gastroenterology Hepatology and Nutrition; European Society for Pediatric Gastroenterology Hepatology and Nutrition . Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498-547. [DOI] [PubMed] [Google Scholar]
- 46.De Bruyne P, Christiaens T, Vander Stichele R, Van Winckel M. Changes in prescription patterns of acid-suppressant medications by Belgian pediatricians: analysis of the national database, [1997-2009]. J Pediatr Gastroenterol Nutr. 2014;58(2):220-225. doi: 10.1097/MPG.0b013e3182a3b04e [DOI] [PubMed] [Google Scholar]
- 47.Barron JJ, Tan H, Spalding J, Bakst AW, Singer J. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45(4):421-427. doi: 10.1097/MPG.0b013e31812e0149 [DOI] [PubMed] [Google Scholar]
- 48.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7(6):e015735. doi: 10.1136/bmjopen-2016-015735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomm W, von Holt K, Thomé F, et al. . Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416. doi: 10.1001/jamaneurol.2015.4791 [DOI] [PubMed] [Google Scholar]
- 50.Lazarus B, Chen Y, Wilson FP, et al. . Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238-246. doi: 10.1001/jamainternmed.2015.7193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Targownik LE, Leslie WD, Davison KS, et al. ; CaMos Research Group . The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos). Am J Gastroenterol. 2012;107(9):1361-1369. doi: 10.1038/ajg.2012.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirsch AW, Monuteaux MC, Fruchtman G, Bachur RG, Neuman MI. Characteristics of children hospitalized with aspiration pneumonia. Hosp Pediatr. 2016;6(11):659-666. doi: 10.1542/hpeds.2016-0064 [DOI] [PubMed] [Google Scholar]
- 53.Thomson J, Hall M, Ambroggio L, et al. . Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. doi: 10.1542/peds.2015-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]