Key Points
Question
Can a hospital-initiated program result in reduced acute care use and better quality of life for patients hospitalized for chronic obstructive pulmonary disease (COPD)?
Findings
In this single-site randomized clinical trial that included 240 patients with COPD, a 3-month program that combined transition and long-term management support compared with usual care resulted in fewer COPD-related hospitalizations and emergency department visits (0.72 vs 1.40 per participant) and better change in health-related quality of life (−1.53 vs +5.44 in the 100-point St George’s Respiratory Questionnaire) at 6 months. Both comparisons were statistically significant.
Meaning
This type of program may offer benefit to patients with COPD, but requires further evaluation in other settings.
Abstract
Importance
Patients hospitalized for chronic obstructive pulmonary disease (COPD) exacerbations have high rehospitalization rates and reduced quality of life.
Objective
To evaluate a hospital-initiated program that combined transition and long-term self-management support for patients hospitalized due to COPD and their family caregivers.
Design, Setting, and Participants
This single-site randomized clinical trial was conducted in Baltimore, Maryland, with 240 participants. Participants were patients hospitalized due to COPD, randomized to intervention or usual care, and followed up for 6 months after hospital discharge. Enrollment occurred from March 2015 to May 2016; follow-up ended in December 2016.
Interventions
The intervention (n = 120) was a comprehensive 3-month program to help patients and their family caregivers with long-term self-management of COPD. It was delivered by COPD nurses (nurses with special training on supporting patients with COPD using standardized tools). Usual care (n = 120) included transition support for 30 days after discharge to ensure adherence to discharge plan and connection to outpatient care.
Main Outcomes and Measures
The primary outcome was number of COPD-related acute care events (hospitalizations and emergency department visits) per participant at 6 months. The co-primary outcome was change in participants’ health-related quality of life measured by the St George’s Respiratory Questionnaire (SGRQ) at 6 months after discharge (score, 0 [best] to 100 [worst]; 4-point difference is clinically meaningful).
Results
Among 240 patients who were randomized (mean [SD] age, 64.9 [9.8] years; females, 61.7%), 203 (85%) completed the study. The mean (SD) baseline SGRQ score was 63.1 (19.9) in the intervention group and 62.6 (19.3) in the usual care group. The mean number of COPD-related acute care events per participant at 6 months was 0.72 (95% CI, 0.45-0.97) in the intervention group vs 1.40 (95% CI, 1.01-1.79) in the usual care group (difference, 0.68 [95% CI, 0.22 to 1.15]; P = .004). The mean change in participants’ SGRQ total score at 6 months was −1.53 in the intervention and +5.44 in the usual care group (adjusted difference, −6.69 [95% CI, −12.97 to −0.40]; P = .04). During the study period, there were 15 deaths (intervention: 7; usual care: 8) and 337 hospitalizations (intervention: 135; usual care: 202).
Conclusions and Relevance
In a single-site randomized clinical trial of patients hospitalized due to COPD, a 3-month program that combined transition and long-term self-management support resulted in significantly fewer COPD-related hospitalizations and emergency department visits and better health-related quality of life at 6 months after discharge. Further research is needed to evaluate this intervention in other settings.
Trial Registration
ClinicalTrials.gov Identifier: NCT02036294
This randomized clinical trial compares the effects of a program combining transition and long-term self-management support vs transition support alone on 6-month hospitalizations and emergency department visits among patients with chronic obstructive pulmonary disease (COPD).
Introduction
Chronic obstructive pulmonary disease (COPD) was the fourth leading cause of death in the United States in 2016, and has been a leading cause of morbidity and disability.1,2,3 Patients with COPD receiving emergency department (ED) and hospital care are more likely to have lower education and income and comorbidities.4 A review of discharge bundle interventions to improve outcomes of hospitalized patients with COPD showed a modest effect on reducing hospitalizations, and no effects on mortality or quality of life.5 Transitional care studies of patients with COPD are few, often limited to addressing postacute care needs in the 30-day postdischarge period, and did not focus on long-term chronic disease self-management skills.6,7,8,9,10 However, this may be insufficient for improving patient outcomes and reducing future acute care use.
Studies on COPD self-management support, mostly conducted in outpatient settings, have demonstrated improvements in health-related quality of life (HRQOL) and reductions in COPD-related acute care events.11,12 These studies have included ongoing education, action plans, and long-term self-management support.11,12,13,14 To our knowledge, no similar interventions have been tested among hospitalized patients with COPD, although hospitalization may offer a unique opportunity to engage patients and family caregivers in self-management of this condition.
In this study, a patient-centered, hospital-initiated, 3-month program that combines transition support and chronic disease self-management (the BREATHE Program) was developed and evaluated. The program aimed to improve quality of life and reduce acute care use among patients with COPD.15 The study’s primary hypothesis was that compared with participants who received usual transitional care, participants randomized to receive this program would have a lower number of COPD-related acute care events and better HRQOL at 6 months after hospital discharge.15
Methods
The Johns Hopkins Institutional Review Board approved this study. Written consent was obtained from all participants. Detailed study methods are described elsewhere.15 The trial protocol and statistical analysis plans are available in Supplement 1 and Supplement 2, respectively.
Study Design and Setting
This single-blinded randomized clinical trial had 2 groups (intervention and usual transitional care). The study took place at Johns Hopkins Bayview Medical Center, a 447-bed academic community hospital in Baltimore, Maryland, that serves the largest number of patients with COPD within the Johns Hopkins Health System (JHHS). Most hospitalized patients with COPD are treated at 1 of 4 medical units at the hospital. All patients admitted to these units starting in March 2015 were screened for eligibility.15 Enrollment ended in May 2016 and follow-up ended in December 2016.
Patients were eligible if they were either admitted to the Johns Hopkins Bayview Medical Center with a diagnosis of acute COPD exacerbation or had a previous COPD diagnosis and were receiving additional treatment to control COPD symptoms in the current hospitalization.15 Additional eligibility criteria included being aged older than 40 years, having a smoking history of more than 10 pack-years, understanding the English language, having no terminal illness (<6-month life expectancy) other than COPD, having no severe cognitive dysfunction (able to follow simple instructions), not being homeless, and expecting discharge to home.15 Eligibility was confirmed via medical record review and clinician confirmation. No participants were excluded based on discharge diagnosis.
Participants were randomized in a 1:1 ratio to either study group based on a pregenerated sequence of assignments. Randomization was stratified by hospital unit, and a computer algorithm was used to perform a blocked randomization assignment within strata with randomly selected block sizes of 2, 4, or 6. Data collectors and outcomes assessors were blinded to group allocation; however, due to the nature of the intervention, participants and clinicians were not blinded.
Study Groups Description
Intervention Group
The study intervention was co-developed with patients who have COPD, caregivers, and other stakeholders, with a focus on improving hospitalized patients’ HRQOL and reducing their future need for seeking emergency care.15,16 It included 3 components deemed necessary and complementary to achieving study goals15:
Transition support to try to ensure that patients and caregivers were prepared for discharge and understood the postdischarge plan of care.
Individualized COPD self-management support to help patients take medications correctly, recognize exacerbations signs and follow action plan, practice breathing exercises and energy conservation techniques, maintain an active lifestyle, seek help as needed, and stop smoking.
Facilitated access to community programs and treatment services.
The intervention was delivered by COPD nurses (ie, nurses with special training on supporting patients with COPD using standardized tools). The nurses met with the patient (and caregiver whenever possible) during the hospital stay and for 3 months after discharge. They provided self-management support and addressed barriers to care. The program followed a patient-centered partnership approach, and was delivered during a series of sessions held at the hospital and after discharge via home visit or telephone.15
Comparison Group
Participants in the comparison group received the usual transitional care provided at the study site. This included assigning a general transition coach to follow the patient for 30 days after discharge, focusing on adherence to the discharge plan, and connecting to outpatient care. eTable 1 in Supplement 3 compares the intervention and usual care groups.
Data Collection
Patient consent, baseline assessment, and randomization were completed during hospital stay. The assessment included patient report on education, income, and race/ethnicity (collected to report on minorities’ representation in randomized clinical trials, using 2 separate questions with specified response categories). Data collection telephone calls were conducted at 1 week and 1, 3, and 6 months after discharge. Acute care visits were assessed via medical record review for all visits within JHHS. Participants were asked at each telephone call if they had visited any non-JHHS hospital or ED and, if so, their records were requested and reviewed. For participants who could not be reached at 6 months, visits within the JHHS and any outside visits reported previously were reviewed. Records of each visit were independently reviewed by 2 physicians to determine whether the visit was COPD-related using predefined criteria.15 A third physician adjudicated any unresolved conflicts. Data on deaths were collected via medical record and death certificate reviews.15
Outcomes
The study’s prespecified primary outcome was the number of COPD-related acute care events, defined as hospitalizations and ED visits, per participant over the 6 months after discharge. A co-primary outcome was the change in participants’ HRQOL as measured by the SGRQ score at 6 months after discharge. The COPD-related events outcome was the primary design variable to power the study, and we would not have considered this study positive without inferring a benefit on this outcome. The SGRQ outcome was chosen as a key supportive outcome given its patient-centeredness and importance to interpreting intervention effects. The SGRQ is a valid instrument for measuring HRQOL in patients with respiratory disease, with a total score and scores for symptom, physical activity, and impact domains (score range, 0 [best]-100 [worst]).17 Total score’s minimum clinically important difference is 4 points.17,18
Prespecified secondary outcomes were (1) 6-month mortality rate and (2) time to death or first COPD-related hospitalization or ED visit. As part of post hoc supplementary analyses, the following outcomes were compared: (1) mean number of COPD-related and all-cause acute care events per participant and the individual components (hospital and ED visits separately) at each time point; (2) percentage of participants who had at least 1 COPD-related acute care event; (3) change in participants’ SGRQ domain scores; and (4) percentage of participants whose HRQOL improved, stayed the same, or deteriorated within each group.
Intervention implementation was tracked and adverse events, including hospitalizations, deaths, and falls resulting in an acute care visit, were monitored.
Statistical Analyses
Sample size was calculated to detect a difference of 0.25 in the mean cumulative number of COPD-related visits per participant between study groups, with 80% power and type I error of 0.05 (2-sided). The 0.25 difference, which was based on results of an earlier COPD self-management trial, is considered clinically meaningful.13,19,20,21 The estimated sample size was 120 per group.15
Unadjusted analyses of the treatment effect under intention to treat were performed using negative binomial regression for the cumulative count of events, and linear regression for change in SGRQ total and domain scores. Additional analyses were conducted, adjusting for baseline SGRQ and unit for change in SGRQ scores and predictors of hospitalization (age, home oxygen use, and hospitalization within past year) and unit for acute care event counts.22,23,24
All analyses included robust estimates of variance and accounted for within-unit correlation. Normality and homoskedasticity of residuals were evaluated for linear regression models. SGRQ scores for patients who died were substituted with 100. Missingness effect on primary outcomes was evaluated by comparing the patient characteristics of those with and without the missing outcome. Survival analyses and Cox proportional hazard models were used to compare the probability of not dying or having a COPD-related acute care event, by study group, adjusting for predictors of hospitalization. The proportional hazard assumption was evaluated with a test of a zero slope for the log-hazard ratio.
Post hoc analyses included logistic regression to compare the odds of having at least 1 COPD-related event, and responder analysis of participants whose HRQOL improved, stayed the same, or deteriorated (categorized change in SGRQ score as improved if ≤−4, and deteriorated if ≥4).17,18,21 A sensitivity analysis was also performed to evaluate missingness effect on HRQOL using a mixed-effects generalized linear model with robust variance estimates, clustering within hospital units, with patient as random effect, and using all available SGRQ total scores (baseline, 3 months, and 6 months). Analysis was performed in Stata versions 14 and 15 (StataCorp). Main analyses were prespecified.15 Statistical significance was considered for P < .05 (2-sided). No adjustment for multiple comparisons was performed, and subsequently all analyses of secondary and other supplementary outcomes should be considered exploratory.
Results
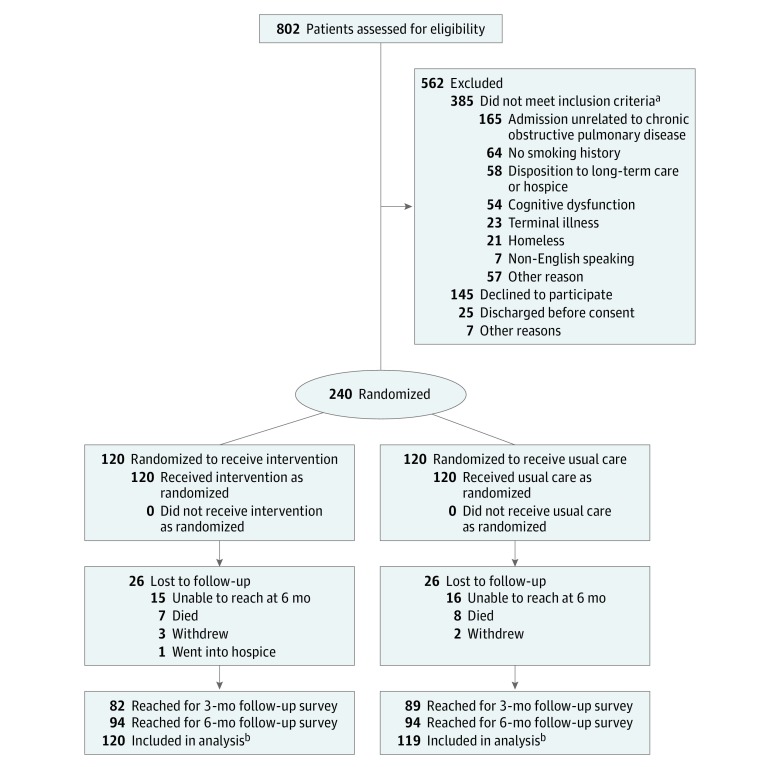
Figure 1 depicts participant recruitment, enrollment, and follow-up. Of 802 patients screened for participation, 417 met eligibility criteria and 240 provided consent. Participants’ baseline characteristics are presented in Table 1 and were similar between study groups. No data were missing for baseline covariates. The mean (SD) participant age was 64.9 (9.8) years and most participants were white (82.5%) and female (61.7%). eTable 2 in Supplement 3 summarizes study participants’ comorbidities. The cumulative number of events at 1, 3, and 6 months after discharge were counted based on medical record review for participants living at those time points. Sixteen participants (14%) in the usual care group and 15 participants (13%) in the intervention group were lost to follow-up. No significant differences were found in baseline characteristics of these participants compared with the overall study sample.
Figure 1. Recruitment, Randomization, and Retention of Participants.
aPatients may have more than 1 reason for not being eligible for the study.
bData on hospitalizations, emergency department visits, and deaths were collected via medical record and vital statistics record review, and were analyzed for all study participants except for 1 participant in the usual care group who withdrew from the study shortly after hospital discharge due to moving out of state.
Table 1. Study Participants’ Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Intervention | Usual Care | |
| No. of participants | 120 | 120 |
| Age, mean (SD), y | 66.0 (10.0) | 63.9 (9.6) |
| Race/ethnicity | ||
| White | 100 (83.3) | 98 (81.6) |
| African American | 18 (15.0) | 20 (16.7) |
| American Indian/Alaska Native | 2 (1.7) | 2 (1.7) |
| Sex | ||
| Male | 44 (36.7) | 48 (40.0) |
| Female | 76 (63.3) | 72 (60.0) |
| Education <12th grade | 53 (44.2) | 44 (36.6) |
| Income ≤$20 000 | 76 (63.3) | 75 (62.5) |
| Living alone | 23 (19.2) | 21 (17.5) |
| Has someone who helps with health care | 77 (64.7) | 75 (62.5) |
| Hospitalized in the past year | 100 (83.3) | 95 (79.2) |
| Body mass index, median (IQR)a | 27.5 (23.7-34.1) | 28.8 (23.1-35.3) |
| No. of years with COPD, median (IQR) | 3 (2-3) | 3 (2-3) |
| Continuous home oxygen therapyb | 41 (34.2) | 58 (48.3) |
| FEV1 % predicted, mean (SD) | 35.8 (14.2) | 33.3 (16.0) |
| FEV1/FVC % predicted, mean (SD) | 57.4 (15.7) | 56.1 (17.4) |
| Respiratory medicine class | ||
| Inhaled steroids | 57 (52.3) | 56 (50.9) |
| Combined β-agonist and anticholinergic | 20 (18.3) | 29 (26.4) |
| Anticholinergic | 13 (11.9) | 3 (2.7) |
| Short-acting β-agonist | 10 (9.2) | 14 (12.7) |
| Theophylline or similar treatment | 7 (6.4) | 7 (6.4) |
| Long-acting β-agonist | 2 (1.8) | 1 (0.9) |
| Currently smoking | 43 (35.8) | 49 (40.8) |
| Charlson Comorbidity Index score, median (IQR)c | 2 (1-4) | 2.5 (1-4) |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in first second of expiration; FVC, forced vital capacity; IQR, interquartile range.
Calculated as weight in kilograms divided by height in meters squared.
Continuous home oxygen therapy refers to using supplemental oxygen at home, during both daytime and nighttime.
Charlson Comorbidity Index score ranges from 0 to 29; higher numbers indicate increased morbidity.
Primary Outcomes
Effect on COPD-Related Acute Care Events
The total number of COPD-related acute care events in this study was 237 (195 COPD-related hospitalizations and 42 COPD-related ED visits). The study primary outcome was the number of COPD-related acute care events per patient over 6 months after discharge. This outcome was assessed for all participants living at 6 months (intervention: n = 113; usual care: n = 112). The mean number of COPD-related events per participant at 6 months was 0.72 (95% CI, 0.45-0.97) in the intervention group and 1.40 (95% CI, 1.01-1.79) in the usual care group (difference, 0.68 [95% CI, 0.22-1.15]; P = .004).
Effect on HRQOL
Mean (SD) baseline SGRQ scores were 63.1 (19.9) in the intervention group and 62.6 (19.3) in the usual care group (Table 2). Data were available to calculate the 6-month change in SGRQ scores for 94 participants within each group (84% of living participants). No significant differences were found in baseline characteristics for age, hospital unit, home oxygen use, hospitalization in past year, and forced expiratory volume among patients with missing change in SGRQ scores compared with other study participants (eTable 3 in Supplement 3). At 6 months after discharge, the mean change in SGRQ total score was −1.53 and +5.44 for the intervention and usual care groups, respectively (difference, −6.97 [95% CI, −14.05 to 0.12]; P = .05). The difference was similar after adjustment for hospital unit and baseline SGRQ score (adjusted difference, −6.69 [95% CI, −12.97 to −0.40]; P = .04) (Table 2). Differences in SGRQ scores had wide confidence intervals, increasing uncertainty in estimating intervention effects on HRQOL.
Table 2. Mean Change in Health-Related Quality of Life, as Measured by St George’s Respiratory Questionnaire, at 6 Months After Discharge by Study Groupa.
| Measure | Intervention | Usual Care | Adjusted Difference, Mean Change (95% CI)b |
P Valuec | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) Score | Change in Score, Mean (95% CI) | Mean (SD) Score | Change in Score, Mean (95% CI) | |||||
| Baseline | At 6 mo | Baseline | At 6 mo | |||||
| Co-primary Outcomed | ||||||||
| Total score | 64.0 (17.8) | 62.5 (22.1) | −1.53 (−5.20 to 2.14) |
62.8 (18.9) | 68.3 (23.2) | 5.44 (0.82 to 10.05) |
−6.69 (−12.97 to −0.40) |
.04 |
| Post Hoc Outcomesd | ||||||||
| Symptom score | 67.1 (20.4) | 61.6 (23.9) | −5.54 (−10.59 to −0.49) |
64.7 (22.1) | 68.0 (24.2) | 3.32 (−2.40 to 9.03) |
−7.16 (−13.39 to −0.93) |
.04 |
| Activity score | 83.0 (17.4) | 79.2 (21.9) | −3.72 (−7.67 to 0.22) |
80.3 (20.8) | 82.1 (21.2) | 1.82 (−2.64 to 6.28) |
−4.57 (−11.67 to 2.53) |
.13 |
| Impact score | 52.1 (21.5) | 52.6 (26.2) | 0.50 (−3.62 to 4.63) |
52.1 (22.2) | 60.3 (27.8) | 8.23 (2.77 to 13.70) |
−7.87 (−15.37 to −0.38) |
.04 |
St George’s Respiratory Questionnaire measures health-related quality of life for patients with respiratory disease; provides a total score and 3 domain scores for symptom, activity, and impact (measuring respiratory symptoms, ability to do physical activity, and impact of illness on life, respectively); and the score range for total and domain scores is 0 (best) to 100 (worst), with a 4-point difference being clinically meaningful.17,18,19,20,21
Adjusted for hospital enrollment unit and St George’s Respiratory Questionnaire score at baseline. Negative numbers suggest the intervention group did better.
Analysis completed with linear regression. Normality of residuals was good. There was no evidence of heteroskedasticity of residuals with respect to group (P = .34, >.99, .85, and .15 for total, symptom, activity, and impact scores, respectively).
Data were available for patients as follows: Total score: n = 94 in usual care, n = 94 in intervention; symptom score: n = 94 in usual care, n = 97 in intervention; activity score: n = 94 in usual care, n = 94 in intervention; and impact score: n = 94 in usual care, n = 96 in intervention.
Secondary Outcomes
There were 7 deaths (5.8%) in the intervention group and 8 deaths (6.7%) in the usual care group (P > .99).
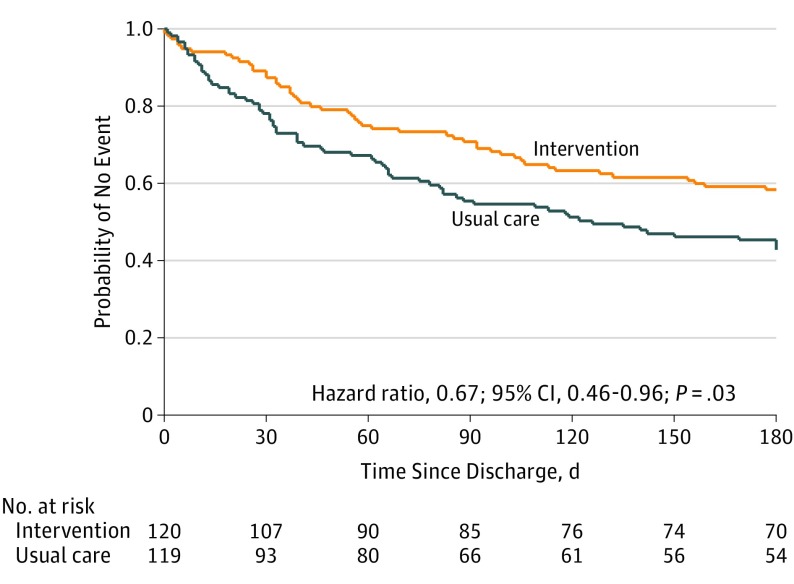
Figure 2 depicts Kaplan-Meier plots for time to first COPD-related acute care event or death. The 6-month event-free survival probability for no death or COPD-related acute care event was 0.58 and 0.43 in the intervention and usual care groups, respectively (log rank P = .01). The adjusted hazard ratio was 0.67 (95% CI, 0.46-0.96; P = .03) (test of proportional hazard assumption met; P = .21) (eFigures 1 and 2 in Supplement 3 depict Kaplan-Meier plots by type of acute care event).
Figure 2. Time to First Chronic Obstructive Pulmonary Disease–Related Acute Care Event (Hospitalization or Emergency Department Visit) or Death.
The median time to first event for the usual care group is 126 days (95% CI, 81-180) compared with greater than 180 days for the intervention group (exact value is not computed because it is beyond the 6-month observation period). Cox proportional hazards model adjusted for hospital unit, age, oxygen use, and prior hospitalization.
Supplementary Post Hoc Analyses
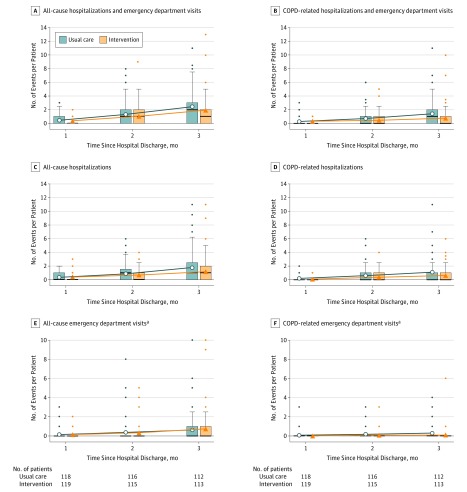
Figure 3 depicts the cumulative number of COPD-related and all-cause events per participant by event type and study group at 1, 3, and 6 months after discharge. The incidence rate ratio of COPD-related events at 6 months for the intervention compared with usual care group was 0.51 (95% CI, 0.41-0.64; P < .001) before adjustment, and 0.63 (95% CI, 0.53-0.76; P < .001) after adjustment for age, home oxygen use, discharge unit, and hospitalization in the prior year. The incidence rates and incidence rate ratios of COPD-related and all-cause events by event type and study group before and after adjustment are depicted in eTable 4 in Supplement 3. The treatment effect was similar after adjustment for age, home oxygen use, discharge unit, and hospitalization in prior year (eTable 4 in Supplement 3).
Figure 3. Cumulative Number of Chronic Obstructive Pulmonary Disease (COPD)–Related and All-Cause Events per Participant by Event Type and Study Group at 1, 3, and 6 Months After Discharge.
The boxes in the graphs show the median and interquartile range (IQR) of the data, with the bottom and top indicating the 25th and 75th percentiles, respectively; the upper whisker extends from the top of the box to the largest value no further than 1.5 times the IQR. The bottom whisker extends from the bottom of the box to the smallest value no further than 1.5 times the IQR; outliers outside the whiskers range are also presented (dots). The circles and triangles indicate the mean number of events for usual care and the intervention, respectively. The black line across the box indicates the median. Boxplots at each time point are staggered to avoid superimposition. Boxplots do not show when the 75th percentile of all data is zero. Whiskers do not show when all data points except for outliers are at zero.
aEmergency department visits that led to a hospitalization are not included in the emergency department visit counts.
The percentage of participants who experienced at least 1 COPD-related acute care event was 38% in the intervention group and 52% in the usual care group (odds ratio, 0.57 [95% CI, 0.34-0.97]; P = .04). Of those participants, 20%, 12%, 3%, and 3% had 1, 2, 3, and 4 or more COPD-related acute care events in the intervention group compared with 22%, 10%, 5%, and 14% in the usual care group, respectively (Fisher exact P = .01).
Table 2 depicts differences in SGRQ domain scores between study groups. There were significant differences in symptom score (adjusted difference, −7.16 [95% CI, −13.39 to −0.93]; P = .04) and impact score (adjusted difference, −7.87 [95% CI, −15.37 to −0.38]; P = .04). The responder analysis showed that at 6 months, the HRQOL improved, stayed the same, or deteriorated among 49%, 24%, and 27% of patients in the intervention group compared with 33%, 19%, and 48% of patients in the usual care group, respectively (Pearson χ2 P = .01). eFigure 3 in Supplement 3 depicts a parallel line plot of change in SGRQ scores by study group. A sensitivity analysis, performed to assess effect of missing values on HRQOL using mixed-effects generalized linear model, allowed for examination of 603 SGRQ total scores across 231 patients and showed findings that were consistent with the primary analysis. (Estimated differences in SGRQ at 6 months compared with baseline for the intervention and usual care groups were −1.42 [95% CI, −5.05 to 2.20] and +5.03 [95 % CI, 0.95 to 9.12], respectively; see the eAppendix and eTable 5 in Supplement 3 for more details.)
Intervention Implementation
The COPD nurse visited 103 of 120 participants at least once in the hospital. Intervention group participants had a mean of 6.1 sessions (interquartile range, 4-8) (eTable 6 in Supplement 3).
Adverse Events
Adverse events were reviewed at 3- to 6-month intervals. There were 337 hospitalizations and 3 falls resulting in acute care visits. No adverse events were attributed to the study intervention.
Discussion
In a single-site randomized clinical trial of patients hospitalized due to COPD, a 3-month program that combined transition and long-term self-management support resulted in significantly fewer COPD-related hospitalizations and ED visits and better HRQOL at 6 months. Studies evaluating discharge bundles for patients with COPD have shown modest effects on reducing hospitalizations and no effects on quality of life.5 COPD self-management interventions, mostly implemented in outpatient settings, have shown similar benefits to this study.11 Prior studies have shown progressive reduction in HRQOL among patients with COPD over time, particularly after COPD exacerbations.13,25 In this study, similar reductions were detected in the usual care group, while patients in the intervention group maintained their HRQOL.
There are several novel aspects to this study. The study intervention was co-developed with patients, caregivers, and stakeholders. It combined transition support with long-term COPD self-management support, and focused on engaging both patients and caregivers. Unlike earlier COPD self-management studies, patients with comorbidities were not excluded and the intervention used an action plan that does not include provision of steroid or antibiotic prescriptions. An earlier study using a COPD action plan with self-initiated treatment was stopped prematurely for concerns of increased mortality.26
There are several features that may have increased program effectiveness. First, starting COPD self-management conversations in a hospitalization due to COPD may have increased patient engagement in his or her care. Second, connecting patients with the COPD nurse while still at the hospital and continuing follow-up for 3 months may have helped with continuity of care and relationship building, and facilitated patient engagement in the intervention. Third, providing support at home or via telephone increased outreach to patients who are more ill and find it difficult to leave home. Fourth, the program was individualized according to patient needs and priorities, allowing flexibility for the nurse to work with the patient on using standardized program tools, rather than following a more rigid and prescriptive intervention approach. In addition, early communications with clinicians, which were encouraged whenever signs of exacerbation were detected, may have provided a mechanism for reducing hospitalizations as clinic-based clinicians may be less likely to admit patients who have an acute COPD exacerbation than ED clinicians.
Limitations
This study has several limitations. First, being a single-site study, the results may not be generalizable to all patients with COPD. Second, the study included a high proportion of low-income and less-educated participants, and these participants may have a greater need for the study’s intervention. Third, for participants unreachable at 6 months after discharge, it was not possible to measure their QOL and verify acute events treated outside the JHHS. However, the number of these participants were similar between study groups and their baseline characteristics were not different from the rest of study participants. Fourth, spirometry evidence of airflow obstruction was not required for enrollment into the study and it is possible that some participants may have been incorrectly diagnosed as having COPD.
Conclusions
In a single-site randomized clinical trial of patients hospitalized due to COPD, a 3-month program that combined transition and long-term self-management support resulted in significantly fewer COPD-related hospitalizations and emergency department visits and better health-related quality of life at 6 months after discharge. Further research is needed to evaluate this intervention in other settings.
Trial Protocol
Statistical Analysis Plan
eTable 1. Comparison of Transitional Support Features of the BREATHE Program and Usual Transitional Care
eTable 2. Select Comorbidities of Study Participants, by Study Group
eTable 3. Comparison of Baseline Characteristics of Participants with Missing Change in SGRQ Scores to Rest of Study Participants
eTable 4. Incidence Rates and Ratios of COPD-related and All-cause Hospitalizations and ED Visits per Participant at 1 Month, 3 Months, and 6 Months Post-discharge
eTable 5. Mean Change in Quality of Life as measured by SGRQ at 3 and 6 months Post-discharge Compared to Baseline
eTable 6. Intervention Implementation
eAppendix. Sensitivity Analysis Examining Intervention Effects on Health-related Quality of Life
eFigure 1. Time to First COPD-Related ED Visit
eFigure 2. Time to First COPD-Related Hospitalization
eFigure 3. Baseline and 6-Month SGRQ Score by Treatment
Data Sharing Statement
References
- 1.Kochanek KD, Murphy SL, Xu JQ, Arias E. Mortality in the United States, 2016: NCHS Data Brief, No. 293. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 2.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance: United States, 1999-2011. Chest. 2013;144(1):284-305. doi: 10.1378/chest.13-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brault MW, Hootman J, Helmick CG, Theis KA, Armour BS; Centers for Disease Control and Prevention (CDC) . Prevalence and most common causes of disability among adults: United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58(16):421-426. [PubMed] [Google Scholar]
- 4.Kumbhare SD, Beiko T, Wilcox SR, Strange C. Characteristics of COPD patients using United States emergency care or hospitalization. Chronic Obstr Pulm Dis. 2016;3(2):539-548. doi: 10.15326/jcopdf.3.2.2015.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ospina MB, Mrklas K, Deuchar L, et al. A systematic review of the effectiveness of discharge care bundles for patients with COPD. Thorax. 2017;72(1):31-39. doi: 10.1136/thoraxjnl-2016-208820 [DOI] [PubMed] [Google Scholar]
- 6.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. doi: 10.7326/0003-4819-155-8-201110180-00008 [DOI] [PubMed] [Google Scholar]
- 7.Prieto-Centurion V, Markos MA, Ramey NI, et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations: a systematic review. Ann Am Thorac Soc. 2014;11(3):417-424. doi: 10.1513/AnnalsATS.201308-254OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926. doi: 10.1016/j.chest.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen PU, Ersgard KB, Soerensen TB, Larsen P. Effectiveness of structured planned post discharge support to patients with chronic obstructive pulmonary disease for reducing readmission rates: a systematic review. JBI Database System Rev Implement Rep. 2017;15(8):2060-2086. doi: 10.11124/JBISRIR-2016-003045 [DOI] [PubMed] [Google Scholar]
- 10.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746-754. doi: 10.1377/hlthaff.2011.0041 [DOI] [PubMed] [Google Scholar]
- 11.Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3):CD002990. doi: 10.1002/14651858.CD002990.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newham JJ, Presseau J, Heslop-Marshall K, et al. Features of self-management interventions for people with COPD associated with improved health-related quality of life and reduced emergency department visits: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:1705-1720. doi: 10.2147/COPD.S133317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice KL, Dewan N, Bloomfield HE, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182(7):890-896. doi: 10.1164/rccm.200910-1579OC [DOI] [PubMed] [Google Scholar]
- 14.Bourbeau J, Julien M, Maltais F, et al. ; Chronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du Québec . Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585-591. doi: 10.1001/archinte.163.5.585 [DOI] [PubMed] [Google Scholar]
- 15.Aboumatar H, Naqibuddin M, Chung S, et al. ; BREATHE Study Patient Family Partners Group . Better Respiratory Education and Treatment Help Empower (BREATHE) study: methodology and baseline characteristics of a randomized controlled trial testing a transitional care program to improve patient-centered care delivery among chronic obstructive pulmonary disease patients. Contemp Clin Trials. 2017;62:159-167. doi: 10.1016/j.cct.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 16.Aboumatar H, Shattuck E An integrative multilevel study for improving patient-centered care delivery among patients with COPD: the BREATHE Study. Presented at: Bringing the Engagement in Research Rubric to Life- Engagement of Patients and Other Stakeholders in Research. PCORI Annual Meeting; Washington, DC; October 8, 2015. [Google Scholar]
- 17.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 18.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398-404. doi: 10.1183/09031936.02.00063702 [DOI] [PubMed] [Google Scholar]
- 19.Chapman KR, Bergeron C, Bhutani M, et al. Do we know the minimal clinically important difference (MCID) for COPD exacerbations? COPD. 2013;10(2):243-249. doi: 10.3109/15412555.2012.733463 [DOI] [PubMed] [Google Scholar]
- 20.Calverley PM. Minimal clinically important difference: exacerbations of COPD. COPD. 2005;2(1):143-148. doi: 10.1081/COPD-200050647 [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250-255. doi: 10.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]
- 22.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748-1755. doi: 10.1378/chest.06-3018 [DOI] [PubMed] [Google Scholar]
- 23.Hurst JR, Vestbo J, Anzueto A, et al. ; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators . Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128-1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 24.Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation: systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2(3):241-251. [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23(5):698-702. doi: 10.1183/09031936.04.00121404 [DOI] [PubMed] [Google Scholar]
- 26.Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156(10):673-683. doi: 10.7326/0003-4819-156-10-201205150-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Comparison of Transitional Support Features of the BREATHE Program and Usual Transitional Care
eTable 2. Select Comorbidities of Study Participants, by Study Group
eTable 3. Comparison of Baseline Characteristics of Participants with Missing Change in SGRQ Scores to Rest of Study Participants
eTable 4. Incidence Rates and Ratios of COPD-related and All-cause Hospitalizations and ED Visits per Participant at 1 Month, 3 Months, and 6 Months Post-discharge
eTable 5. Mean Change in Quality of Life as measured by SGRQ at 3 and 6 months Post-discharge Compared to Baseline
eTable 6. Intervention Implementation
eAppendix. Sensitivity Analysis Examining Intervention Effects on Health-related Quality of Life
eFigure 1. Time to First COPD-Related ED Visit
eFigure 2. Time to First COPD-Related Hospitalization
eFigure 3. Baseline and 6-Month SGRQ Score by Treatment
Data Sharing Statement