Key Points
Question
Is a 3-dimensionally printed, bioresorbable nasal implant safe and effective in caudal septal reconstruction?
Findings
In this multicenter clinical trial of 20 patients with septal deviations who underwent septoplasty, the 3-dimensionally printed polycaprolactone nasal implants demonstrated proper mechanical support and thinness as well as excellent biocompatibility and surgical manipulability.
Meaning
Polycaprolactone could serve as a clinically biocompatible material in various craniofacial reconstructions in the future.
Abstract
Importance
Studies have shown the controllability and porosity of polycaprolactone as well as the use of 3-dimensional (3-D) printing for nasal reconstruction in animal models. The utility of polycaprolactone with 3-D technology in nasal cartilaginous framework reconstruction in humans remains unknown.
Objective
To investigate the safety and efficacy of 3-D printed, bioresorbable polycaprolactone nasal implants.
Design, Setting, and Participants
This multicenter clinical trial comprised 20 patients with caudal septal deviations who underwent septoplasty, which used a 3-D printed polycaprolactone mesh, at 2 centers in South Korea. Patients were included if they were aged 18 to 74 years and had nasal septal deviations, Nasal Obstruction Symptom Evaluation scores greater than 20, and persistent nasal obstructions. Twenty-two patients met the inclusion criteria, but 2 patients were excluded before the operation. The study was conducted from July 1, 2016, to June 30, 2017.
Main Outcomes and Measures
The change in total Nasal Obstruction Symptom Evaluation score between the preoperative examination and the week 12 postoperative examination was the primary outcome. Changes in bilateral nasal cavity minimum cross-sectional area and volume on acoustic rhinometry at weeks 4 and 12 after the operation as well as changes in the nasal cavity cross-sectional area at the osteomeatal unit and nasal septum angle in the paranasal sinus on computed tomography after week 12 were among the secondary outcomes.
Results
Of the 20 patients included in the study, 4 (20%) were female, 16 (80%) were male, with a mean (SD) age of 34.95 (11.96) years. The preoperative and week 12 postoperative results revealed significant changes in the minimal cross-sectional areas on acoustic rhinometry (0.41 [SD, 0.39] vs –0.11 [SD, 0.18]; difference, 0.42; 95% CI, 0.23-0.61), nasal septum angles on computed tomography (11.22 [SD, 6.57] vs 2.89 [SD, 3.12]; difference, 8.33; 95% CI, 5.08-11.58), and Nasal Obstruction Symptom Evaluation scores (73.50 [SD, 19.88] vs 3.75 [SD, 6.26]; difference, 69.75; 95% CI, 59.22-80.28). The surgeons’ convenience level with the procedure was favorable (visual analog scale score [SD], 90.90 [9.45]), and so were the patients’ symptom improvements and satisfaction after 12 weeks (visual analog scale score [SD], 88.30 [9.87]).
Conclusions and Relevance
The 3-D printed, homogeneous, composite, microporous polycaprolactone nasal implant demonstrated proper mechanical support and thinness with excellent biocompatibility and surgical manipulability. Polycaprolactone may be a clinically biocompatible material for use in various craniofacial reconstructions in the future.
This multicenter study examines the clinical application of a 3-D printed biomaterial implant in septoplasty for patients with caudal septal deviations.
Introduction
Nasal reconstruction presents a substantial challenge to otolaryngologists and facial plastic surgeons. Autologous septal cartilage is accepted as the criterion standard nasal grafting material. However, trauma, infection, severely deviated septal cartilage, and previous septal operation often render adequate septal cartilage unavailable for the required reconstruction. Moreover, the acquisition of cartilage is associated with complications, such as saddle nose. Other autologous cartilage donor sources are associated with more donor-site morbidity than septal cartilage sources. In addition, auricular cartilage has an innate curvilinear shape, and costal cartilage is associated with an unpredictable degree of absorption and warping.1 Therefore, various alloplastic materials have been developed to replace autologous implants. However, despite advances in alloplastic materials, alloplastic materials are still associated with infection, extrusion, implant shifts, surgical manipulation problems, and poor biocompatibility.2
Three-dimensional (3-D) printing technology has been used in a variety of medical fields, including plastic surgery and craniofacial reconstruction.3,4 The advantage of 3-D printing is its capability to not only produce patient-specific scaffold but also design homogeneous standardized microporous composite scaffold. Synthetic polymers may not precisely mimic the properties of the native scaffold but do allow for greater control in their fabrication process and more reliable reproducibility.5 Therefore, the material properties, bioactivities, and porosities of synthetic grafts can be controlled and customized for specific applications. Recently, several animal models, including that of the present preclinical study, have demonstrated that 3-D tissue engineering technology may solve the nasal cartilage problem.6,7 Previous animal tests have demonstrated that 3-D printed mesh properly supports the nasal septum after the operation, and the surrounding tissue is well-fused between the pores without inflammatory responses.6 Using the existing knowledge regarding the controllability and porosity of polycaprolactone (PCL) properties and the use of 3-D printing for nasal reconstruction in animal models, we conducted a multicenter clinical trial involving patients with nasal septal deformities to evaluate the efficacy and safety of a PCL mesh.
To our knowledge, this is the first study to use 3-D printing to investigate the safety and efficacy of a bioresorbable PCL nasal implant with a homogeneous composite porous microarchitecture for nasal cartilaginous framework reconstruction. Furthermore, to our knowledge, this study is the first to report multicenter clinical results of the application of a 3-D printed biomaterial.
Methods
This clinical trial (ClinicalTrials.gov Identifier: NCT03088618) was approved by the institutional review boards of the Seoul St Mary’s Hospital and the Bucheon St Mary’s Hospital (South Korea), and it was conducted in accordance with the Declaration of Helsinki.8 Written informed consent was obtained from all patients (n = 22) before they were recruited. This study was conducted from July 1, 2016, to June 30, 2017.
Preparation of the PCL Mesh Using 3-D Printing Technology
Polycaprolactone (Puracsorb PC, Corbion Purac Biomaterials) was used to fabricate a mesh using a 3-D printing system (3DP Printer, T&R Biofab Co Ltd). The 3-D printing system was operated at a temperature of 120°C. The fabricated PCL mesh (TnR Mesh, T&R Biofab Co Ltd) consisted of twelve 30 × 10 × 1-mm layers with triangular pores, and its line width and pore size were both 500 μm. The mesh was irradiated with gamma rays at 15 kGy.
Preparation of the Porcine Septal Cartilage
The head of a pig slaughtered for food was purchased. The nose was separated from the head, and the nasal bone and soft tissue near the porcine septal cartilage were removed using a blade and scissors. The bending strength was tested using a specimen from the perichondrium with the septal cartilage, and a specimen from the perichondrium was removed with a blade.
Scanning Electron Microscope Analysis of the PCL Mesh
The morphological structure of the 3-D printed PCL mesh was confirmed using a field emission scanning electron microscope (S-4700, Hitachi) with an acceleration voltage of 10 kV. The mesh was coated with platinum for 3 minutes using a sputter coater.
In Vitro Analysis of the PCL Mesh Characteristics
Tensile Strength Testing
A test of the tensile strength of the PCL mesh was performed using a universal testing instrument (Model 3343, Instron) operated at a constant speed of 1 mm · min−1. During the test, the load and displacement were monitored. Two different groups of PCL mesh were used to study how exposures to body fluid and blood affected tensile strength. The mesh was placed in a plastic container with saline solution for 30 minutes, and the hydrated group was compared with the unhydrated group.
Bending Strength Testing
In the bending strength test, porcine septal cartilage and PCL mesh were compared to determine whether the mesh exhibited a strength that corresponded to that of the porcine septal cartilage. The test was performed using a universal testing instrument and an acrylonitrile butadiene styrene jig. The jig was designed and manufactured using a fused deposition modeling 3-D printer (SE 3D printer, Stratasys). The porcine septal cartilage, measuring 30 mm in length and 10 mm in width, was obtained from the head of a 6-month-old pig.
Clinical Trial
Study Population and Eligibility Criteria
To be eligible for inclusion, patients had to meet the following criteria: aged 18 to 74 years and with nasal septal deviations (especially caudal septal cartilage deviations), scores greater than 20 on the Nasal Obstruction Symptom Evaluation (NOSE) scale9 (score range: 0-100, with the highest score indicating severe problem), and nasal obstructions persisting for more than 3 months and symptoms persisting for more than 4 weeks despite proper medical treatment. Women with childbearing potential were required to have a negative urine pregnancy test and to have no plan to become pregnant during the course of the study.
The exclusion criteria were as follows: known allergic reaction to PCL medical devices; pregnancy or lactation; history of surgical procedure in the nasal or paranasal sinuses prior to screening or radiation treatment in the head and neck; history of participation in other clinical trials of drugs or medical devices within 3 months; untreated nasal bone fractures or trauma; surgical-site infection caused by nasal bone fractures or trauma; untreated perforation of the septum, sinusitis, nasal cavity polyposis, nasal cavity sarcoidosis, nasal valve collapse, or Wegener granulomatosis; and inflammation in the nasal cavity, a sarcoma or carcinoma in the nasal cavity, asthma, untreated palate-facial disfigurement or cleft palate, systemic inflammatory disease, or sepsis.
Twenty-two patients who met the inclusion criteria were enrolled. Of the 22 patients, 2 patients (9%) were excluded. One patient was taking cold medicine that included pseudoephedrine hydrochloride, and the other patient refused general anesthesia just before the operation. Twenty patients were included in the study and underwent septoplasty at 2 centers (Seoul St Mary’s Hospital and Bucheon St Mary’s Hospital, South Korea).
Outcomes and Assessments
The primary outcome was the change in total NOSE score between the examination before the operation and at week 12 after the operation. Secondary outcomes were as follows: NOSE scores by topic at weeks 4 and 12 after the operation; changes in the bilateral nasal cavity minimal cross-sectional area on acoustic rhinometry at weeks 4 and 12 after the operation; changes in the bilateral nasal cavity volume on acoustic rhinometry at weeks 4 and 12 after the operation; changes in the nasal cavity cross-sectional area (at the osteomeatal unit level in the coronal section) on computed tomography at week 12 after the operation; changes in the nasal septum angle4 (at the most deviated section in the coronal plane) on computed tomography at week 12 after the operation; and surgeon’s comfort level with performing the operation (with 0 indicating very uncomfortable and 100 indicating very comfortable) as well as patient’s level of symptom improvement and satisfaction with the procedure (with 0 indicating no symptom change and dissatisfaction, and 100 indicating resolved symptom and satisfaction) on the visual analog scale.
Surgical Technique
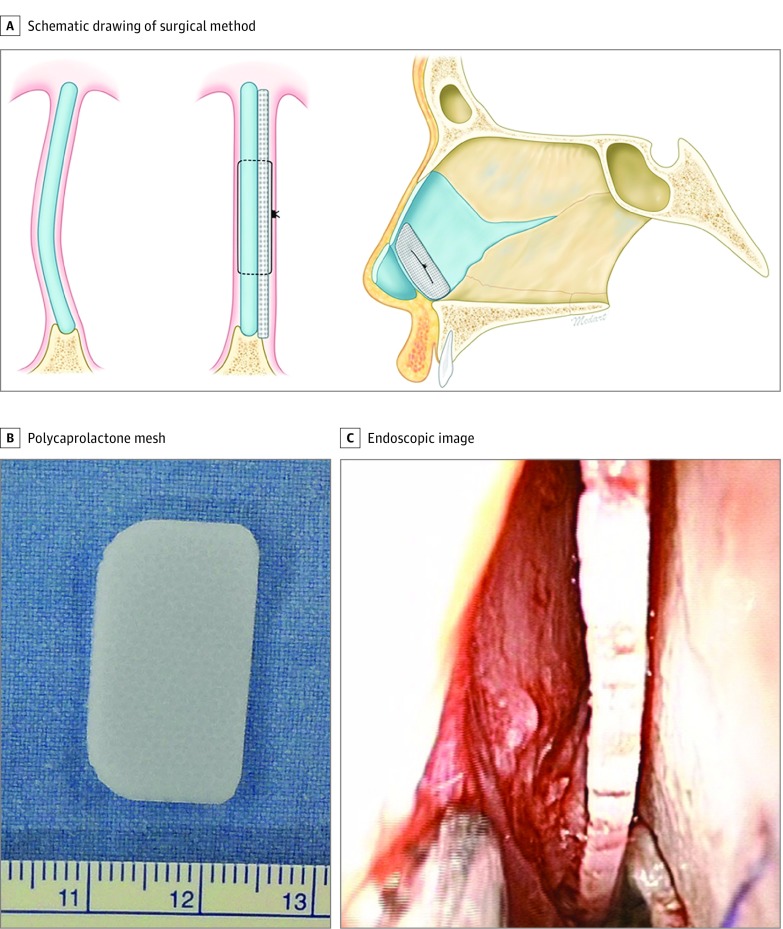
After general anesthesia was administered, we injected lidocaine hydrochloride, 1%, with epinephrine into the mucosa for the hydrodissection of the mucoperichondrium of the septal cartilage. A hemitransfixion incision was created with a No. 15 scalpel blade at the caudal septum. A mucoperichondrial flap was raised broadly from the cartilaginous septum to the perpendicular plate of the ethmoid, vomer, and maxillary crest. A contralateral mucoperichondrial flap was raised through the bone-cartilage junction. The PCL mesh was fashioned into a batten graft with sufficient length to span the distance from the anterior nasal spine to the cartilaginous dorsum. After all of the areas of the caudal septal deviation had been adequately addressed, the prepared mesh was placed in the caudal septum. The mesh was placed in the concave side of the caudal septal cartilage, and the mesh and the septum were attached together with size 5-0 polydioxanone sutures (Figure 1). Subsequently, the caudal septum complex was affixed to the anterior nasal spine with polydioxanone sutures, and quilting sutures were placed through the mucosal layers using size 5-0 polydioxanone sutures.
Figure 1. Schematic Drawing of the Surgical Method and Gross Morphological Structure of the Polycaprolactone Mesh.
A, The mesh was placed in the concave side of the caudal septal cartilage, and the mesh and septum were sutured together. This schematic drawing by Kwan Hyun Youn, PhD, is reproduced with permission. B, The mesh before insertion. The ruler is measured in millimeters. C, An intraoperative endoscopic image of the positioning of the mesh at the concave side of the caudal septal cartilage.
Sample Size Calculation and Statistical Analysis
The sample size calculations are published elsewhere.10,11,12,13 The changes between the preoperative and postoperative NOSE scores in previous studies ranged from 23.75 to 48.92, with a pooled mean (SD) of 37.18 (24.13). In this clinical trial, the expected change in the mean (SD) NOSE score was 37.00 (25.00). The minimum expected postoperative variation was set to 15 (95% CI, 14.79-32.71), which is superior to the expected results in the literature.10,11,12,13 Considering a 20% dropout rate, a total of 22 patients were included in the sample of this study.
Paired, 2-tailed t tests or Wilcoxon signed rank tests were used to compare the preoperative and postoperative values. Effect size metrics were used to describe the differences, and 95% CIs14,15 were used to describe the precision of the estimates and, where possible and appropriate, to determine the compatibility of data with clinically meaningful effects. Cohen d was used to describe the magnitude of the effect: d = 0.2 was considered a small effect size; 0.5, medium; and 0.8, large. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
In total, 20 patients underwent septoplasty that used a 3-D printed polycaprolactone mesh. Of these 20 patients, 4 (20%) were female, 16 (80%) were male, with a mean (SD) age of 34.95 (11.96) years.
The PCL line of the final version of the mesh was 500 μm. The interconnected triangular pores had 50% porosity, which played an important role in the induction of the ingrowth of the surrounding tissues.
Tensile Strength Test Results
In the tensile strength tests, 2 different groups (hydrated [mesh with saline solution] and unhydrated [mesh without saline solution]) were compared. The mean (SD) tensile strength of the hydrated group (n = 5) was 7.83 (0.47) MPa (megapascal) and the unhydrated group (n = 5) was 8.27 (0.50) MPa. The difference between the 2 groups was 0.44 MPa (95% CI, –0.268 to 1.148 MPa), and the Cohen d was 0.9, suggesting a large effect size.
Bending Strength Results
Bending strength tests were performed. The bending test process applied to the PCL mesh and porcine septal cartilage. The mean (SD) bending strength of the porcine septal cartilage without perichondrium was 5.46 (0.48) MPa. The porcine septal cartilage with perichondrium exhibited a mean (SD) bending strength of 6.32 (1.70) MPa. The difference between the bending strength was 0.86 MPa (95% CI, −0.962 to 2.682 MPa) and the Cohen d was 0.7, suggesting a large effect size. The mean (SD) bending strength of the PCL mesh was 24.10 (1.06) MPa; the difference in bending strength between this and the porcine septal cartilage without perichondrium was 18.64 MPa (95% CI, 17.44-19.84 MPa), and the Cohen d was 22.6, indicating a very large effect size. The difference in bending strength between the PCL mesh and the porcine septal cartilage with perichondrium was 17.78 MPa (95% CI, 15.71-19.85 MPa), and the Cohen d was 12.5.
Clinical Outcomes
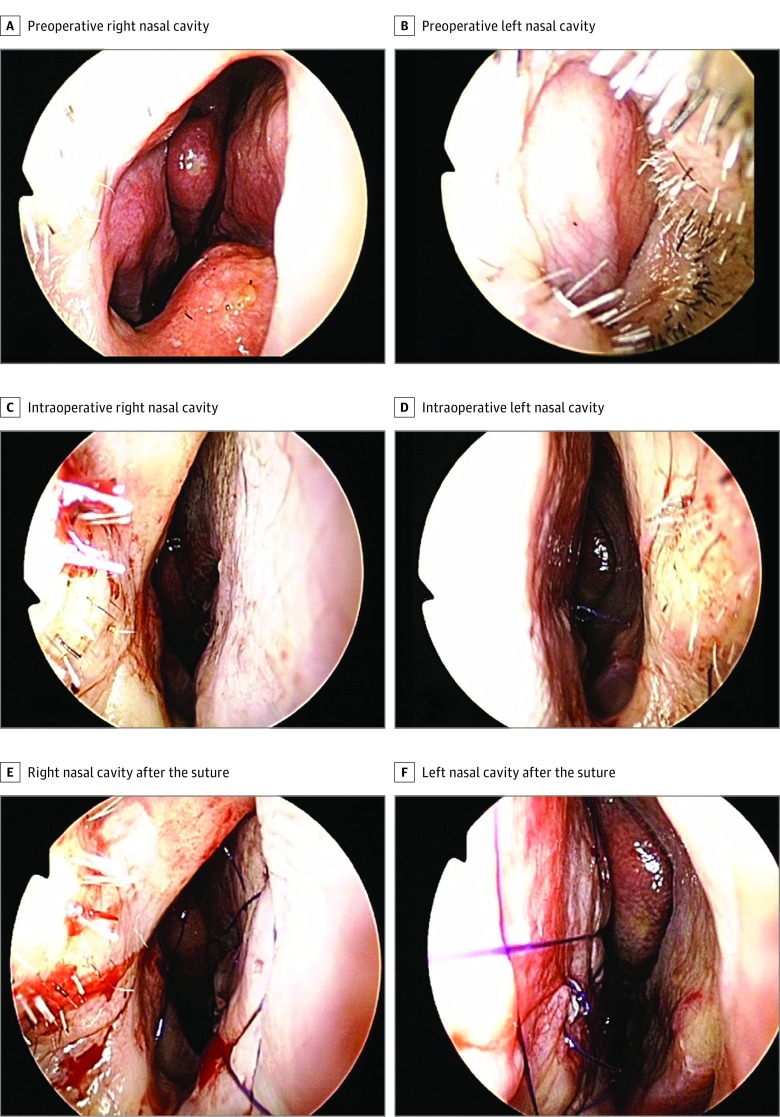
Figure 2A provides intraoperative images of the caudal septal correction using the PCL mesh. Preoperative and postoperative endoscopic (Figure 2B and C) and paranasal sinus computed tomographic (Figure 3) images revealed good surgical results, and the mesh was easily combined with the quilting sutures during the operations. The preoperative and week 12 postoperative examination results revealed a substantial difference of changes in the minimal cross-sectional area on acoustic rhinometry (0.41 [SD, 0.39] vs –0.11 [SD, 0.18]; difference, 0.42; 95% CI, 0.23-0.61) and Cohen d for paired data of 1.05; in the nasal septum angle at the paranasal sinus on computed tomography (11.22 [SD, 6.57] vs 2.89 [SD, 3.12]; difference, 8.33; 95% CI, 5.08-11.58) and Cohen d of 1.20; and in the NOSE scale score (73.50 [SD, 19.88] vs 3.75 [SD, 6.26]; difference, 69.75; 95% CI, 59.22-80.28) and Cohen d of 3.10 (Table). No complications were reported during the study. Moreover, the convenience level of the surgeons (visual analog scale score [SD], 90.90 [9.45]) and the symptom improvement and satisfaction of the patients (visual analog scale score [SD], 88.30 [9.87]) at 12 weeks after the operation exhibited favorable results.
Figure 2. Preoperative and Intraoperative Endoscopic Images.
Preoperative endoscopic images of the right (A) and left (B) nasal cavity. Intraoperative endoscopic images after implant: right (C) and left (D) nasal cavity before the quilting suture and right (E) and left (F) nasal cavity after the suture.
Figure 3. Computed Tomographic Coronal Images of the Paranasal Sinus .
Table. Preoperative and Postoperative Results.
| Variable | Preoperative to Week 4 Postoperative | Preoperative to Week 12 Postoperative | ||
|---|---|---|---|---|
| Absolute Difference (95% CI) | Cohen d | Absolute Difference (95% CI) | Cohen d | |
| Acoustic rhinometry | ||||
| MCA, mm | 0.40 (0.20-0.59) | 0.95 | 0.42 (0.23 to 0.61) | 1.05 |
| Volume, mm3 | 1.36 (0.87-3.17) | 0.35 | 0.84 (−0.84 to 2.52) | 0.23 |
| PNS CT | ||||
| OMU, mm2 | NA | NA | 19.92 (−31.70 to 71.54) | 0.18 |
| Deviated angle, degrees | NA | NA | 8.33 (5.08 to 11.58) | 1.20 |
| NOSE scale scorea | 55.75 (41.50-70.00) | 1.83 | 69.75 (59.22 to 80.28) | 3.10 |
Abbreviations: MCA, minimum cross-sectional area; NA, not applicable; NOSE, Nasal Obstruction Symptom Evaluation; OMU, osteomeatal unit; PNS CT, paranasal sinus computed tomography.
The NOSE scale scores range from 0 to 100; higher scores indicate a more severe problem.
Discussion
Septoplasty or nasal functional reconstruction is one of the most commonly performed operations in the world.16,17 It usually requires reestablishing the shape and integrity of the nasal framework and correcting the caudal septal deviation or dislocation.18 Many surgeons prefer autologous nasal septal cartilage for nasal reconstruction.19 However, nasal septal cartilage harvesting weakens the supporting structure of the nasal framework and, over time, causes nasal dorsum collapse because of mechanical tension and loading from the skin and soft tissue.6,20 Even with the use of septal batten grafts with septal cartilage during septoplasty or rhinoplasty, several cases of saddle nose (resulting from the inability to withstand the dorsal nasal weight and tension) have been reported. In addition, because of the thickness of the cartilage strut, the internal nasal valve tends to narrow. Therefore, as an alternative to autologous septal cartilage, the ideal alloplastic material for nasal septal reconstruction should be sufficiently strong to resist the inherent septal coiling power and to provide mechanical support. However, material that is too hard might cause nasal tip necrosis and decrease surgical manipulation. The material should be sufficiently thin to avoid affecting the internal nasal valve. Furthermore, it should exhibit excellent biocompatibility to facilitate the rapid ingrowth of host tissue and significant integration with the surrounding soft tissue.21
To date, several alloplastic implants have been introduced to replace autologous implants. Silicone, Gore-Tex (W. L. Gore and Associates), Supramid (Ethicon), Proplast (Vitek), Mersiline (Ethicon), and Medpor (Stryker Corporate) are currently available and have been used for nasal reconstruction with variable success rates. Polydioxanone flexible plates (Ethicon) have also been used for nasal septal reconstruction.
Some materials are too stiff and require several holes for sutures, which cannot be placed near the nasal tip area because nasal tip necrosis may develop. Other materials are so flexible that they require a nasal septal cartilage buttress for reconstruction. Most materials are not sufficient in terms of the ease of surgical manipulation. Therefore, the demand for a new material for nasal implants and craniofacial reconstruction has been consistent.
Polycaprolactone has been widely used for drug delivery devices and in the tissue engineering field because it is biocompatible and slowly degrades in the body.9 The long-term biodegradability of PCL mesh can resist deformations because of contractions of the skin and scars that occur during the healing process. This mesh is completely absorbed after several years, which is helpful for avoiding skin thinning and ulcerations from excessive local mechanical pressure.6 In addition, PCL is an ideal material for application in scaffolds and implants because it can be melted at low temperatures without the need for dissolution in toxic solvents. It is also ideal for the 3-D printing process because this technology enables the precise control of material properties, shape, bioactivity, and porosity as well as customization and reproducibility for specific applications.20 Therefore, proper mechanical property, fibrovascular ingrowth degree, and suture-friendly microporous design can be controlled by the 3-D printing technique.
A previous preclinical animal study and additional subsequent in vitro tests found that 50% of these interconnected triangular pores were appropriate for implants and were associated with accelerated healing.6 Subsequently, the proper porosity for enhancing human nasal septal cartilage growth and improved cartilage properties in terms of being thinner, maintaining tensile strength on exposure to body fluids and blood, being more bend-resistant, and exhibiting enhanced surgical manipulability were found. The microporosity of PCL controlled by 3-D printing promotes rapid fibrovascular ingrowth without granulation-tissue formation.6 Once fibrovascular ingrowth occurs, the mesh is stabilized, which decreases the likelihood of extrusion and/or infection of the implant.22 After the clinical trial, we plan to obtain operative results that include the maintenance of the planned structural shape without complications and patient satisfaction at up to 12 weeks of follow-up. In the present study, we targeted patients with intractable caudal septal deviations and used 3-D printing technology to optimize a septal batten graft material. The minimal cross-sectional area on acoustic rhinometry and deviated angle results, which directly affect the internal nasal valve and the patient’s nasal symptoms, had excellent outcomes. In contrast, the osteomeatal unit dimension and nasal cavity volume exhibited relatively smaller changes. Regarding surgical convenience, this 3-D printed PCL mesh does not require holes for anchoring or quilting sutures. The mesh was easily affixed to the nasal septum with various suture techniques during the operation.
Limitation
One important limitation of this study was the relatively short follow-up duration. Previous reports on patients with implanted cartilage grafts after a long-term follow-up have demonstrated that the grafts have been completely replaced with fibrous tissue without external structural changes.23 A clinical trial with long-term follow-up is necessary to determine the outcome after complete resorption of the mesh has occurred.
Conclusions
In this clinical trial, the 3-D printed microporous PCL nasal implant exhibited appropriate mechanical support and thinness and excellent biocompatibility and surgical manipulability. Polycaprolactone may serve as a clinically biocompatible material in various craniofacial reconstructions in the future.
References
- 1.Chang AA, Reuther MS, Briggs KK, et al. In vivo implantation of tissue-engineered human nasal septal neocartilage constructs: a pilot study. Otolaryngol Head Neck Surg. 2012;146(1):46-52. doi: 10.1177/0194599811425141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HS, Park SS, Kim MH, Kim MS, Kim SK, Lee KC. Problems associated with alloplastic materials in rhinoplasty. Yonsei Med J. 2014;55(6):1617-1623. doi: 10.3349/ymj.2014.55.6.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-Dimensional Printing and Its Applications in Otorhinolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi: 10.1177/0194599816678372 [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Jang J, Lee J-S, Cho D-W. Current advances in three-dimensional tissue/organ printing. Tissue Eng Regen Med. 2016;13(6):612-621. doi: 10.1007/s13770-016-8111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grayson WL, Fröhlich M, Yeager K, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107(8):3299-3304. doi: 10.1073/pnas.0905439106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SH, Yun BG, Won JY, et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017;127(5):1036-1043. doi: 10.1002/lary.26400 [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Fan F, Kang N, et al. Tissue engineering of human nasal alar cartilage precisely by using three-dimensional printing. Plast Reconstr Surg. 2015;135(2):451-458. doi: 10.1097/PRS.0000000000000856 [DOI] [PubMed] [Google Scholar]
- 8.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 9.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157-163. doi: 10.1016/j.otohns.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 10.Erickson B, Hurowitz R, Jeffery C, et al. Acoustic rhinometry and video endoscopic scoring to evaluate postoperative outcomes in endonasal spreader graft surgery with septoplasty and turbinoplasty for nasal valve collapse. J Otolaryngol Head Neck Surg. 2016;45:2. doi: 10.1186/s40463-016-0115-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahveci OK, Miman MC, Yucel A, Yucedag F, Okur E, Altuntas A. The efficiency of Nose Obstruction Symptom Evaluation (NOSE) scale on patients with nasal septal deviation. Auris Nasus Larynx. 2012;39(3):275-279. doi: 10.1016/j.anl.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Kim JN, Choi HG, Kim SH, et al. The efficacy of bioabsorbable mesh as an internal splint in primary septoplasty. Arch Plast Surg. 2012;39(5):561-564. doi: 10.5999/aps.2012.39.5.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004;130(3):283-290. doi: 10.1016/j.otohns.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 14.Cumming G. The new statistics: why and how. Psychol Sci. 2014;25(1):7-29. doi: 10.1177/0956797613504966 [DOI] [PubMed] [Google Scholar]
- 15.Ellis PD. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results. Cambridge, UK: Cambridge University Press. 2015;1:40-41. [Google Scholar]
- 16.Eskandarlou M, Motamed S. Evaluation of frequency of four common nasal anatomical deformities in primary rhinoplasty in a Tehran plastic surgery center. World J Plast Surg. 2014;3(2):122-128. [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh A, Friedman O. Surgical treatment of nasal obstruction in rhinoplasty. Clin Plast Surg. 2016;43(1):29-40. doi: 10.1016/j.cps.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Watson D. Tissue engineering for rhinoplasty. Facial Plast Surg Clin North Am. 2009;17(1):157-165, viii. doi: 10.1016/j.fsc.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 19.Maas CS, Monhian N, Shah SB. Implants in rhinoplasty. Facial Plast Surg. 1997;13(4):279-290. doi: 10.1055/s-0028-1082427 [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Lee DC, Ha DH, Kim SW, Cho DW. Redefining the septal L-strut to prevent collapse. PLoS One. 2016;11(4):e0153056. doi: 10.1371/journal.pone.0153056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romo T III, Sclafani AP, Jacono AA. Nasal reconstruction using porous polyethylene implants. Facial Plast Surg. 2000;16(1):55-61. doi: 10.1055/s-2000-7326 [DOI] [PubMed] [Google Scholar]
- 22.Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1(1):3-13. doi: 10.1089/ten.1995.1.3 [DOI] [PubMed] [Google Scholar]
- 23.Lee DC, Shin JH, Kim SW, et al. Anatomical analysis of nasal obstruction: nasal cavity of patients complaining of stuffy nose. Laryngoscope. 2013;123(6):1381-1384. doi: 10.1002/lary.23841 [DOI] [PubMed] [Google Scholar]