Key Points
Question
Do maternal plasma cholesterol levels influence the epigenetic signature of genes that are relevant for cholesterol metabolism and atherogenesis in fetal aortas?
Findings
In this autopsy study of 78 fetal human aorta samples from a single institution, maternal cholesterol level was inversely associated with the methylation of SREBP2 promoter.
Meaning
The influence of maternal cholesterol levels during pregnancy on early atherogenesis in fetal life could involve epigenetic mechanisms, and fetal epigenetic signatures could influence the risk of atherosclerosis in the adult offspring of mothers with hypercholesterolemia and their possible development of cardiovascular disease.
This autopsy study tests whether epigenetic signatures characterize early fetal atherogenesis associated with maternal hypercholesterolemia and provides a quantitative estimate of the contribution of maternal cholesterol level to fetal lesion size.
Abstract
Importance
Although increasingly strong evidence suggests a role of maternal total cholesterol and low-density lipoprotein cholesterol (LDLC) levels during pregnancy as a risk factor for atherosclerotic disease in the offspring, the underlying mechanisms need to be clarified for future clinical applications.
Objective
To test whether epigenetic signatures characterize early fetal atherogenesis associated with maternal hypercholesterolemia and to provide a quantitative estimate of the contribution of maternal cholesterol level to fetal lesion size.
Design, Setting, and Participants
This autopsy study analyzed 78 human fetal aorta autopsy samples from the Division of Human Pathology, Department of Advanced Biomedical Sciences, Federico II University of Naples, Naples, Italy. Maternal levels of total cholesterol, LDLC, high-density lipoprotein cholesterol (HDLC), triglycerides, and glucose and body mass index (BMI) were determined during hospitalization owing to spontaneous fetal death. Data were collected and immediately processed and analyzed to prevent degradation from January 1, 2011, through November 30, 2016.
Main Outcomes and Measurements
Results of DNA methylation and messenger RNA levels of the following genes involved in cholesterol metabolism were assessed: superoxide dismutase 2 (SOD2), low-density lipoprotein receptor (LDLR), sterol regulatory element binding protein 2 (SREBP2), liver X receptor α (LXRα), and adenosine triphosphate–binding cassette transporter 1 (ABCA1).
Results
Among the 78 fetal samples included in the analysis (59% male; mean [SD] fetal age, 25 [3] weeks), maternal cholesterol level explained a significant proportion of the fetal aortic lesion variance in multivariate analysis (61%; P = .001) independently by the effect of levels of HDLC, triglycerides, and glucose and BMI. Moreover, maternal total cholesterol and LDLC levels were positively associated with methylation of SREBP2 in fetal aortas (Pearson correlation, 0.488 and 0.503, respectively), whereas in univariate analysis, they were inversely correlated with SREBP2 messenger RNA levels in fetal aortas (Pearson correlation, −0.534 and −0.671, respectively). Epivariations of genes controlling cholesterol metabolism in cholesterol-treated human aortic endothelial cells were also observed.
Conclusions and Relevance
The present study provides a stringent quantitative estimate of the magnitude of the association of maternal cholesterol levels during pregnancy with fetal aortic lesions and reveals the epigenetic response of fetal aortic SREBP2 to maternal cholesterol level. The role of maternal cholesterol level during pregnancy and epigenetic signature in offspring in cardiovascular primary prevention warrants further long-term causal relationship studies.
Introduction
Prevention of atherosclerosis and associated atherosclerotic cardiovascular diseases (including ischemic stroke, aortic and/or carotid atherosclerosis, coronary heart disease, and peripheral artery disease) is fundamental to promote healthy longevity.1 The presence of early stages of atherosclerosis in fetuses and youth indicates the potential importance of adopting preventive measures at very early stages of human development.2,3,4 Moreover, cardiovascular risk factors may be associated with atherosclerotic disease at a young age, and maternal hypercholesterolemia may be associated with atherosclerotic disease in the fetus or infant offspring.2,3,5,6 In addition, maternal diet and birth weight affect the lifetime development of coronary heart disease.7 Recently, the Framingham Heart Study demonstrated that low-density lipoprotein cholesterol (LDLC) levels of adult offspring were associated with maternal prepregnancy LDLC levels independently of anthropometric, lifestyle, and genetic factors.8
Experimental models provide compelling evidence about the risk of fetal exposure to elevated maternal plasma cholesterol levels. In particular, low-density lipoprotein receptor (LDLR) knockout mice, which were exposed in utero to maternal hypercholesterolemia, developed early atherosclerosis and altered aortic gene expression.9 The exposure to maternal hypercholesterolemia was also a risk factor for susceptibility to postnatal atherosclerosis in the offspring of apolipoprotein E–deficient mice.10 Furthermore, treatment of hypercholesterolemic pregnant mice with statins lowered the level of cardiovascular risk factors in the offspring.11
Epigenetic mechanisms could be central mediators in the onset and development of metabolic diseases and subsequent cardiovascular disease.12,13,14,15 Such mechanisms are defined as mitotic and meiotic changes in gene expression that do not involve DNA sequence changes. In particular, fetal adaption to maternal environment may involve epigenetic reprogramming, which could play a role in primary prevention of atherosclerosis.12,16,17 Herein, we analyze epigenetic modifications of a set of genes related to cholesterol levels and atherogenesis in human fetal aortas and the association of such changes with maternal cholesterol levels and atherosclerotic lesion size.
Methods
Fetal Samples
Autopsy samples of fetal aortas (n = 78) were obtained from spontaneously aborted fetuses from January 1, 2011, through November 30, 2016. Fetuses routinely underwent autopsy at the Department of Human Pathology of Federico II University of Naples, Naples, Italy. Causes of spontaneous abortion and premature death included trauma, eclampsia, and major fetal genetic defects (often associated with cerebral malformations). Mean (SD) maternal age at spontaneous fetal death was 35 (5) years. Maternal levels of total cholesterol, LDLC, high-density lipoprotein cholesterol (HDLC), triglycerides, and glucose and maternal body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) were determined during hospitalization owing to spontaneous fetal death. The LDLC level was calculated with the Friedewald formula ([Total Cholesterol Level − HDLC Level − Triglyceride Level]/5) in milligrams per deciliter (to convert cholesterol levels to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.113). The extension of the ongoing research protocol was approved by the institutional review board of the Federico II University of Naples on the basis of the first approved research protocol (#09/03),2,3,17,18,19,20 and informed written consent was obtained from each mother.
Fetuses from mothers with types 1 and 2 diabetes and diseases affecting hematopoiesis or the immune system (including human immunodeficiency virus infection) were excluded from the study. We also excluded fetuses with cerebral ischemic processes.
Preparation of Aortic Sections
Samples were immediately processed after collection to prevent their degradation. Fetal aortas were exposed, the branching arteries were cut off, and loose adventitial tissue was removed in situ. Isolated aortas were then cut open, thoroughly washed with ice-cold, sterile phosphate-buffered saline solution (PBS) containing 2mM EDTA to remove adherent blood cells, and placed in ice-cold PBS containing 50mM butylated hydroxytoluene, 0.001% aprotinin, 50mM EDTA, and 0.008% chloramphenicol. Samples were immersed in optimum cutting temperature medium and flash frozen in liquid nitrogen. Portions of each tissue sample were sectioned with a cryotome, and the resulting 5- to 7-μm-thick sections were prepared for immunohistochemistry by fixation in buffered 10% formalin and paraffin embedding. To extract total DNA and RNA, other portions of each tissue sample were cut into pieces of approximately 10 to 100 mg and disrupted with a tissue homogenizer (Mikro Dismembrator S; B Braun Biotech International) using chromium steel beads.
Measurement of Lesion Size
Fatty streak lesion size in each fetal aorta was determined with oil red O staining and counterstaining with hematoxylin. In addition, tissue sections were also stained with alcian blue to determine the density of smooth muscle cells, as previously described in detail.2 Briefly, we obtained microphotographs of stained lesions, and digital pictures were morphometrically evaluated by computer-assisted image analysis. For some analyses, fetal aortic samples were dichotomized according to the lesion size, and we considered 120 × 103 μm2/section to be the cutoff.
DNA Extraction and Methylated DNA Immunoprecipitation
Extraction of DNA from fetal tissues and cultured cells and immunoprecipitation of methylated DNA was performed with the methylated DNA immunoprecipitation (MeDIP) kit (Diagenode) according to manufacturer’s instructions. Homogenized tissues (from the 78 fetal aortas) were suspended in genomic digestion buffer with proteinase K and incubated at 50°C overnight. Genomic DNA was extracted with a combination of phenol, chloroform, and isoamyl alcohol, and 30 μg of purified DNA was sheared (10 cycles of 30 seconds with ultrasonography on and 30 seconds with ultrasonography off) in fragments ranging from 100 to 800 base pairs (bp) using a sonication system (Bioruptor UCD-200; Diagenode). Sheared DNA was analyzed by means of agarose gel electrophoresis. Four micrograms of each sheared DNA sample was stored as input. Another comparable amount underwent MeDIP using an anti-5′methylcytosine antibody; samples were rotated overnight at 4°C in the presence of MeDIP-blocked beads. After several washes, immunoprecipitated DNA was eluted and purified using the phenol-chloroform–isoamyl alcohol combination. The amount of methylated DNA enrichment in MeDIP samples compared with input samples was verified by real-time polymerase chain reaction (qPCR) using a set of specific primers. Real-time PCR was performed with PCR master mix (SYBR Greeen; Biorad) and by applying the following conditions: an initial denaturation step at 95°C for 3 minutes; followed by 40 cycles consisting of 30 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C; and a final step of 1 minute at 95°C. Melting curves were obtained by incubating the amplification products at 65°C and at increasing temperatures (increments of 0.5°C for 5 seconds). These conditions were used for all set primers except for the annealing temperature of superoxide dismutase 2 (SOD2) complementary DNA amplification, which was set at 62°C. Real-time PCR data were expressed as percentage of immunoprecipitated DNA per total input.
The regulatory regions and the CpG islands analyzed in this study were selected using the Integrated Regulation from ENCODE (Encyclopedia of DNA Elements) Tracks and the ENCODE DNA Methylation Tracks data sets, respectively, which are available on the University of California Santa Cruz Genome Browser (https://genome.ucsc.edu/index.html). Primer pairs for the methylation analysis of fetal DNA were designed by using Genome Browser and the 2009 genome assembly GRCh37/hg19 (https://genome.ucsc.edu/). The following regions were included:
Superoxide dismutase 2 (SOD2 [NM_001024465]), a promoter region located 1033 to 1175 bp upstream of the transcriptional start site (TSS) (chr6:160115386-160115528) and an enhancer region located 610 to 786 bp downstream of the TSS (chr6:160113567-160113743);
Sterol regulatory element binding protein 2 (SREBP2 [NM_004599]), a promoter region located 382 to 549 bp upstream of the TSS (chr22:42228557-42228724);
Adenosine triphosphate–binding cassette transporter 1 (ABCA1 [NM_005502]), a promoter region located 11 to 160 bp upstream of the TSS (chr9:107690538-107690687);
Low-density lipoprotein receptor (LDLR [NM_000527]), a promoter region located 158 bp upstream to 61 bp downstream of the TSS (chr19:11199880-11200099) and an enhancer region located 1069 to 1281 bp downstream of the TSS (chr19:11201107-11201319); and
Liver X receptor α (LXRα [also NR1H3] [NM_001251934]), a region consisting of the 5′-UTR sequence and located 437 to 607 bp downstream of the TSS (chr11:47270288-47270458).
Primer pairs used are shown in eTable 1 in the Supplement.
We used Methyl Primer Express (version 1.0) and Genome Browser software to select important gene regulatory regions. Data are reported as the percentage of input DNA ([amount of enriched sample DNA material in nanograms divided by the amount of fragmented DNA used in immunoprecipitation reaction in nanograms] × 100), using the threshold cycle value of qPCR to extrapolate DNA concentration of the sample with a standard curve plot. To determine the total amount of enriched DNA in the sample, multiply the DNA concentration by the volume of enriched DNA. Protocol methods are available at MeDIP catalog No. 55009 (Active Motif Nord America [www.activemotif.com]).
Immunohistochemistry
Immunohistochemistry was performed on 56 of 78 samples owing to the low quantity of some samples. Sections of each fetal aorta were deparaffinized in xylene and dehydrated in graded concentrations of ethanol. After blocking the endogenous peroxidase activity with 3% hydrogen peroxide for 15 minutes, the sections were heated in 10mM citrate buffer in a microwave pressure cooker for 20 minutes. Slides were allowed to cool to room temperature, and nonspecific sites were blocked with horse serum (Dako Corporation) for 30 minutes at room temperature. Sections were incubated for 60 minutes at room temperature with the following primary antibodies: anti–trimethyl-histone H3 Lys9 (H3K9me3) at a dilution of 1:500 (Millipore); anti–trimethyl-histone H3 Lys27 (H3K27me3) at a dilution of 1:200 (Millipore); and anti–trimethyl-histone H3 Lys4 (H3K4me3) at a dilution of 1:100 (Millipore). The sections were then stained using an immunoperoxidase technique and avidin-biotin complex. Slides were rinsed with PBS, incubated for 15 minutes with biotinylated anti–rabbit IgG at a dilution of 1:500 (Dako Corporation), washed in PBS, and incubated for 30 minutes with avidin-biotin peroxidase (Dako Corporation). Bound antibodies were visualized by 20 minutes of incubation with 3,3-diaminobenzidine tetrahydrochloride. All sections were counterstained with Mayer hematoxylin solution. The labeling index was calculated as the percentage of positive cells among 100 cells observed under the microscope by analyzing 5 representative fields. To discriminate high methylation from low methylation status, the number of positive cells was evaluated in each microscopic field at original magnification ×20, and a cutoff value of 75% positive cells was chosen.
RNA Extraction and qPCR Assay
Total RNA was extracted from homogenized tissues and cultured cells using total RNA isolation reagent (TRIzol solution; Life Technologies) according to the manufacturer’s instructions. RNA was quantified with a spectrophotometer (NanoDrop ND-1000; Thermo Scientific), and its integrity was assessed by denaturing agarose gel electrophoresis. RNA from tissues (500 ng) and from cells (1 μg) underwent reverse transcription (SuperScript III; Life Technologies). Real-time PCR was performed using a qPCR detection system (CFX96; BioRad Laboratories, Ltd) with iQ SYBR Green Supermix (BioRad Laboratories, Ltd). The specificity of each oligonucleotide pair was verified with the BLAST (Basic Local Alignment Search Tool) program, and the amplification products were analyzed by agarose gel electrophoresis. We have analyzed the expression levels of a gene set involved in the cholesterol pathway, particularly SOD2, LDLR, SREBP2, LXRα, and ABCA1. Quantitative results were normalized to GAPDH as a housekeeping gene. We used the primer sequences described in eTable 1 in the Supplement. Each sample was analyzed in triplicate, and threshold cycle values were determined for the target gene. Melting curve analysis was performed to verify a single product species. Relative changes in gene expression were analyzed using the comparative threshold cycle method and reported as relative expression compared with the value of control complementary DNA.
Cell Culture
Human aortic endothelial cells (HAECs) (Lonza) were cultured in endothelial growth medium 2 (Lonza) in a 37°C incubator with 5% carbon dioxide. Human aortic endothelial cells (2 × 104 cells/well) were seeded in 24-well culture plates coated with basement membrane matrix (Matrigel; Corning Life Sciences) for 96 hours in the presence or absence of 10/μg/mL of cholesterol (Sigma-Aldrich).
Statistical Analysis
Univariate correlation analysis was performed to identify the association of the methylation status of 7 fetal genomic loci with maternal levels of total cholesterol, LDLC, HDLC, triglycerides, and glucose, maternal BMI, and fetal lesion area. The same variables were used in multivariate regression analysis to evaluate the potential association of the methylation status of the 7 fetal genomic loci with the fetal lesion areas, taking into account the confounding effect of the maternal levels of total cholesterol, HDLC, triglycerides, and glucose and maternal BMI. The effect of maternal LDLC level was not assessed in multivariate analysis because it was calculated with the Friedewald formula and therefore equal to the value obtained from maternal total cholesterol level. Unless otherwise indicated, data are expressed as mean (SD).
Data from immunohistochemical studies were evaluated by χ2 test, and data from cell culture studies were evaluated by paired t test. All analyses were performed with 0.05 type I error using Stata software (version 11.2; StataCorp, LP). Two-tailed P < .05 was considered statistically significant.
Results
Study Population
We assessed human aortic samples from 78 fetuses (46 male [59%] and 32 female [41%]; mean fetal age, 25 [3] weeks). Mean fetal aortic lesion area was 307.5 (236.8) μm2. No statistically significant association was found between fetal or maternal age and fetal lesion area (Pearson correlation coefficient for fetal age and lesion area, 0.177 [P = .12]; Pearson correlation coefficient for maternal age and lesion area, 0.036 [P = .75]). Plasma lipid levels were assessed during the hospitalization related to the spontaneous fetal death. Mean maternal plasma total cholesterol level was 256.5 (60.2) mg/dL. Total cholesterol level was at least 200 mg/dL in 64 mothers (82%) and at least 280 mg/dL in 24 mothers (31%). Mean LDLC level was 181.1 (52.0) mg/dL; mean plasma HDLC level, 36 (7) mg/dL; and mean plasma triglyceride level, 188 (23) mg/dL. After 12 weeks of pregnancy, mean maternal BMI was 24.5 (2.8). Mean fasting plasma glucose level was 81.2 (11.8) mg/dL (to convert to millimoles per liter, multiply by 0.0555). A positive correlation was found for maternal cholesterol level with BMI and with glucose level (Pearson correlation coefficient, 0.276 [P = .02] and 0.269 [P = .02], respectively).
Correlation of Methylation of 7 Fetal Genomic Loci, Fetal Lesion Area, and Maternal Plasma Cholesterol Levels
We used a bivariate Pearson correlation to evaluate the strength and direction of associations among the methylation status of 7 fetal genomic loci, maternal plasma total cholesterol and LDLC levels, and fetal aortic lesion area (Table 1). The results demonstrated a strong positive correlation (Pearson correlation coefficient, 0.782; P < .001) between mean maternal plasma cholesterol level (256.5 [60.2] mg/dL) and mean fetal aortic lesion area (307.5 [236.8] μm2). Similar results were observed also for maternal LDLC level and fetal aortic lesion area (Pearson correlation coefficient, 0.733; P < .001). Moreover, only the methylation status of fetal SREBP2 among the other analyzed genes was correlated with fetal aortic lesion area (Pearson correlation coefficient, 0.293 [P = .01]) and maternal plasma cholesterol level (Pearson correlation coefficient, 0.488 [P < .001]). Results of the multivariable regression analysis indicated that the methylation density was not significantly associated with lesion area (Table 2). The multivariate model explains a relevant fraction (61%) of the association between fetal aortic lesion area and maternal plasma cholesterol level independent of maternal HDLC, triglyceride, and glucose levels and maternal BMI.
Table 1. Univariate Analysis Evaluating the Association Among the Methylation Status of 7 Fetal Genomic Loci, Maternal Plasma Cholesterol and LDLC Levels, and Fetal Aortic Lesion Areaa.
| Gene Locus | DNA Methylation, Mean (SD)b | Lesion Area | Maternal Cholesterol Level | Maternal LDLC Level | |||
|---|---|---|---|---|---|---|---|
| Pearson Correlation Coefficient | P Value | Pearson Correlation Coefficient | P Value | Pearson Correlation Coefficient | P Value | ||
| SOD2 | |||||||
| Enhancer | 1.6 (4.0) | 0.135 | .25 | −0.092 | .43 | −0.102 | .38 |
| Promoter | 1.9 (3.7) | −0.108 | .36 | −0.032 | .79 | −0.041 | .73 |
| LDLR | |||||||
| Promoter | 0.9 (3.2) | −0.170 | .89 | 0.072 | .55 | 0.069 | .56 |
| Enhancer | 1.6 (3.6) | −0.071 | .55 | 0.054 | .65 | 0.057 | .63 |
| LXRα 5′UTR | 1.4 (3.3) | −0.137 | .25 | − 0.093 | .43 | −0.096 | .42 |
| ABCA1 promoter | 1.0 (3.1) | −0.135 | .26 | −0.098 | .42 | −0.113 | .35 |
| SREBP2 promoter | 1.3 (2.3) | 0.293 | .01 | 0.488 | <.001 | 0.503 | <.001 |
Abbreviations: ABCA1, adenosine triphosphate–binding cassette transporter 1; LDLC, low-density lipoprotein cholesterol; LDLR, low-density lipoprotein receptor; LXRα, liver X receptor α; SOD2, superoxide dismutase 2; SREBP2, sterol regulatory element binding protein 2.
Includes autopsy samples of 78 fetal aortas and maternal data.
Table 2. Multivariate Regression Analysis Evaluating the Independent Association Between Fetal Lesion Area and the Methylation Status of 7 Fetal Genomic Locia .
| Variable | Regression Coefficient | P Value |
|---|---|---|
| Maternal cholesterol level | 3.098 | ≤.001 |
| Maternal HDLC level | −1.187 | .87 |
| Maternal triglyceride level | −1.126 | .64 |
| Maternal glucose level | −1.504 | .49 |
| Maternal BMI | 15.302 | .25 |
| SREBP2 promoter | −0.520 | .43 |
| SOD2 | ||
| Enhancer | −0.007 | .49 |
| Promoter | −0.009 | .74 |
| LDLR | ||
| Promoter | 0.003 | .80 |
| Enhancer | −0.006 | .51 |
| LXRα 5′UTR | −0.002 | .73 |
| ABCA1 promoter | 0.002 | .94 |
Abbreviations: ABCA1, adenosine triphosphate–binding cassette transporter 1; BMI, body mass index; HDLC, high-density lipoprotein cholesterol; LDLR, low-density lipoprotein receptor; LXRα, liver X receptor α; SOD2, superoxide dismutase 2; SREBP2, sterol regulatory element binding protein 2.
Analysis takes into account the confounding effect of the maternal levels of plasma cholesterol, HDLC, triglycerides, and glucose and BMI. Analysis includes autopsy samples of 78 fetal aortas and maternal data.
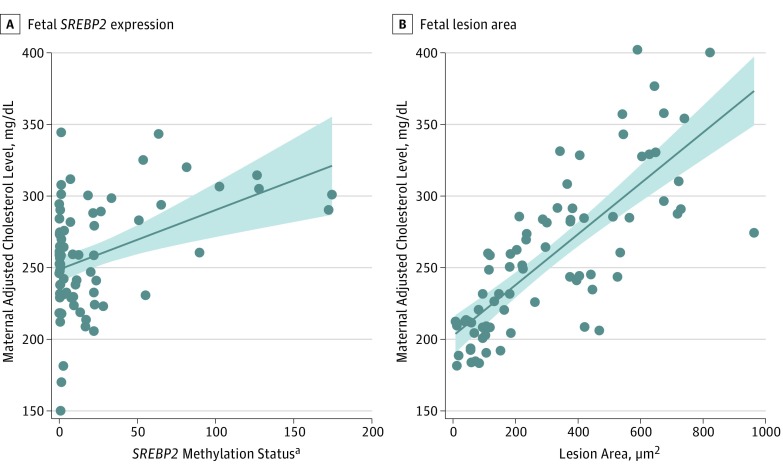
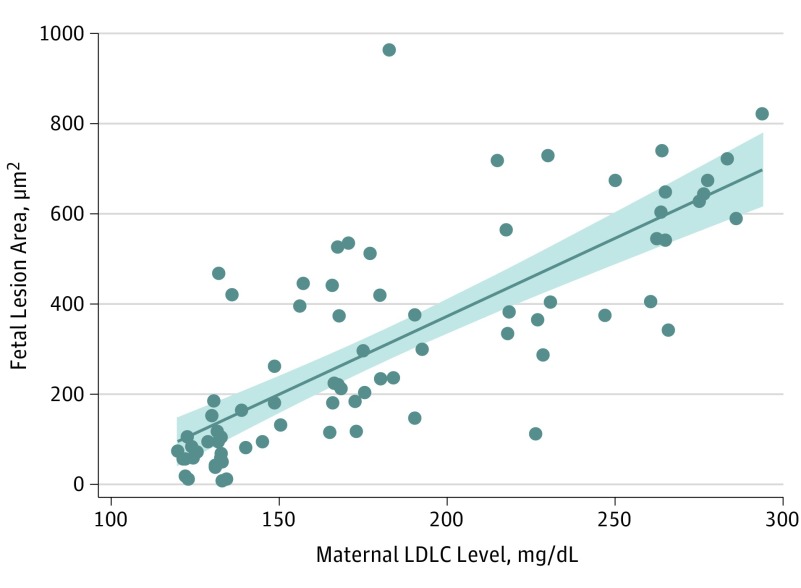
Figure 1 shows the positive association of adjusted maternal cholesterol level vs the fetal lesion area (regression coefficient, 3.098 [P < .001]) and fetal SREBP2 (regression coefficient, 0.380 [P = .002]). In each panel, cholesterol levels are adjusted for the mean value of the other factor; thus, both graphs show the association of each factor independent of the confounding effect of the other. Figure 2 shows the positive association of adjusted maternal LDLC level vs the fetal lesion area (regression coefficient, 3.461 [P < .001]). Overall, these data show, in univariate analysis, that SREBP2 is associated with maternal total cholesterol and LDLC levels.
Figure 1. Multivariate Adjusted Maternal Plasma Cholesterol Level vs Fetal SREBP2 Gene and Fetal Lesion Area.
In each plot, the cholesterol levels are adjusted for the mean value of the other factor; thus, both graphs show the association of each factor independent from the confounding effect of the other. Shaded area indicates 95% CI for the fit (solid line). To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
aMeasured as fold change compared with controls, calculated as the measured methylation levels of selected DNA regions by calculating the fold change of the enrichment of methylated sequences between the evaluated sample and a reference value following the 2−ΔΔct formula.21,22,23,24
Figure 2. Multivariate Adjusted Maternal Plasma Low-Density Lipoprotein Cholesterol (LDLC) Level vs Fetal Lesion Area.
The LDLC levels are adjusted for the mean value of the other factors; thus, the association of each factor is shown independent from the confounding effect of the other. Shaded area indicates 95% CI for the fit (solid line). To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
Collectively, these data suggest that maternal plasma total cholesterol and LDLC levels affect the size of fetal aortic atherosclerotic lesions and the methylation status of the fetal SREBP2 gene. In addition, none of the other 6 CpG-rich areas within the regulative elements of the analyzed genes were significantly associated with the fetal lesion area.
Immunohistochemical Studies
To verify whether other types of epigenetic modifications in fetal aortas were associated with maternal cholesterol levels or fetal aortic lesion size, we performed immunohistochemical studies using primary antibodies against different types of histone methylations (trimethylated Lys4, Lys9, and Lys27 of histone H3) and against DNA 5-methylcytosine. The analysis was performed only on 56 fetal aortas, due to the scarce quantities of several samples. We did not find any significant correlation between maternal cholesterol values and H3K4m3, H3K9m3, H3K27me3, or global CpG DNA methylation. Similarly, we did not find an association between global DNA methylation and fetal lesion area. On the contrary, higher positivity for H3K27me3 staining was significantly associated with a fetal lesion area of at least 120 × 103 μm2 (χ2 = 6.75; P = .009) (Table 3).
Table 3. Association Between the Level of Histone Methylation and Dichotomized Fetal Lesion Areaa.
| Histone Methylation | No. of Samples by Fetal Lesion Area | |
|---|---|---|
| <120 × 103 μm2 | ≥120 × 103 μm2 | |
| Lower positivity for H3K27me3 | 10 | 9 |
| Higher positivity for H3K27me3 | 7 | 30 |
Abbreviation: H3K27me, anti–trimethyl-histone H3 Lys27.
Includes autopsy samples of 56 fetal aortas. χ2 = 6.75; P = .009.
Gene Expression Study
We obtained RNA from only 21 fetal aortas owing to the scarcity of the other samples. Consistent with the direct correlation between maternal plasma cholesterol levels and methylation of the fetal SREBP2 gene, SREBP2 messenger RNA (mRNA) was inversely correlated both with maternal plasma cholesterol and LDLC levels (eTable 2 in the Supplement). In contrast, the expression of the other analyzed genes did not correlate with maternal plasma cholesterol levels or LDLC levels (eTable 2 in the Supplement). The correlation between SREBP2 methylation and expression was not statistically significant (Pearson correlation coefficient, −0.173; P = .39).
Primary Human Aortic Endothelial Cells
To provide further evidence that variations of extracellular cholesterol levels could induce epigenetic changes in genes that are directly or indirectly related to cholesterol metabolism, we analyzed the methylation status of regulatory regions of SREBP2, SOD2, LDLR, LXRα, and ABCA1 genes in cultured HAECs, treated with cholesterol for 96 hours. Cholesterol induced a modest hypermethylation of the promoter (mean [SD], 0.41 [0.09] vs 0.07 [0.14]; P = .048), a marked demethylation of the 3′ UTR, and a decrease of the transcript of SREBP2 compared with controls (mean [SD], 1.80 [0.26] vs 0.60 [0.14]; P = .002) (eFigure, B in the Supplement). Accordingly, the mRNA of LDLR, one of the SREBP2 target genes, decreased (eFigure, G in the Supplement). Hypermethylation after cholesterol treatment was observed in the 5′UTR of the LXRα gene (mean [SD], 27.70 [5.60] vs 5.50 [1.30]; P = .002) (eFigure, D in the Supplement) in accordance with a decrease of its mRNA (mean [SD], 2.21 [0.50] vs 0.75 [0.10]; P = .01) (eFigure, G in the Supplement). In contrast, the promoter of ABCA1 was demethylated in the presence of cholesterol (mean [SD], 124.70 [54.00] vs 21.18 [4.90]; P = .03) (eFigure, E in the Supplement) with no significant change of its mRNA level vs controls (eFigure, G in the Supplement). A 3-fold cholesterol-dependent demethylation, compared with controls, was observed in the SOD2 enhancer, and its gene expression was increased in a cholesterol-dependent manner (mean [SD], 11.76 [4.90] vs 3.53 [0.90]; P = .05) (eFigure, F and G in the Supplement). Therefore, these data from cell culture experiments are partly consistent with those obtained in fetal tissue, in that the addition of extracellular cholesterol is associated with epigenetic and expression variations of the SREBP2 gene.
Discussion
The primary findings of the present study include the following. First, maternal plasma total cholesterol and LDLC levels are positively associated with methylation of SREBP2 in fetal aortas. Second, maternal total cholesterol and LDLC levels are negatively associated with the expression of SREBP2 in fetal aortas. Third, epivariations of genes control cholesterol metabolism and atherogenesis in cholesterol-treated HAECs. Fourth, maternal total cholesterol and LDLC levels are associated with development of fetal aortic atherosclerotic lesions.
After the previous observation that maternal hypercholesterolemia during pregnancy is associated with the development of early atherosclerotic lesions in human fetal aortas,2,3,17,18 this study quantified the effect of maternal plasma total cholesterol and LDLC levels on fetal aortic lesion size. Moreover, since epigenetics is increasingly recognized as a central mechanism in fetal programming with potentially associated health consequences in adulthood, we sought to understand whether epigenetic modifications were taking place in fetal aortas in the presence of increasing concentrations of maternal plasma cholesterol. In addition, we observed that, in fetal aortas, only the methylation of SREBP2 was associated with maternal total cholesterol and LDLC levels and the fetal aortic lesion area. However, regression analysis indicated that the methylation of SREBP2 is indeed independently associated with maternal cholesterolemia, but its correlation with fetal lesion area is not significant when taking into account maternal cholesterol level as a confounding variable. Regarding other epigenetic modifications, we observed an association between increased H3K27m3 and larger fetal lesion areas. Therefore, these data are consistent with the concept that maternal cholesterol levels elicit epigenetic modifications in the fetus and suggest that the development of the initial atherosclerotic lesion in fetal tissue could be associated with these epigenetic modifications. Moreover, increasing maternal cholesterol level is associated not only with increased methylation of SREBP2, but also decreased levels of its mRNA in fetal aortas. Hence, the observed epigenetic modifications in fetal aortas may be functionally relevant. In addition, the changes of methylation and the reduced expression of SREBP2 in cholesterol-treated HAECs support the finding that, in fetal aortas, extracellular cholesterol is able to influence the epigenetic and transcriptional regulation of SREBP2.
Limitations
Epigenetic modifications of certain genes that are involved in cholesterol homeostasis could be a relevant mechanism in the fetal atherogenic response to elevated maternal cholesterol level. Atherosclerosis is a pathologic degeneration of the artery wall that is difficult to reverse, and regenerative medicine is not yet a viable option to cure atherosclerotic disease25,26,27,28; thus, prevention is necessary to reduce the burden of cardiovascular disease.5 Of note, in experimental models of maternal in utero programming of atherosclerosis, pharmacologic treatment of maternal hypercholesterolemia during pregnancy reduced atherogenesis and cardiovascular risk factors in the offspring.10,29 MicroRNAs (miRNAs) are involved in atherosclerotic initiation and progression, including lipid metabolism. Given the fact that maternal health can also influence miRNA profiles of offspring via amniotic fluid, the role of miRNA-33a and -33b and miRNA-200c as circulating miRNA associated with hypercholesterolemia should also be investigated.30,31 We acknowledge that a genome-wide methylation analysis could have been the best strategy in identifying maternal cholesterol level and fetal atherogenesis. However, the analysis included only candidate genes and regulatory loci owing to scarce fetal tissue availability. This tissue scarcity also made it difficult for us to reliably separate the medial content from the endothelium and intimal portion to perform separate DNA and RNA analyses. Moreover, a chromatin immunoprecipitation sequencing experiment and transcriptome analysis could be performed to further understand the significance of SREBP2 dysregulation. We recognize the limitations related to study of premature aborted fetal issue, because certain intrinsic epigenetic abnormalities might exist that could be associated with developmental defects and thus complicate the findings of the study.
Conclusions
The landmark epidemiologic study in the Framingham Heart Study population recently confirmed the crucial role of maternal dyslipidemias in the pathogenesis of cardiovascular disease in the adult offspring.8 Further pathogenic and network studies studies can expand our fetal hypothesis on atherogenesis to obese pregnant mothers and mothers with gestational diabetes as well as type 1 or type 2 diabetes. We speculate that other dysplipidemias involving triglycerides would not be causally involved in the pathogenesis of early atherogenesis in humans. Indeed, several animal models developed by investigators5,9,12,16,29 and confirmed by other groups on the fetal hypothesis of atherogenesis10,11 provided the background that maternal cholesterol (including LDLC and many oxidized lipids) may promote fetal atherogenesis. The present study supports the possibility that epigenetic mechanisms, including methylation of SREBP2, may be involved in early human atherogenesis. Future attempts to design more effective long-term programs of primary cardiovascular prevention in the offspring of mothers with hypercholesterolemia should invest more efforts to elucidate the potential epigenetic mechanisms underlying the association between early atherogenesis and maternal hypercholesterolemia during pregnancy.
eTable 1. Primers Used for Quantitative Real-Time PCR
eTable 2. Univariate Analysis Evaluating the Association Among the mRNA Expression of 5 Genes in Fetal Aortas and Maternal Plasma Cholesterol
eFigure. Data in Cultured Cells
References
- 1.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535-546. doi: 10.1161/CIRCRESAHA.115.307611 [DOI] [PubMed] [Google Scholar]
- 2.Napoli C, D’Armiento FP, Mancini FP, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia: intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100(11):2680-2690. doi: 10.1172/JCI119813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354(9186):1234-1241. doi: 10.1016/S0140-6736(99)02131-5 [DOI] [PubMed] [Google Scholar]
- 4.Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Natural history of aortic and coronary atherosclerotic lesions in youth: findings from the PDAY Study. Arterioscler Thromb. 1993;13(9):1291-1298. doi: 10.1161/01.ATV.13.9.1291 [DOI] [PubMed] [Google Scholar]
- 5.Napoli C, Lerman LO, de Nigris F, Gossl M, Balestrieri ML, Lerman A. Rethinking primary prevention of atherosclerosis-related diseases. Circulation. 2006;114(23):2517-2527. doi: 10.1161/CIRCULATIONAHA.105.570358 [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650-1656. doi: 10.1056/NEJM199806043382302 [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13(9):364-368. doi: 10.1016/S1043-2760(02)00689-6 [DOI] [PubMed] [Google Scholar]
- 8.Mendelson MM, Lyass A, O’Donnell CJ, D’Agostino RB Sr, Levy D. Association of maternal prepregnancy dyslipidemia with adult offspring dyslipidemia in excess of anthropometric, lifestyle, and genetic factors in the Framingham Heart Study. JAMA Cardiol. 2016;1(1):26-35. doi: 10.1001/jamacardio.2015.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napoli C, de Nigris F, Welch JS, et al. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation. 2002;105(11):1360-1367. doi: 10.1161/hc1102.106792 [DOI] [PubMed] [Google Scholar]
- 10.Alkemade FE, Gittenberger-de Groot AC, Schiel AE, et al. Intrauterine exposure to maternal atherosclerotic risk factors increases the susceptibility to atherosclerosis in adult life. Arterioscler Thromb Vasc Biol. 2007;27(10):2228-2235. doi: 10.1161/01.ATV.0000282193.31936.fd [DOI] [PubMed] [Google Scholar]
- 11.Elahi MM, Cagampang FR, Anthony FW, Curzen N, Ohri SK, Hanson MA. Statin treatment in hypercholesterolemic pregnant mice reduces cardiovascular risk factors in their offspring. Hypertension. 2008;51(4):939-944. doi: 10.1161/HYPERTENSIONAHA.107.100982 [DOI] [PubMed] [Google Scholar]
- 12.Napoli C, Crudele V, Soricelli A, et al. Primary prevention of atherosclerosis: a clinical challenge for the reversal of epigenetic mechanisms? Circulation. 2012;125(19):2363-2373. doi: 10.1161/CIRCULATIONAHA.111.085787 [DOI] [PubMed] [Google Scholar]
- 13.Sommese L, Zullo A, Mancini FP, Fabbricini R, Soricelli A, Napoli C. Clinical relevance of epigenetics in the onset and management of type 2 diabetes mellitus. Epigenetics. 2017;12(6):401-415. doi: 10.1080/15592294.2016.1278097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimaldi V, De Pascale MR, Zullo A, et al. Evidence of epigenetic tags in cardiac fibrosis. J Cardiol. 2017;69(2):401-408. doi: 10.1016/j.jjcc.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Ordovás JM, Smith CE. Epigenetics and cardiovascular disease. Nat Rev Cardiol. 2010;7(9):510-519. doi: 10.1038/nrcardio.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Napoli C, Infante T, Casamassimi A. Maternal-foetal epigenetic interactions in the beginning of cardiovascular damage. Cardiovasc Res. 2011;92(3):367-374. doi: 10.1093/cvr/cvr201 [DOI] [PubMed] [Google Scholar]
- 17.Napoli C, Witztum JL, de Nigris F, Palumbo G, D’Armiento FP, Palinski W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation. 1999;99(15):2003-2010. doi: 10.1161/01.CIR.99.15.2003 [DOI] [PubMed] [Google Scholar]
- 18.D’Armiento FP, Bianchi A, de Nigris F, et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke. 2001;32(11):2472-2479. doi: 10.1161/hs1101.098520 [DOI] [PubMed] [Google Scholar]
- 19.Liguori A, D’Armiento FP, Palagiano A, et al. Effect of gestational hypercholesterolaemia on omental vasoreactivity, placental enzyme activity and transplacental passage of normal and oxidised fatty acids. BJOG. 2007;114(12):1547-1556. doi: 10.1111/j.1471-0528.2007.01510.x [DOI] [PubMed] [Google Scholar]
- 20.Liguori A, D’Armiento FP, Palagiano A, Palinski W, Napoli C. Maternal C-reactive protein and developmental programming of atherosclerosis. Am J Obstet Gynecol. 2008;198(3):281.e1-281.e5. doi: 10.1016/j.ajog.2007.11.027 [DOI] [PubMed] [Google Scholar]
- 21.Mohn F, Weber M, Schübeler D, Roloff TC. Methylated DNA immunoprecipitation (MeDIP). Methods Mol Biol. 2009;507:55-64. doi: 10.1007/978-1-59745-522-0_5 [DOI] [PubMed] [Google Scholar]
- 22.Gebhard C, Schwarzfischer L, Pham TH, Andreesen R, Mackensen A, Rehli M. Rapid and sensitive detection of CpG-methylation using methyl-binding (MB)-PCR. Nucleic Acids Res. 2006;34(11):e82. doi: 10.1093/nar/gkl437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong Y, Jun Y, Kim J, et al. Integrative analysis of DNA methylation and mRNA expression during differentiation of umbilical cord blood derived mononuclear cells to endothelial cells. Gene. 2017;635:48-60. doi: 10.1016/j.gene.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Zhu J, Zhao M, Zhang B, Jiang C, Yang X. Methylation-level inferences and detection of differential methylation with MeDIP-seq data. PLoS One. 2018;13(8):e0201586. doi: 10.1371/journal.pone.0201586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommese L, Zullo A, Schiano C, Mancini FP, Napoli C. Possible muscle repair in the human cardiovascular system. Stem Cell Rev. 2017;13(2):170-191. doi: 10.1007/s12015-016-9711-3 [DOI] [PubMed] [Google Scholar]
- 26.Grimaldi V, Schiano C, Casamassimi A, et al. Imaging techniques to evaluate cell therapy in peripheral artery disease: state of the art and clinical trials. Clin Physiol Funct Imaging. 2016;36(3):165-178. doi: 10.1111/cpf.12210 [DOI] [PubMed] [Google Scholar]
- 27.Elshazly MB, Stegman B, Puri R. Regression of coronary atheroma with statin therapy. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):131-137. doi: 10.1097/MED.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 28.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5(2):91-102. doi: 10.1038/ncpcardio1086 [DOI] [PubMed] [Google Scholar]
- 29.Napoli C, Witztum JL, Calara F, de Nigris F, Palinski W. Maternal hypercholesterolemia enhances atherogenesis in normocholesterolemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy: an experimental model of atherogenic mechanisms in human fetuses. Circ Res. 2000;87(10):946-952. doi: 10.1161/01.RES.87.10.946 [DOI] [PubMed] [Google Scholar]
- 30.D’Agostino M, Martino F, Sileno S, et al. Circulating miR-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with miR-33a/b levels: implication of a ZEB1-dependent mechanism. Clin Sci (Lond). 2017;131(18):2397-2408. doi: 10.1042/CS20171121 [DOI] [PubMed] [Google Scholar]
- 31.Martino F, Carlomosti F, Avitabile D, et al. Circulating miR-33a and miR-33b are up-regulated in familial hypercholesterolaemia in paediatric age. Clin Sci (Lond). 2015;129(11):963-972. doi: 10.1042/CS20150235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Primers Used for Quantitative Real-Time PCR
eTable 2. Univariate Analysis Evaluating the Association Among the mRNA Expression of 5 Genes in Fetal Aortas and Maternal Plasma Cholesterol
eFigure. Data in Cultured Cells