Abstract
Importance
Prevention of obesity during childhood is critical for children in underserved populations, for whom obesity prevalence and risk of chronic disease are highest.
Objective
To test the effect of a multicomponent behavioral intervention on child body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) growth trajectories over 36 months among preschool-age children at risk for obesity.
Design, Setting, and Participants
A randomized clinical trial assigned 610 parent-child pairs from underserved communities in Nashville, Tennessee, to a 36-month intervention targeting health behaviors or a school-readiness control. Eligible children were between ages 3 and 5 years and at risk for obesity but not yet obese. Enrollment occurred from August 2012 to May 2014; 36-month follow-up occurred from October 2015 to June 2017.
Interventions
The intervention (n = 304 pairs) was a 36-month family-based, community-centered program, consisting of 12 weekly skills-building sessions, followed by monthly coaching telephone calls for 9 months, and a 24-month sustainability phase providing cues to action. The control (n = 306 pairs) consisted of 6 school-readiness sessions delivered over the 36-month study, conducted by the Nashville Public Library.
Main Outcomes and Measures
The primary outcome was child BMI trajectory over 36 months. Seven prespecified secondary outcomes included parent-reported child dietary intake and community center use. The Benjamini-Hochberg procedure corrected for multiple comparisons.
Results
Participants were predominantly Latino (91.4%). At baseline, the mean (SD) child age was 4.3 (0.9) years; 51.9% were female. Household income was below $25 000 for 56.7% of families. Retention was 90.2%. At 36 months, the mean (SD) child BMI was 17.8 (2.2) in the intervention group and 17.8 (2.1) in the control group. No significant difference existed in the primary outcome of BMI trajectory over 36 months (P = .39). The intervention group children had a lower mean caloric intake (1227 kcal/d) compared with control group children (1323 kcal/d) (adjusted difference, −99.4 kcal [95% CI, −160.7 to −38.0]; corrected P = .003). Intervention group parents used community centers with their children more than control group parents (56.8% in intervention; 44.4% in control) (risk ratio, 1.29 [95% CI, 1.08 to 1.53]; corrected P = .006).
Conclusions and Relevance
A 36-month multicomponent behavioral intervention did not change BMI trajectory among underserved preschool-age children in Nashville, Tennessee, compared with a control program. Whether there would be effectiveness for other types of behavioral interventions or implementation in other cities would require further research.
Trial Registration
ClinicalTrials.gov Identifier: NCT01316653
Key Points
Question
What is the effect of a 36-month multicomponent behavioral intervention for obesity prevention on body mass index (BMI) trajectories in underserved preschool-age children at risk for obesity but not yet obese?
Findings
In this randomized clinical trial that included 610 parent-child pairs from underserved communities, the mean BMI in both the intervention and control groups was 17.8 at 36 months, with no significant difference in BMI trajectories.
Meaning
The behavioral intervention was not effective in this low-income minority population.
This randomized clinical trial compares the effects of a 36-month multicomponent behavioral intervention for obesity prevention vs a school-readiness control on body mass index (BMI) trajectories in underserved preschool-age children at risk for obesity but not yet obese.
Introduction
Obesity often begins in childhood, with the highest rates among minority populations.1,2 Numerous randomized clinical trials (RCTs) address childhood obesity treatment by targeting child health behaviors such as diet, physical activity, sleep, and media use.3 These behavioral interventions have met with variable efficacy and often yield small, if any, effect sizes on child weight outcomes.4,5 In addition, whereas several interventions have been successful at producing short-term reductions in child body mass index (BMI), few trials have been of sufficient length to assess sustainability.6
Obesity is a complex problem, affected by the dynamic interaction of biology, behavior, and children’s social and physical environments.7,8 Given the challenges associated with effective obesity treatment, recent focus has been on childhood obesity prevention.5,9 The developmental origins of disease hypothesis suggests that early life influences can alter a person’s life-long health trajectory, linking early obesogenic exposures and rapid weight trajectory to common chronic adult conditions including coronary artery disease and type 2 diabetes.10,11 These problems are especially salient for families from traditionally underserved minorities,12 who are confronted with significant barriers such as poverty.
The Growing Right Onto Wellness (GROW) RCT tested a theoretically grounded, 36-month behavior change intervention focused on childhood obesity prevention among preschool-aged children from underserved communities. It was hypothesized that the intervention would attenuate child BMI growth trajectories compared with the control group over 36 months.
Methods
This study was conducted within the Childhood Obesity Prevention and Treatment Research consortium, a National Heart, Lung, and Blood Institute/National Institute of Child Health and Human Development–sponsored collaborative effort to develop and evaluate novel approaches to prevent or treat childhood obesity. The study was supported by an independent coordinating center at the University of North Carolina at Chapel Hill.
The Vanderbilt University Medical Center institutional review board and a National Heart, Lung, and Blood Institute–appointed data and safety monitoring board approved the study protocol and conducted routine evaluations of participant safety and protocol adherence throughout the trial. Written informed consent was obtained by bilingual data collectors in participants’ language of choice using an enhanced, low-literacy approach.13
The intervention focused on changing behavior and featured several key strategies hypothesized to maximize health behavior change, including (1) considering the health behaviors of both parent and child, (2) using the built environment of existing community centers, (3) implementing a tiered-intensity intervention to maximize sustainability of participation, and (4) using an adaptive intervention.7 The study’s design and methodology have been previously reported.14 The protocol and statistical analysis plan are available in Supplement 1.
Setting and Participants
Parent-child pairs were recruited from 54 physicians’ offices and community settings and enrolled from August 2012 to May 2014 in Nashville, Tennessee. The final 36-month follow-up was conducted between October 2015 and June 2017. Recruitment efforts included posters, mailed brochures, radio commercials, community events, in-person recruitment, and word of mouth. Child eligibility criteria included age 3 to 5 years, English or Spanish speaking, and high normal weight to overweight but not yet obese (BMI ≥50th and <95th percentile based on US Centers for Disease Control and Prevention standardized growth curves).15 Caregiver eligibility included commitment to participate in the 36-month study, English or Spanish speaking, and consistent telephone access.
Participants also had to qualify for at least 1 service for underserved populations (eg, Medicaid; Special Supplemental Nutrition Program for Women, Infants, and Children). Participants were excluded if a medical condition precluded routine physical activity or if participants lived or worked outside an 8-km radius of participating community centers. Because obesity disproportionately affects children from underserved minorities,12 race and ethnicity information was collected. Race and ethnicity for both parent and child were assessed by parent report, using fixed categories with an open-ended option.
Randomization
Participants were randomized using a computer-generated schedule that was stratified by community center and parent language preference (English or Spanish). Randomly permuted block sizes varied from 2 to 6. Assignment was implemented through an electronic interface that concealed group assignment until each individual was enrolled. Only study staff not involved in data collection implemented randomization, and group assignment could not be changed.
Intervention Description
Informed by effective adult obesity treatment behavioral trials16,17 and innovative concepts proposed by the National Institutes of Health,18 the intervention (GROW Healthier) was a tiered-intensity program of decreasing intensity: (1) a 12-week intensive phase with weekly 90-minute skills-building sessions via either in-person groups or telephone calls; (2) a 9-month maintenance phase with monthly coaching telephone calls; and (3) a 24-month sustainability phase providing frequent cues to action (eg, texts, personalized letters, monthly calls) to use parks and recreation programming for healthy family behaviors. The intervention was based on social cognitive theory and the socioecological model, focusing on behavior change techniques including goal setting, self-monitoring, and problem solving in the context of participants’ home and community environments.19,20
Intervention content included skills building for parents and children regarding nutritional choices, physical activity habits, use of the family and built environment, engaged parenting, healthy sleep, and reduced media time.21 Each week (intensive phase) or month (maintenance phase), participants created a self-defined goal about family health behaviors targeted in the intervention (diet, physical activity, sleep, media use, engaged parenting). The intervention included an adaptive component, an additional coaching telephone call14 that provided BMI results and additional guided goal setting and problem solving; this occurred when a child’s BMI category increased or remained obese at a data collection time point.
The control condition (GROW Smarter) was a school-readiness program developed and delivered by the Nashville Public Library. The curriculum consisted of six 30-minute group-based activities delivered concurrently with data collection sessions. Participants in the intervention and control groups received the school-readiness program. Therefore, the only difference between conditions was the obesity prevention intervention.
Blinding
Data collectors were blinded to individual participant study condition and aggregated study results by group. All study staff, including the primary investigator and statisticians, were blinded to postbaseline data aggregated by group until all study data had been collected and cleaned.
Outcomes
The prespecified primary outcome was child BMI trajectory across 36 months modeled using linear and quadratic terms. BMI was calculated as weight in kilograms divided by height in meters squared. The protocol identified 7 prespecified secondary outcomes: (1) child mean daily energy intake (kcal); mean percentage of energy intake from (2) fat, (3) carbohydrates, and (4) protein; mean daily minutes spent in (5) rest and sedentary behavior and (6) moderate and vigorous physical activity (MVPA); and (7) community center use with child (never or at least once). Prevalence of child obesity (BMI ≥95th percentile) was the only post-hoc outcome analyzed. Parent anthropometrics and child waist circumference/triceps skinfold were also collected but not included as secondary outcomes in this analysis.
Data were collected at baseline and 3, 9, 12, 24, and 36 months by trained, blinded, bilingual data collectors in participant homes or local community centers. Data collected included parent and child height (without shoes, to nearest 0.1 cm) and weight (to nearest 0.1 kg) using wall-mounted stadiometers and research-grade scales. Annually, children were asked to wear a GT3X+ accelerometer (ActiGraph) on their waist for 24 hours daily for 7 consecutive days to assess physical activity. Previously validated cut points determined time spent in sedentary behaviors and light, moderate, and vigorous activities.22 Data obtained annually from 24-hour diet recall on 2 weekdays and 1 weekend day assessed parent-reported child dietary intake (using NDS-R software).
Survey data were collected in the participant’s chosen language via guided administration and included demographic, behavioral, and psychosocial domains. Parent-reported community center use with the child was assessed through the survey item: “How often do you go to your community recreation center with your children to be active together?” (6-point scale from “never” to “every day”), and the responses were dichotomized into “never” or “at least once” for analysis. Food insecurity was assessed using the 6-item short form of the US Household Food Security Survey Module.23
Participants were encouraged to complete all data collection elements within 45 days of anthropometric data collection. Process measures included fidelity of implementation and dose received. Fidelity was assessed using standardized tools14 for both in-person and telephone call sessions. Dose received was measured by attendance at in-person sessions or participation in telephone calls throughout the study.
Adverse Event Reporting
Adverse events were identified throughout the study period by encouraging participants to contact the study team if an event occurred and by asking participants about adverse events using a structured questionnaire at all data collection time points.
Sample Size
A power analysis was conducted with a 2-tailed α = .05 and 90% power to detect a standardized effect size of 0.4. Results suggested that a final sample of 480 pairs was required. Therefore, 600 pairs were planned, with an anticipated 80% retention rate. The effect size of 0.4 was selected based on data from a previous behavioral intervention conducted in Nashville among Latino participants (trial protocol in Supplement 1).24
Statistical Methods
Baseline characteristics were expressed as mean (SD) or median (quartile 1, quartile 3), depending on their distribution, or number (%) for categorical variables. This trial used an intention-to-treat approach to test the difference in the BMI growth trajectory between children in the intervention and control groups. The analysis fit a 2-level (time nested within child) mixed-effects regression model, using a maximum likelihood procedure to handle missing data and an unstructured variance-covariance matrix.25 Data were assumed to be missing at random. Because clinical literature about childhood obesity indicates that the shape of the BMI trajectory across ages 3 to 8 years is curvilinear, a quadratic model was selected a priori, defining trajectory using both linear and quadratic terms.15,26 Time varied individually and was measured as a continuous variable defined as years since baseline. Two child-level variables, age at baseline (mean-centered) and intervention condition, were covariates for the intercept, linear, and quadratic BMI growth trajectory terms. Child sex was a covariate for the intercept only. Success was evaluated by a likelihood ratio test to determine whether the linear and quadratic intervention effects were jointly equal to zero (df = 2; .05 level). The coordinating center independently replicated the primary outcome analysis and confirmed the findings.
Analysis of secondary and post-hoc outcomes used ordinary least squares regression for continuous outcomes and Poisson regression with robust standard errors for binary outcomes. Models were prespecified and accounted for covariates thought to be associated with each outcome, such as baseline outcome value, child age, and sex. The Benjamini-Hochberg procedure was used to control the false-discovery rate for multiple comparisons based on the number of time points analyzed for each outcome. The P values before and after correction are presented. Residual diagnostics were performed to ensure distributional assumptions were met.
Prior literature informed selection of a series of post-hoc moderator analyses based on the child BMI growth trajectory mixed-effects model to determine whether the intervention’s effect on growth trajectory varied across different baseline values of child age; child sex; parent BMI; parent race/ethnicity; child energy intake; percentage of energy intake from fat, carbohydrates, and protein; percentage of wear time in sedentary behavior and MVPA; baseline child BMI percentile; child birth weight; food security status; and community center.27,28
Statistical analyses were conducted using Stata version 14.2 (StataCorp). Statistical significance was defined using a 2-sided test with α = .05.
Results
Of the 2126 participants assessed for eligibility, 610 parent-child pairs were randomized, with 304 assigned to the intervention group and 306 to the control group. The 36-month retention rate was 91.4% for the intervention and 88.9% for the control (Figure 1). At baseline, the child mean (SD) age was 4.3 (0.9) years, 51.9% were female, 91.4% were Hispanic/Latino, 65.7% were between the 50th and 85th Centers for Disease Control and Prevention BMI percentiles, and 34.3% were between the 85th and 95th percentiles. Most study children were born in the United States (96.4%), whereas most adults were born outside the United States, including Mexico (63.6%), El Salvador (9.4%), Honduras (6.6%), and Guatemala (6.1%). The Special Supplemental Nutrition Program for Women, Infants, and Children and/or Supplemental Nutrition Assistance Program was used by 87.5% of families, and 42.6% of families reported food insecurity. Table 1 shows baseline data by study group.
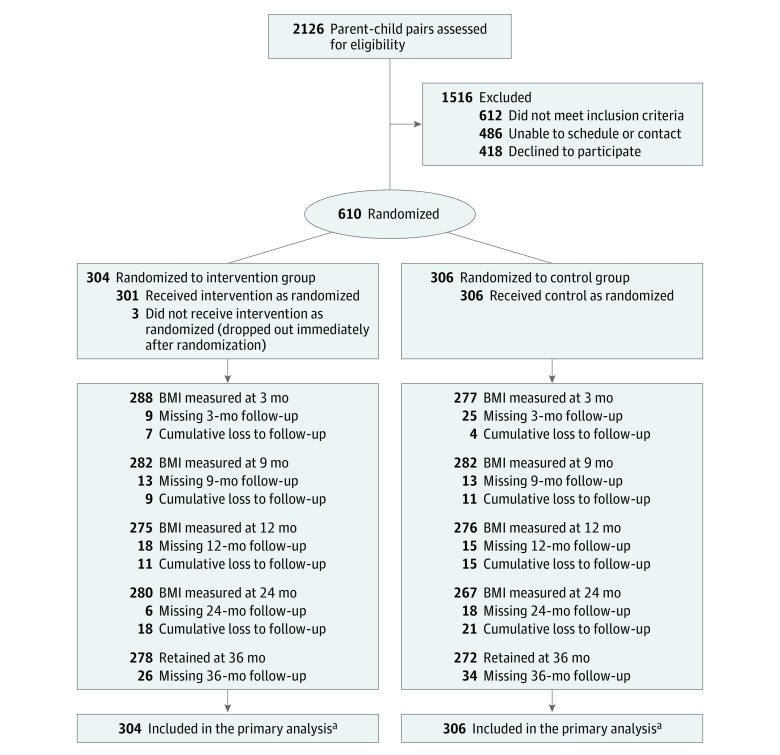
Figure 1. CONSORT Flow Diagram of the Parent-Child Pairs.
Of the 610 parent-child pairs randomized, 304 were randomized to the intervention group and 306 were randomized to the control group. At each time point, the number retained represents the number of children for whom BMI was collected. Missing body mass index (BMI) falls into 2 categories: BMI measure missing but BMI was collected at a later time point vs permanently lost to follow-up with no further BMI measures collected. The cumulative number of parent-child pairs permanently lost to follow-up is indicated at each time point above, and was 26 for the intervention group and 34 for the control group at year 3 follow-up.
aBecause the primary analysis used an intention-to-treat approach, all participants were analyzed in the group to which they were randomized, regardless of missing data.
Table 1. Characteristics of the Participants at Baselinea.
| Characteristic | Intervention (n = 304)b |
Control (n = 306)b |
|---|---|---|
| Child Characteristics | ||
| Female, No. (%) | 154 (50.7) | 162 (52.9) |
| Age at anthropometry collection, y | 4.3 (0.9) | 4.3 (0.9) |
| Anthropometry | ||
| BMI | 16.7 (0.8) | 16.6 (0.8) |
| BMI category percentiles, No. (%)c | n = 302 | n = 301 |
| 50-84.9 | 193 (63.9) | 203 (67.4) |
| 85-94.9 | 109 (36.1) | 98 (32.6) |
| BMI z score | 0.83 (0.48) | 0.82 (0.46) |
| Waist circumference, cm | 53.0 (3.4) (n = 303) | 53.1 (3.0) (n = 305) |
| Triceps skinfold, mm | 9.5 (2.7) (n = 300) | 9.7 (2.3) (n = 304) |
| Mean daily physical activity, mind | n = 302 | n = 302 |
| Total wear time, median (Q1, Q3) | 1077 (954, 1122) | 1070 (959, 1121) |
| Rest/sedentary behavior | 638.1 (120.2) | 634.3 (119.9) |
| Light physical activity | 288.4 (59.4) | 290.1 (56.6) |
| Moderate/vigorous activity | 84.1 (30.3) | 86.0 (31.4) |
| Diet | n = 304 | n = 305 |
| Mean daily total energy intake, kcal | 1184 (334) | 1202 (429) |
| Mean daily percentage of energy from fat, % | 28.5 (5.2) | 28.2 (5.3) |
| Mean daily percentage of energy from carbohydrates, % | 55.4 (6.1) | 56.1 (6.6) |
| Mean daily percentage of energy from protein, % | 16.1 (3.2) | 15.7 (3.3) |
| Race/ethnicity, No. (%) | n = 303 | n = 304 |
| Hispanic, Mexican origin | 187 (61.7) | 202 (66.5) |
| Hispanic, Non-Mexican origin | 92 (30.4) | 74 (24.3) |
| Non-Hispanic black | 19 (6.3) | 17 (5.6) |
| Non-Hispanic white | 2 (0.7) | 4 (1.3) |
| Non-Hispanic other | 3 (1.0) | 7 (2.3) |
| Adult Characteristics | ||
| Female, No. (%) | 300 (98.7) | 300 (98.0) |
| Age at anthropometry collection, y | 32.5 (6.2) | 31.6 (5.8) |
| Anthropometry | ||
| BMI | 29.8 (6.2) | 29.4 (5.3) |
| Waist circumference, cme | 97.7 (13.4) (n = 285) | 96.7 (11.9) (n = 283) |
| Triceps skinfold, mm | 31.5 (9.2) | 31.3 (8.7) |
| Race/ethnicity, No. (%) | ||
| Hispanic, Mexican origin | 183 (60.2) | 204 (66.7) |
| Hispanic, Non-Mexican origin | 95 (31.3) | 74 (24.1) |
| Non-Hispanic black | 19 (6.3) | 20 (6.5) |
| Non-Hispanic white | 4 (1.3) | 5 (1.6) |
| Non-Hispanic other | 3 (1.0) | 3 (1.0) |
| Time in the United States, median (Q1, Q3), y | 10.0 (8.0, 14.0) (n = 303) | 10.0 (7.0, 13.0) (n = 306) |
| Brief acculturation scale for Hispanics, median (Q1, Q3)f | 4.0 (4.0, 7.0) (n = 274) | 4.0 (4.0, 6.0) (n = 272) |
| Employment status, No. (%) | n = 303 | n = 306 |
| Working full time | 51 (16.8) | 57 (18.6) |
| Working part time | 52 (17.2) | 67 (21.9) |
| Not working for pay | 200 (66.0) | 182 (59.5) |
| Marital status, No. (%) | n = 303 | n = 305 |
| Married or living as married | 260 (85.8) | 244 (80.0) |
| Single | 43 (14.2) | 61 (20.0) |
| Relation to child, No. (%) | n = 303 | n = 306 |
| Mother | 293 (96.7) | 296 (96.7) |
| Father | 3 (1.0) | 6 (2.0) |
| Other | 7 (2.3) | 4 (1.3) |
| Use of WIC and/or SNAP, No. (%) | 257 (85.1) (n = 302) | 273 (89.8) (n = 304) |
| Household income, No. (%), $ | ||
| ≤14 999 | 85 (28.0) | 89 (29.1) |
| 15 000-24 999 | 90 (29.6) | 82 (26.8) |
| 25 000-34 999 | 39 (12.8) | 37 (12.1) |
| 35 000-49 999 | 7 (2.3) | 9 (2.9) |
| ≥50 000 | 2 (0.7) | 2 (0.7) |
| Don’t know or no answer | 81 (26.6) | 87 (28.4) |
| Education, No. (%) | ||
| <High school diploma | 182 (59.9) | 192 (62.7) |
| ≥High school graduate | 122 (40.1) | 114 (37.3) |
| CES-Depression (high = 16 or higher), No. (%)g | 71 (23.4) (n = 303) | 59 (19.3) (n = 306) |
| Food insecurity level, No. (%)h | n = 302 | n = 304 |
| Food secure [0-1] | 165 (54.6) | 183 (60.2) |
| Food insecure without hunger [2-4] | 86 (28.5) | 87 (28.6) |
| Food insecure with hunger [5-6] | 51 (16.9) | 34 (11.2) |
| Community center use with child, No. (%)i | n = 303 | n = 305 |
| Never | 216 (71.3) | 211 (69.2) |
| At least once | 87 (28.7) | 94 (30.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-Depression, Center for Epidemiological Studies–Depression; SNAP, Supplemental Nutrition Assistance Program; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Data are expressed as mean (SD) unless No. (%) or median (Q1, Q3) are indicated. Data that were not normally distributed are expressed as median (Q1, Q3). All randomized patients are included.
Some variables had a small amount of missing data due to not meeting the minimum criteria for inclusion (eg, insufficient wear time or not enough diet recalls), refusing to answer the question, or another unique issue.
By design, all children were to be between the 50th and 95th percentiles based on population-standardized growth curves developed by the US Centers for Disease Control and Prevention. However, 2 participants were below the 50th percentile, and 5 participants were at or above the 95th. These participants are not included in either BMI category but are included in the intention-to-treat analyses for all variables for which they provided data.
Physical activity measured with triaxial accelerometers. Individual wear time was averaged across valid wear days to produce a mean daily wear time and time in each physical activity category for each child.
Adult waist circumference: Summary is based on a total of 568 nonpregnant adults. Forty-two adults were not measured due to pregnancy.
A total of 556 Hispanic participants were eligible for assessment (intervention = 278, control = 278). The survey consists of 4 questions, and scores range from 4 to 20, with higher scores indicating greater acculturation.
The CES-Depression survey consists of 20 questions, and scores range from 0 to 60, with higher scores indicating greater depressive symptoms. Scores of 16 or greater can aid in identifying individuals at risk for clinical depression.29
Standard 6-question short-form survey for classifying households into food security status levels. The scale ranges from 0 to 6, with higher scores indicating greater food insecurity. Survey instructions were used to code raw scores into categories: 0-1, food secure; 2-4, food insecure without hunger; and 5-6, food insecure with hunger.23
Dichotomized from original 6-point scale: never, once per month or less, more than once per month, once per week, more than once per week, and every day.
Primary Outcome
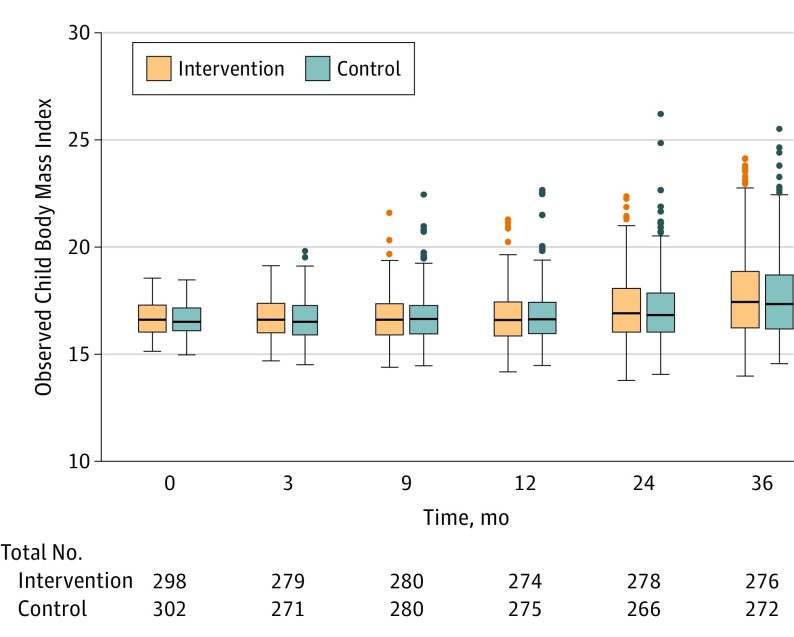
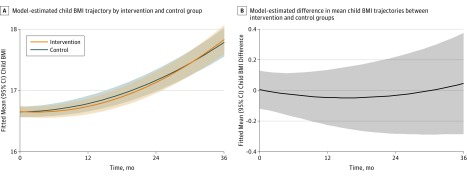
The mean (SD) child BMI at 36 months was 17.8 (2.2) in the intervention group and 17.8 (2.1) in the control group. Adjusted models showed no significant BMI difference (B = 0.05 [95% CI, −0.29 to 0.38]; P = .79) at 36 months (eTable 1 in Supplement 2). Box plots of child BMI at each time point for the intervention and control groups are presented in Figure 2. No meaningful intervention effect was detected on the prespecified primary outcome of child BMI trajectory over 3 years (joint likelihood ratio test, P = .39). Neither the linear intervention effect (BMI difference per year) (B = −0.082 [95% CI, −0.246 to 0.082]; P = .33) nor the quadratic effect (BMI difference per year squared) (B = 0.032 [95% CI, −0.014 to 0.078]; P = .18) was statistically significant (Figure 3). Model-estimated child BMI trajectories by sex and age are presented in eFigure 1 in Supplement 2.
Figure 2. Child BMI by Group at Each Follow-up Time Point.
Box plots are shown where the middle line represents the median observed child body mass index (calculated as weight in kilograms divided by height in meters squared), boxes represent the interquartile range, whiskers extend to the most extreme observed values with 1.5*IQR of the nearer quartile, and dots represent observed values outside that range.
Figure 3. Intervention Effect on Child Body Mass Index (BMI).
A, Model-estimated child BMI trajectory for the intervention (n = 304) and control (n = 306) groups. Shaded regions represent 95% CIs around each trajectory. Model-based estimates indicate the BMI linear intervention effect (BMI difference/year) was −0.082 (95% CI, −0.246 to 0.082; P = .33) and the BMI quadratic intervention effect (BMI difference/yearsquared) was 0.032 (95% CI, −0.014 to 0.078; P = .18). The joint likelihood ratio test failed to reject the null hypothesis that the linear and quadratic terms were jointly different from zero (P = .39).
B, Model-estimated difference in the mean child BMI trajectories between intervention and control groups, where a value of zero indicates no difference. Shaded region represents 95% CIs around this difference. BMI was calculated as weight in kilograms divided by height in meters squared.
Secondary Outcomes
Analyses of secondary outcomes are shown in Table 2. At 36 months, the mean (SD) child daily energy intake was 1227 (363) kcal for children in the intervention group and 1323 (397) kcal for children in the control group. The intervention resulted in a statistically significant reduction in mean child daily energy intake compared with the control group, which persisted across the 3 yearly time points. At 36 months, regression models indicated that parents in the intervention group reported their children consumed 99.4 kcal fewer than the control group (95% CI, 38.0-160.7; P = .002; corrected P = .003) and a slightly greater percentage of energy intake from protein at 24 and 36 months. No statistically significant intervention effects were detected for percentage of energy from fat or carbohydrates or mean daily time in sedentary behavior or MVPA. At 36 months, 56.8% of parents in the intervention group reported use of a community center with their child compared with 44.4% in the control group. Adjusted models indicated that participants in the intervention group were more likely to use a community center with their child vs those in the control group at all yearly time points (36-month adjusted risk ratio, 1.29 [95% CI, 1.08-1.53]; P = .004; corrected P = .006).
Table 2. Intervention Effect on Secondary Outcomesa.
| Prespecified Secondary Outcomes | Intervention (n = 304) | Control (n = 306) | Adjusted Difference (95% CI)b |
P Value | Corrected P Valuec | ||
|---|---|---|---|---|---|---|---|
| No. With Data (%) | Mean (SD) | No.With Data (%) | Mean (SD) | ||||
| Dietary Intake | |||||||
| Mean daily energy intake, kcal | |||||||
| 12 mo | 227 (74.7) | 1157 (306) | 225 (73.5) | 1261 (351) | −88.5 (−142.1 to −34.9) |
.001 | .003 |
| 24 mo | 229 (75.3) | 1212 (380) | 209 (68.3) | 1296 (372) | −82.8 (−144.6 to −21.1) |
.009 | .009 |
| 36 mo | 227 (74.7) | 1227 (363) | 219 (71.6) | 1323 (397) | −99.4 (−160.7 to −38.0) |
.002 | .003 |
| Mean energy from fat, % | |||||||
| 12 mo | 227 (74.7) | 28.2 (5.0) | 225 (73.5) | 28.5 (4.7) | −0.3 (−1.2 to 0.5) | .45 | .45 |
| 24 mo | 229 (75.3) | 27.8 (5.5) | 209 (68.3) | 28.4 (4.8) | −0.6 (−1.6 to 0.3) | .20 | .45 |
| 36 mo | 227 (74.7) | 28.6 (5.1) | 219 (71.6) | 28.9 (5.2) | −0.4 (−1.4 to 0.5) | .36 | .45 |
| Mean energy from carbohydrates, % | |||||||
| 12 mo | 227 (74.7) | 55.1 (5.9) | 225 (73.5) | 55.2 (5.8) | 0.1 (−0.9 to 1.2) | .83 | .83 |
| 24 mo | 229 (75.3) | 54.9 (6.3) | 209 (68.3) | 55.5 (5.6) | −0.4 (−1.5 to 0.7) | .45 | .80 |
| 36 mo | 227 (74.7) | 54.2 (6.0) | 219 (71.6) | 54.7 (5.8) | −0.3 (−1.4 to 0.7) | .53 | .80 |
| Mean energy from protein, % | |||||||
| 12 mo | 227 (74.7) | 16.7 (3.1) | 225 (73.5) | 16.3 (3.2) | 0.2 (−0.3 to 0.8) | .46 | .46 |
| 24 mo | 229 (75.3) | 17.3 (3.3) | 209 (68.3) | 16.1 (3.1) | 1.0 (0.4 to 1.5) | .001 | .003 |
| 36 mo | 227 (74.7) | 17.2 (3.3) | 219 (71.6) | 16.4 (3.0) | 0.7 (0.2 to 1.3) | .01 | .02 |
| Physical Activityd | |||||||
| Mean daily rest/sedentary time, min | |||||||
| 12 mo | 230 (75.7) | 619.9 (130.0) | 232 (75.8) | 618.5 (131.9) | −2.2 (−12.8 to 8.4) | .68 | .77 |
| 24 mo | 252 (82.9) | 635.5 (121.7) | 222 (72.5) | 646.8 (124.6) | −1.5 (−11.7 to 8.6) | .77 | .77 |
| 36 mo | 248 (81.6) | 663.6 (117.5) | 234 (76.5) | 660.0 (120.5) | 3.6 (−6.5 to 13.6) | .49 | .77 |
| Mean daily moderate/vigorous physical activity time, min | |||||||
| 12 mo | 230 (75.7) | 85.2 (32.2) | 232 (75.8) | 83.5 (31.9) | 1.7 (−2.7 to 6.1) | .45 | .68 |
| 24 mo | 252 (82.9) | 80.9 (31.0) | 222 (72.5) | 83.3 (33.1) | −0.2 (−4.7 to 4.4) | .95 | .95 |
| 36 mo | 248 (81.6) | 76.2 (31.8) | 234 (76.5) | 78.6 (29.3) | −1.7 (−6.0 to 2.5) | .43 | .68 |
| Community Center Use | No. With Data (%) | No. Attending (%) | No. With Data (%) | No. Attending (%) | Adjusted Risk Ratio (95% CI)e | ||
| Center use with child (never vs at least once)e | |||||||
| 12 mo | 259 (85.2) | 147 (56.8) | 258 (84.3) | 101 (39.1) | 1.47 (1.22-1.76)f | <.001 | <.001 |
| 24 mo | 263 (86.5) | 145 (55.1) | 243 (79.4) | 110 (45.3) | 1.21 (1.02-1.44)f | .03 | .03 |
| 36 mo | 259 (85.2) | 147 (56.8) | 248 (81.0) | 110 (44.4) | 1.29 (1.08-1.53)f | .004 | .006 |
Residual diagnostics were performed on each of the secondary outcome models to ensure distributional assumptions were met.
Dietary intake and physical activity adjusted differences are model estimates adjusting for baseline value of the outcome variable, age at baseline, and sex. Physical activity models also adjusted for mean daily wear time.
P values corrected for 3 comparisons using the Benjamini-Hochberg procedure.
Physical activity data are from vector magnitude output from triaxial accelerometers.
Dichotomized from original 6-point scale: never, once per month or less, more than once per month, once per week, more than once per week, and every day.
Community center use adjusted risk ratios (95% CIs) are model estimates from Poisson regression models with robust standard errors and adjusting for baseline center use.
Post-Hoc Outcomes
At study end, when children were ages 6 to 8 years, 25.4% of children in the intervention group and 23.5% of children in the control group were overweight, and 35.5% of children in the intervention group and 34.2% of children in the control group were obese. Children eligible for the adaptive intervention increased over time: n = 39 at 3-month; n = 45 at 9-month; n = 46 at 12-month; and n = 102 at 24-month follow-up. In post-hoc analysis, children in the intervention group had a significantly lower risk of developing obesity at 3-month follow-up compared with the control group before correcting for multiple comparisons (adjusted risk ratio, 0.51 [95% CI, 0.29-0.92]; P = .02; corrected P = .10). The intervention effect on reducing the estimated risk of obesity at 3 months increased as child baseline BMI increased above the mean (eFigure 2 in Supplement 2). The lower risk of obesity was not sustained at other time points (Table 3).
Table 3. Prevalence of Child Obesity at Each Time Point.
| Post Hoc Outcome: Child Obesitya | Intervention (n = 304) | Control (n = 306) | Adjusted Risk Ratio (95% CI)b | P Value for Difference | Corrected P Value for Differencec | ||
|---|---|---|---|---|---|---|---|
| No. With Data (%) | No. With Obesity (%) | No. With Data (%) | No. With Obesity (%) | ||||
| 3 mo | 279 (91.8) | 16 (5.7) | 271 (88.6) | 25 (9.2) | 0.51 (0.29- 0.92) | .02 | .10 |
| 9 mo | 280 (92.1) | 22 (7.9) | 280 (91.5) | 30 (10.7) | 0.70 (0.42- 1.15) | .16 | .27 |
| 12 mo | 274 (90.1) | 30 (10.9) | 275 (89.9) | 39 (14.2) | 0.73 (0.48- 1.10) | .13 | .27 |
| 24 mo | 278 (91.4) | 63 (22.7) | 266 (86.9) | 61 (22.9) | 0.92 (0.70- 1.21) | .57 | .71 |
| 36 mo | 276 (90.8) | 98 (35.5) | 272 (88.9) | 93 (34.2) | 0.99 (0.80- 1.22) | .90 | .90 |
Obesity defined as 95th percentile or above based on population-standardized growth curves developed by the US Centers for Disease Control and Prevention.
Risk ratios are from Poisson regression models with robust standard errors and adjusting for child baseline body mass index, age, and sex.
P values corrected for 5 multiple comparisons using the Benjamini-Hochberg procedure.
Post-hoc exploratory moderator analyses indicated statistically significant intervention effects on the linear and quadratic growth of BMI of children who were food insecure with hunger at baseline (eFigure 3 in Supplement 2). Significant quadratic intervention effects were also found for males and baseline child energy intake. No other statistically significant moderator effects were found (eTable 2 in Supplement 2).
Adherence to Study Protocol
The mean dose received among intervention participants was 92% for the intensive phase, 87% for the maintenance phase, and 85% for the sustainability phase. School-readiness dose receipt in both conditions was 83% throughout the study period. No crossover occurred between conditions. Fidelity to the intervention curriculum was 99% over the 3 years.
Adverse Events
One parent fractured an ankle while roller-skating during an event at a local community center. No additional intervention-related adverse events occurred.
Discussion
This 36-month community-based, family-centered, behavioral intervention did not change BMI trajectory in underserved preschool children who were not yet obese. The primary outcome of child BMI trajectory was selected in lieu of other standardized outcomes (eg, BMI z score) to capture potential differences in child growth curve shapes known to be predictive of later cardiovascular risk.11,30 Throughout the 36-month trial, the intervention and control groups demonstrated nearly identical growth trajectories and rates of obesity. The prevalence of obesity observed in both the intervention and control groups was similar to the regional prevalence of obesity for Latino children (37.7%), indicating that the behavioral intervention did not alter the usual pattern of obesity in this low-income minority population.31 The study was adequately powered to detect differences in child BMI trajectory and achieved 90% retention with little differential attrition. In addition, the precision of the effect estimates (ie, confidence intervals for the difference in mean child BMI trajectories) is sufficient to conclude that no meaningful difference existed in the primary outcome between the intervention and control groups.
Of the more than 350 RCTs conducted to prevent or treat childhood obesity, few have demonstrated successful BMI change and most have studied higher-resourced populations, had small effect sizes not always of clinical significance, and/or lacked long-term follow-up. RCTs conducted with high quality and an independent coordinating center have consistently failed to produce meaningful, sustained results in childhood obesity prevention.32,33 This pattern of unsuccessful childhood obesity prevention interventions is consistent with the findings of this study, which, to our knowledge, is the largest and longest obesity prevention RCT of its kind to date. This study was consistent with recent recommendations from the US Preventive Services Task Force to achieve 26 hours of contact time in year 1 but then it decreased in subsequent years.34 The US Preventive Services Task Force examined the dose required for obesity treatment for children 6 years and older. There are no guidelines yet for what is needed to achieve effective obesity prevention. Prevention of childhood obesity in low-income, underserved populations could require an increased intensity of behavioral interventions over longer periods of time.
While there was no effect on the primary outcome, the multicomponent intervention demonstrated effects on the secondary outcomes of diet and use of the community center for physical activity. Children in the intervention condition consumed almost 100 fewer kcal per day and had a higher percentage of energy from protein compared with children in the control condition. While the intervention did not change the already high levels of child MVPA, children in the intervention used their local community centers for family physical activity more frequently than the control group, although the control group participants increased their use as well. Previous research has hypothesized that health behavior changes of this magnitude would result in modest improvements in BMI.35 However, previous population-based modeling studies have indicated that populations from different socioeconomic strata as well as racial and ethnic subgroups have different thresholds for achieving healthy weight, with Latino and African American populations requiring more than 100-kcal differences.35
There are several potential explanations for why a close to 100-kcal reduction and increased use of the built environment would not result in child BMI change. First, measurement bias due to the reliance on parent-report measures may have led to these results, suggesting the need for confirmation in controlled settings.36,37 However, the multipass protocol required for 24-hour diet recalls and the absence of direct nutrition education during the more passive 24-month sustainability phase might make a systematic measurement bias less likely. Second, while some individual behavior change can result from interventions such as these, achieving a sufficient amount of individual-level behavior changes in the family and community environment may not be feasible for these extremely low-income minority populations.
A notable characteristic of this trial was the exclusive enrollment of parent-child pairs from significant poverty. Parental depression was reported by 21.4% of these families, and 42.6% reported food insecurity at baseline. Previous literature suggests that biologically embedded obesity phenotypes can be produced by toxic stress, altering homeostatic regulation of pathways that influence resting metabolic rate, satiety set points, and epigenetics even before obesity manifests.38 Research has also found that diversity of microbiome species and exposure to endocrine disruptors early in life affect changes in metabolic function, in some cases, regardless of calories consumed.39,40 These influences could potentially alter an individual’s energy balance so that even a statistically significant reduction in daily energy intake might not be clinically meaningful. Further evaluation may be warranted and underscores the importance of measuring biological-level mediators in long-term, high-intensity behavioral obesity interventions.
Post-hoc analyses indicated several findings that should be interpreted with caution, generating hypotheses for future research. First, the intervention reduced obesity prevalence after the 3-month intensive phase, but this reduction was not sustained. The effect was most pronounced in children with higher baseline BMI. This finding is consistent with previous literature on childhood obesity treatment in which short-term BMI improvements are achieved for those with higher baseline BMI, but long-term BMI improvements are not realized. Second, post-hoc moderator analyses indicated that the intervention may have been more effective for certain population subgroups, suggesting that tailored interventions may be needed. The moderator analyses indicated that intervention group children who experienced food insecurity with hunger at baseline had a different BMI growth trajectory over 3 years. This finding emphasizes the importance of addressing systemic factors that affect health behaviors to achieve child obesity prevention.
Limitations
This study had several limitations. First, because the study was conducted among low-income minority populations, the findings should not be generalized to other populations. Second, data on biological measures of cardiovascular or diabetes risk were not collected. Third, energy and nutrient intake were assessed by parent report of child diet and subject to social desirability bias that may differ as a result of intervention participation.37 Fourth, this trial focused on the preschool period, which may not be the optimal age for obesity prevention. Future research should clarify the optimal timing of obesity prevention interventions.
Conclusions
A 36-month multicomponent behavioral intervention did not change BMI trajectory among underserved preschool-age children in Nashville, Tennessee, compared with a control program. Whether there would be effectiveness for other types of behavioral interventions or implementation in other cities would require further research.
Trial Protocol and Statistical Analysis Plan
eTable 1. Child BMI at Each Time Point
eTable 2. Intervention Moderation Effects on Child BMI Trajectory
eFigure 1. Model-Estimated Child BMI Trajectories for Intervention and Control Groups by Baseline Age for Males and Females
eFigure 2. Intervention Effect on Child Obesity at 3 Months
eFigure 3. Model-Estimated Child BMI Trajectories for Intervention and Control Groups Moderated by Baseline Food Security Status
References
- 1.Gillman MW, Barker D, Bier D, et al. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatr Res. 2007;61(5, pt 1):625-629. doi: 10.1203/pdr.0b013e3180459fcd [DOI] [PubMed] [Google Scholar]
- 2.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring). 2008;16(7):1651-1656. doi: 10.1038/oby.2008.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heerman WJ, JaKa MM, Berge JM, et al. The dose of behavioral interventions to prevent and treat childhood obesity: a systematic review and meta-regression. Int J Behav Nutr Phys Act. 2017;14(1):157. doi: 10.1186/s12966-017-0615-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waters E, de Silva-Sanigorski A, Hall BJ, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011;(12):CD001871. [DOI] [PubMed] [Google Scholar]
- 5.Seo DC, Sa J. A meta-analysis of obesity interventions among US minority children. J Adolesc Health. 2010;46(4):309-323. doi: 10.1016/j.jadohealth.2009.11.202 [DOI] [PubMed] [Google Scholar]
- 6.Lin JS, O’Connor E, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral counseling to promote a healthy lifestyle in persons with cardiovascular risk factors: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2014;161(8):568-578. doi: 10.7326/M14-0130 [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. Washington, DC: National Academies Press; 2012. [Google Scholar]
- 8.Institute of Medicine Examining a Developmental Approach to Childhood Obesity: The Fetal and Early Childhood Obesity: The Fetal and Early Childhood Years: Workshop Summary. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 9.McGuire S. Institute of Medicine, 2012: accelerating progress in obesity prevention: solving the weight of the nation. Washington, DC: the National Academies Press. Adv Nutr. 2012;3(5):708-709. doi: 10.3945/an.112.002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802-1809. doi: 10.1056/NEJMoa044160 [DOI] [PubMed] [Google Scholar]
- 11.Péneau S, González-Carrascosa R, Gusto G, et al. Age at adiposity rebound: determinants and association with nutritional status and the metabolic syndrome at adulthood. Int J Obes (Lond). 2016;40(7):1150-1156. doi: 10.1038/ijo.2016.39 [DOI] [PubMed] [Google Scholar]
- 12.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723-1725. doi: 10.1001/jama.2018.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heerman WJ, White RO, Barkin SL. Advancing informed consent for vulnerable populations. Pediatrics. 2015;135(3):e562-e564. doi: 10.1542/peds.2014-3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Po’e EK, Heerman WJ, Mistry RS, Barkin SL. Growing Right Onto Wellness (GROW): a family-centered, community-based obesity prevention randomized controlled trial for preschool child-parent pairs. Contemp Clin Trials. 2013;36(2):436-449. doi: 10.1016/j.cct.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959-1968. doi: 10.1056/NEJMoa1108660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratt CA, Stevens J, Daniels S. Childhood obesity prevention and treatment: recommendations for future research. Am J Prev Med. 2008;35(3):249-252. doi: 10.1016/j.amepre.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronfenbrenner U. Ecology of the family as a context for human-development: research perspectives. Dev Psychol. 1986;22(6):723-742. doi: 10.1037/0012-1649.22.6.723 [DOI] [Google Scholar]
- 20.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 21.Gesell SB, Bess KD, Barkin SL. Understanding the social networks that form within the context of an obesity prevention intervention. J Obes. 2012;2012:749832. doi: 10.1155/2012/749832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butte NF, Wong WW, Lee JS, Adolph AL, Puyau MR, Zakeri IF. Prediction of energy expenditure and physical activity in preschoolers. Med Sci Sports Exerc. 2014;46(6):1216-1226. doi: 10.1249/MSS.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulliford MC, Mahabir D, Rocke B. Reliability and validity of a short form household food security scale in a Caribbean community. BMC Public Health. 2004;4:22. doi: 10.1186/1471-2458-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkin SL, Gesell SB, Po’e EK, Escarfuller J, Tempesti T. Culturally tailored, family-centered, behavioral obesity intervention for Latino-American preschool-aged children. Pediatrics. 2012;130(3):445-456. doi: 10.1542/peds.2011-3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harold GT, Kerr DC, Van Ryzin M, DeGarmo DS, Rhoades KA, Leve LD. Depressive symptom trajectories among girls in the juvenile justice system: 24-month outcomes of an RCT of multidimensional treatment foster care. Prev Sci. 2013;14(5):437-446. doi: 10.1007/s11121-012-0317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer J, Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford, England: Oxford University Press; 2003. doi: 10.1093/acprof:oso/9780195152968.001.0001 [DOI] [Google Scholar]
- 27.Huang TT, Drewnosksi A, Kumanyika S, Glass TA. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009;6(3):A82. [PMC free article] [PubMed] [Google Scholar]
- 28.Janicke DM, Steele RG, Gayes LA, et al. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. J Pediatr Psychol. 2014;39(8):809-825. doi: 10.1093/jpepsy/jsu023 [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 30.Sovio U, Kaakinen M, Tzoulaki I, et al. How do changes in body mass index in infancy and childhood associate with cardiometabolic profile in adulthood? findings from the Northern Finland Birth Cohort 1966 Study. Int J Obes (Lond). 2014;38(1):53-59. doi: 10.1038/ijo.2013.165 [DOI] [PubMed] [Google Scholar]
- 31.Trust for America's Health The state of obesity: better policies for a healthier America. http://healthyamericans.org/reports/stateofobesity2017/. Published August 2017. Accessed November 17, 2017.
- 32.Wang Y, Cai L, Wu Y, et al. What childhood obesity prevention programmes work? a systematic review and meta-analysis. Obes Rev. 2015;16(7):547-565. doi: 10.1111/obr.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleich SN, Segal J, Wu Y, Wilson R, Wang Y. Systematic review of community-based childhood obesity prevention studies. Pediatrics. 2013;132(1):e201-e210. doi: 10.1542/peds.2013-0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(23):2427-2444. doi: 10.1001/jama.2017.0332 [DOI] [PubMed] [Google Scholar]
- 35.Wang YC, Orleans CT, Gortmaker SL. Reaching the healthy people goals for reducing childhood obesity: closing the energy gap. Am J Prev Med. 2012;42(5):437-444. doi: 10.1016/j.amepre.2012.01.018 [DOI] [PubMed] [Google Scholar]
- 36.Harnack L, Himes JH, Anliker J, et al. Intervention-related bias in reporting of food intake by fifth-grade children participating in an obesity prevention study. Am J Epidemiol. 2004;160(11):1117-1121. doi: 10.1093/aje/kwh328 [DOI] [PubMed] [Google Scholar]
- 37.Stevens J, Taber DR, Murray DM, Ward DS. Advances and controversies in the design of obesity prevention trials. Obesity (Silver Spring). 2007;15(9):2163-2170. doi: 10.1038/oby.2007.257 [DOI] [PubMed] [Google Scholar]
- 38.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252-2259. doi: 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- 39.Tran V, Tindula G, Huen K, et al. Prenatal phthalate exposure and 8-isoprostane among Mexican-American children with high prevalence of obesity. J Dev Orig Health Dis. 2017;8(2):196-205. doi: 10.1017/S2040174416000763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22(4):117-123. doi: 10.1016/j.tem.2011.01.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Child BMI at Each Time Point
eTable 2. Intervention Moderation Effects on Child BMI Trajectory
eFigure 1. Model-Estimated Child BMI Trajectories for Intervention and Control Groups by Baseline Age for Males and Females
eFigure 2. Intervention Effect on Child Obesity at 3 Months
eFigure 3. Model-Estimated Child BMI Trajectories for Intervention and Control Groups Moderated by Baseline Food Security Status