Introduction
Cryptococcus neoformans is classically an opportunistic pathogen capable of causing a disseminated invasive fungal infection in humans and animals. This encapsulated yeast has been isolated from soil samples worldwide, with the principle source believed to be pigeon droppings1. The prevalence of this infection in immunocompromised hosts is widely documented; however, no discernable underlying comorbidity is found in 20% of affected individuals2. The pathogen is believed to gain entry to the host through the respiratory tract and is presumably disseminated hematogenously. Any organ may be affected; however, primary sites of involvement include the lungs and central nervous system. While systemic infection bone involvement may occur in up to 10% of cases, isolated skeletal involvement remains extremely rare3, 4, 5, 6, 7, 8. Prior to this case only 51 cases of isolated cryptococcal osteomyelitis have been reported, and 40% of these patients suffered from systemic disease9. Here we report a case of isolated cryptococcal osteomyelitis in the proximal femur of a healthy 27‐year‐old male. The authors have obtained the patient's informed written consent for print and electronic publication of the case report.
Case Report
A previously healthy 27‐year‐old male with an admitted history of heroin use was referred to our orthopaedic oncology service following MRI findings for primary bone malignancy involving the right proximal femur (Figs 1, 2, 3). The patient presented with a 3‐week history of progressive resting and activity related right hip and thigh pain. On exam he was afebrile, alert, and in no apparent distress. Significant exam findings included an antalgic gait, painful straight leg raise, and tenderness to palpation over the right greater trochanter. Short arc hip range of motion was painless, and the remainder of his exam was benign. On review of systems he had a “toothache” one‐month prior. He denied constitutional symptoms, and the remainder of a 12‐point review of systems was negative. His past medical history was noncontributory, and pertinent social history included an occupation as a janitor at a local university. When asked in retrospect, the patient did report unmasked exposure to bird droppings as a part of his occupation as a janitor. He initially denied use of alcohol, tobacco, or intravenous drugs but later admitted to heroin use at a later follow‐up visit. Radiographs of the right hip demonstrated a 2 cm purely lytic lesion of the proximal femur without sclerosis or periosteal reaction. MRI demonstrated an intramedullary proximal femur lesion with extensive peripheral edema. The differential diagnosis included infection, Ewing's sarcoma, osteosarcoma, lymphoma, and histiocytosis X. A technetium‐99 bone scan was obtained and demonstrated increased osteoblastic activity of the right proximal femur and mandible that correlated with his history of a “toothache.” Relevant laboratory studies included a white cell count of 11.6 K/μL, erythrocyte sedimentation rate (ESR) of 59 mm/h, and a C‐reactive protein (CRP) of 59.8 mg/L.
Figure 1.
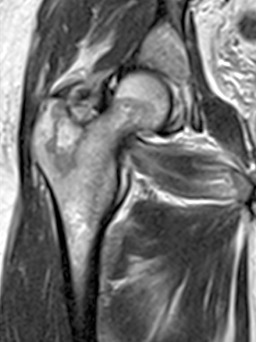
Proton density T2 sequence coronal cut MRI showing infiltrative lesion of the right proximal femur with geographic margins.
Figure 2.
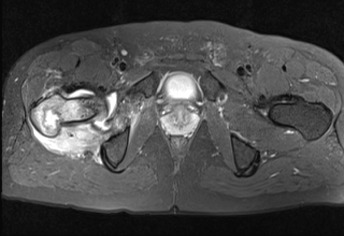
Proton density fat suppressed sequence axial cut MRI demonstrating a proximal femur lesion with erosion of the posteromedial cortex and significant local edema.
Figure 3.
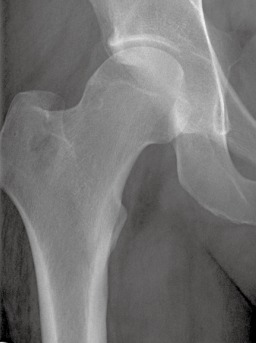
Anterior‐posterior radiograph of the right hip demonstrating geographic osteolytic proximal femur lesion without periosteal reaction.
On the second hospital day the patient was taken to the operating room for an open biopsy of the right greater trochanter lesion. Gross purulence was encountered intraoperatively, and frozen section of the metaphyseal cancellous bone demonstrated heavy purulence consistent with osteomyelitis and, less likely, lymphoma. A biopsy specimen was sent for aerobic, anaerobic, acid fast and fungal cultures. A permanent specimen was sent for pathology and immunohistochemical staining. The lesion was curettaged, and the void was filled with approximately 20 mL of calcium sulfate bone graft substitute impregnated with 0.5 g of vancomycin and 0.6 g of tobramycin (limited dosage secondary to cavity size, Fig. 4). Postoperatively the patient was treated with intravenous vancomycin and zosyn at the recommendation of our infectious disease consultants. A dental consult was obtained to evaluate periodontal disease. However, the patient refused a recommendation for tooth extraction. Anaerobic culture showed early growth of peptostreptococcus and propionibacterium. On the fourth postoperative day fungal cultures demonstrated late growth of Cryptococcus neoformans.
Figure 4.
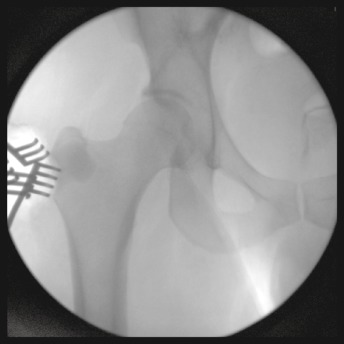
Intraoperative fluoroscopy of the right hip following curettage and injection of antibiotic impregnated bone graft substitute.
The patient's postoperative course was complicated only by pain at the surgical site, which limited his mobility. The compromised bone was protected with toe touch weight bearing. With the exception of one spiked fever to 39.3°C, and on the fourth postoperative day he remained afebrile. Following the late growth of Cryptococcus, the patient had an extensive work up to rule out underling systemic infection. Cerebrospinal fluid was collected and was negative for cryptococcal antigen. Cerebrospinal fluid and blood fungal cultures were negative on discharge. The patient was nonreactive to human immunodeficiency virus‐1 (HIV‐1)/HIV‐2 antibody. The final pathologic diagnosis was acute and chronic osteomyelitis, and immunophenotyping was within normal limits. Long‐term oral antibiotic and antifungal treatment recommendations consisted of fluconazole 400 mg daily for 6 months and Augmentin 875 mg twice daily for 6 weeks. The patient's pain improved, and he was ambulatory with crutches at the time of discharge.
The patient completed his course of augmentin. However, he has been noncompliant with fluconazole therapy since his 2‐month postoperative visit, complaining of financial constraints, but also admitted to resuming the use of heroin. Reinstitution of antifungal therapy was unsuccessful despite the provision of financial aid, which was terminated after he admitted drug use. While he has been weight bearing as tolerated on the affected extremity since the 2‐month postoperative visit, the patient continued to complain of hip pain at his 4‐month visit. Radiographs demonstrated healing of the site of curettage and bone graft substitute (Fig. 5). Physical exam at this time was benign, complete blood count was normal, ESR was 12 mm/h and CRP was 14 mg/L. At this time the patient was referred to our infectious disease consultants for further recommendations. At the eighth month, the infectious disease consultant noted that the patient was clinically asymptomatic and planned to repeat radiographs, complete blood count (CBC), and inflammatory biomarkers to determine whether additional antifungal therapy was warranted. Unfortunately, due to continued IV drug abuse, the patient was lost to follow‐up at this time.
Figure 5.
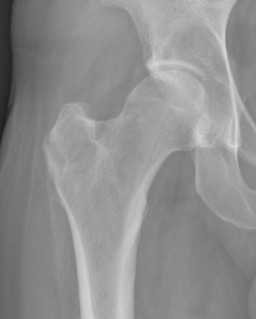
Anterior‐posterior radiograph of the right proximal femur 4 months following curettage.
Discussion
Cryptococcus was first isolated from soil as well as the droppings and nests of pigeons in the 1950s by Emmons, although this ubiquitous pathogen has since been isolated in soil samples from around the world. Severely immunocompromised patients are most commonly affected, particularly HIV patients with CD4 lymphocyte counts less than 100 cells/μL of blood2. The commonly accepted method of host entry is through the respiratory tract; however, traumatic inoculation has been reported. The primary host defense is through cell‐mediated immunity. Following inoculation the infection can take one of several pathways, including clinical disease in the immunosuppressed, elimination of the infection with resultant immunity, and a dormant infection that may be reactivated if immunosuppression occurs at a later date2. The encapsulated yeast may evade host immunity by surviving within macrophage vacuoles. The host inflammatory response and additional monocyte recruitment may propagate infection. In the vast majority of individuals, host immunity is effective at eradicating infection after initial exposure. Surprisingly, most adults possess an antibody to Cryptococcus, and in New York City most children acquire antibodies to cryptococcal antigens before age 10, suggesting that asymptomatic infections are not infrequent2.
In addition to acquired immune deficiency syndrome (AIDS) patients, other groups or conditions associated with increased risk include: malignancy, organ transplant recipients, sarcoidosis, lymphoproliferative disorders, diabetes, systemic lupus erythematosus, cirrhosis, peritoneal dialysis patients, and patients treated with monoclonal antibodies. Despite the association of cryptococcosis and immunosuppression, an estimated 20% of infected patients have no discernible underlying disease2. In a cohort of 109 HIV‐negative patients the two most common sites of infection included the pulmonary (36%) and central nervous systems (51%)2. Specifically, bone involvement occurs in 5–10% of patients, with disseminated disease. Isolated osseous involvement remains very rare4, 5, 6, 7, 8, 10.
Based on a review of the literature, the presentation of this patient is consistent with prior reports. Patients typically complain of focal pain and tenderness. Symptoms are typically progressive and may range from trivial at onset to refusal to bear weight11, 12. When a bony prominence is involved, erythema, soft tissue swelling, and tenderness to palpation were noted9, 11, 13. Constitutional symptoms are infrequently reported. In one case, however, a pediatric patient reported a 9 kg weight loss over 6 months12. An evaluation of the literature also reported a range of duration of symptoms from 2 weeks to 33 months (median 3 months)14.
In the largest review of isolated cryptococcal osteomyelitis with documented radiographic appearance, lesions were uniformly lytic or erosive in nature10. While many of these lesions have well‐defined geographic borders, this is not a constant finding15. Cortical destruction with an associated purulent soft tissue fluid collection is frequent, although periosteal reaction or sclerotic margins are rare. As in this case, the metaphyses of long bones are frequently involved, and some authors have noted a predilection for bony promenences15. While infection of nearly every bone has been described, the ribs and vertebrae appear to be equally affected, accounting for approximately 20% of cases each (10/51). Single bone involvement was predominant in the cases reviewed, but multiple sites of osseous involvement have been reported, and all patients’ musculoskeletal complaints should be investigated.
Open biopsy, fine needle aspiration, and serology have all been reported as diagnostic tools in the literature. Given the history and radiographic appearance the differential diagnosis should include fungal infection, bacterial infection, benign neoplasm, or low‐grade malignancy. For this reason, our institution prefers open biopsy for pathologic and microbiologic diagnosis. This is consistent with the most common method of diagnosis reported by Behrman14. We would caution against isolated serological testing for establishment of diagnosis, as detection of antigen will be negative in 34% of non‐HIV patients with cryptococcal disease16. It is important to send all bone cultures for aerobic, anaerobic, fungal, and mycobacterial analysis, as the diagnosis may be missed if broad‐spectrum cultures are not obtained.
Curettage in combination with antifungals, antifungals alone, and rarely surgical excision alone has all been demonstrated to effect cure in reported cases9, 14, 16. Select cases in which surgical excision alone was curative demonstrate the effectiveness of this modality at rapid elimination of fungal load. While most reported cases have been managed medically, there is no standard with regard to treatment duration. Given the potential side effects of prolonged antifungal therapy, surgery may be an important adjuvant in limiting treatment duration. According to the 2010 guidelines for management of cryptococcosis in an immunocompetent host, if central nervous system disease is ruled out and fungemia is not present, than fluconazole 400 mg for 6–12 months should be considered17. Before this is considered it is imperative to rule out unrecognized disseminated disease. Chest radiographs should be obtained to rule out pulmonary foci, cerebrospinal fluid should be analyzed for cryptococcal antigen, and consent for HIV testing should be sought. Additionally, a CD4 count may assist with diagnosis of idiopathic lymphopenia.
This rare clinical case demonstrates that an infectious etiology of any species must remain in the differential diagnosis when malignancy is considered. Specifically, Cryptococcus neoformans and other fungal infections should be included in the differential diagnosis of a patient presenting with purely lytic lesion on plain radiographs without evidence of periosteal reaction. Tissue samples should be obtained, and cultures and staining specific to fungal, mycobacterial, aerobic and anaerobic pathogens should be sought. In the case of Cryptococcus neoformans, it is imperative to rule out respiratory and central nervous system involvement as well as fungemia, as progression to disseminated disease may occur in up to 62% of HIV‐seronegative patients7, 16. Treatment decisions must be patient‐specific, and involvement of an infectious disease specialist is prudent.
Disclosure: The authors declare no conflict of interest. No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. Wildstein MS, Martin SM Jr, Glaser JA. Cryptococcal osteomyelitis in a 20‐year‐old male with sarcoidosis. Spine J, 2005, 5: 467–470. [DOI] [PubMed] [Google Scholar]
- 2. Perfect JR. Cryptococcus neoformans In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Disease. Vol. 2 6th edn New York: Churchill Livingstone, 2010; 2997–3009. [Google Scholar]
- 3. Collins VP. Bone involvement in cryptococcosis (torulosis). Am J Roentgenol Radium Ther, 1950, 63: 102–112. [PubMed] [Google Scholar]
- 4. Abdul‐Karim FW, Pathria MN, Heller JG, et al Case report 664. Cryptococcus neoformans osteomyelitis. Skeletal Radiol, 1991, 20: 227–229. [DOI] [PubMed] [Google Scholar]
- 5. Amit A, Sudish K, Pople IK. Primary calvarial cryptococcal osteomyelitis in a patient with idiopathic lymphopenia. Acta Neurochir (Wien), 2008, 150: 713–714. [DOI] [PubMed] [Google Scholar]
- 6. Govender S, Mutasa E, Parbhoo AH. Cryptococcal osteomyelitis of the spine. J Bone Joint Surg Br, 1999, 81: 459–461. [DOI] [PubMed] [Google Scholar]
- 7. Levine AM, Meier P, Dorfman HD. Isolated cryptococcus osteomyelitis of the humerus simulating a neoplasm of bone in a patient with sarcoidosis. Case report 329. Skeletal Radiol, 1985, 14: 152–156. [DOI] [PubMed] [Google Scholar]
- 8. Sethi S. Cryptococcal osteomyelitis in the ribs. J Glob Infect Dis, 2010, 2: 63–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu PY. Cryptococcal osteomyelitis: case report and review. Diagn Microbiol Infect Dis, 1998, 30: 33–35. [DOI] [PubMed] [Google Scholar]
- 10. Burch KH, Fine G, Quinn EL, et al Cryptococcus neoformans as a cause of lytic bone lesions. JAMA, 1975, 231: 1057–1059. [PubMed] [Google Scholar]
- 11. Annapureddy SR, Masterson SW, David HG, et al Post partum osteomyelitis due to Cryptococcus neoformans . Scand J Infect Dis, 2007, 39: 354–356. [DOI] [PubMed] [Google Scholar]
- 12. Raftopoulos I, Meller JL, Harris V, et al Cryptococcal rib osteomyelitis in a pediatric patient. J Pediatr Surg, 1998, 33: 771–773. [DOI] [PubMed] [Google Scholar]
- 13. Goldshteyn N, Zanchi A, Cooke K, et al Cryptococcal osteomyelitis of the humeral head initially diagnosed as avascular necrosis. South Med J, 2006, 99: 1140–1141. [DOI] [PubMed] [Google Scholar]
- 14. Behrman RE, Masci JR, Nicholas P. Cryptococcal skeletal infections: case report and review. Rev Infect Dis, 1990, 12: 181–190. [DOI] [PubMed] [Google Scholar]
- 15. Amenta PS, Stead J, Kricun ME. Case report 226: isolated Cryptococcus neoformans osteomyelitis of femur. Skeletal Radiol, 1983, 9: 263–265. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Tawfiq JA, Ghandour J. Cryptococcus neoformans abscess and osteomyelitis in an immunocompetent patient with tuberculous lymphadenitis. Infection, 2007, 35: 377–382. [DOI] [PubMed] [Google Scholar]
- 17. Perfect JR, Dismukes WE, Dromer F, et al Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis, 2010, 50: 291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]


