Abstract
Objective
Treatment of distal femoral fracture in post‐polio patients is difficult because the bone is usually osteopenic, small and deformed. This retrospective study aimed to investigate the outcomes of distal femoral fracture in post‐polio patients treated by locking compression plates (LCP).
Methods
The medical records of 19 post‐polio patients (mean age 49 years at time of surgery) were reviewed and intraoperative data retrieved. Fracture union and callus formation were evaluated on radiographs taken at each postoperative visit. Functional outcome assessments included range of motion and Hospital for Special Surgery (HSS) score of the ipsilateral knee joint.
Results
Sixteen femoral fractures occurred in the poliomyelitis‐affected limbs. The mean duration of operation was 86 min and mean blood loss 120 mL. All fractures healed (mean, four months) but union was delayed in one. At the final follow‐up 2 yrs after surgery, the mean range of knee flexion was 105° (range, 90°–130°), and mean HSS score 76 points (range, 60–93). There were no cases of nonunion, implant cutout, or other complications.
Conclusions
LCP provides stable fixation of distal femoral fractures in post‐polio patients. Bony union and good functional outcomes are achieved, but delayed union and minimal callus may occur.
Keywords: Femur, Internal fixation, Osteopenia, Poliomyelitis
Introduction
Poliomyelitis is an acute viral infectious disease caused by infection of motor neurons with poliovirus, resulting in denervation of muscle fibers1, 2, 3, 4. Most patients who survive acute poliomyelitis have multiple sequelae1 , 5 , 6. A common sequela is paralysis of the lower extremities, characterized by flaccid muscle tone, asymmetric involvement and retarded growth7. With aging, affected limbs may become increasingly flaccid and weakened, resulting in limited ambulation8.
Clinical dilemmas caused by post‐polio syndrome may be encountered by orthopaedic trauma surgeons. Patients are at risk of falling and sustaining fractures2 , 4 , 7 , 9. Morbidity ranges from 35% to 48%2 , 10 , 11. Fractures commonly occur in the distal femur and proximal humerus11. Distal femoral fracture in elderly patients with osteopenia presents a difficult management problem12, especially in post‐polio patients. In addition to the higher incidence of osteopenia in post‐polio patients7 , 10 , 11, the bones on the affected side may be small, deformed and hypovascular because of the decreased bulk and vascularity of the paralyzed muscles, all these factors can contribute to poor fracture healing13, 14, 15. Furthermore, the decreased muscle strength and limited ambulation may negatively affect postoperative rehabilitation11. Although surgical outcomes of distal femoral fracture in adult and elderly patients have been reported16, 17, 18, 19, 20, 21, results of treatment of this fracture in post‐polio patients have not been well documented.
Locking compression plates (LCPs), which provide angular stability by minimizing interference with the fracture site, have been used for treatment of distal femoral fractures in osteopenic bone22 , 23. LCPs have theoretical advantages for post‐polio patients. A retrospective study of femoral fractures in post‐polio patients, including fractures in the proximal part, midshaft and distal part of the femur, has shown that LCPs may address these challenges and provide good outcomes24. Despite this one study, limited information is available about the treatment of femoral fractures with LCP in post‐polio patients.
We postulated that LCPs may provide stable fixation and satisfactory clinical outcomes in distal femoral fractures in post‐polio patients. In the current study, we retrospectively evaluated a series of distal femoral fractures treated with LCPs. The purpose of the study was to evaluate the technique and outcomes of using LCP to treat distal femoral fractures in post‐polio patients.
Materials and Methods
From January 2005 to March 2011, 19 consecutive post‐polio patients (11 men and 8 women; mean age, 49 years; range, 37–63 years) sustained unilateral distal femoral fracture and were treated with LCPs in our hospital. The fractures were caused by falls during activities of daily living (15 patients), motorcycle accidents (3 patients) and a fall from a bicycle (1 patient). The fractures were all closed and classified (Association for Osteosynthesis /Orthopedic Trauma Association classification) as 32‐B2 in four patients, 32‐B3 in three, 32‐C3 in two, 33‐A1 in two, 33‐A2 in one, 33‐B1 in four and 33‐C1 in three25. Surgery was performed within 1 week of injury in 15 fractures and delayed >1 week for 4 fractures because of general health problems.
Surgical Technique
All surgical procedures were done by one senior surgeon (JX). The patient was placed in the supine position on a radiolucent operation table. The leg was freed from traction. No tourniquet was applied. A longitudinal parapatellar incision (8–10 cm) was made over the lateral femoral condyle. The subcutaneous tissues were incised in line with the skin incision to expose the distal femur. Anatomic reduction of the articular surface was achieved for intra‐articular fractures (types 33‐B and 33‐C). Kirschner wires were used for temporary fixation of the fracture fragments. For extra‐articular fractures, indirect reduction was guided by fluoroscopic radiography, with continuous traction on the calf, valgus or varus stress and pointed reduction forceps. For comminuted shaft fractures, only the alignment on coronal and sagittal planes was restored by traction; the fracture sites were not exposed. Strong muscle tension was noted during fracture reduction and distraction rods were utilized when satisfactory alignment could not be achieved with manual traction alone.
Locking compression plates (Synthes, Solothurn, Switzerland) were used for internal fixation after fracture reduction had been achieved. A plate of length that provided ≥ 3 plate holes proximal to the fracture was selected (Fig. 1). A submuscular tunnel along the femur was created using an osteotome. The LCP was inserted through the tunnel and the level of the distal end of the plate checked by palpation and fluoroscopy. Locking screws were inserted to fix the distal part of plate to the distal femur. An additional incision was made to expose the proximal end of the plate. A conventional cortical screw was usually inserted through the combination hole to compress the fracture and adjust the alignment, then locking fixation screws were inserted with insertion guides. When femoral deformity was present and a precise fit of the LCP on bone was not feasible, the LCP was placed on the bone and locking screws were inserted so that the plate could function as an internal fixator. Where deformation or a small diameter bone shaft prevented use of proximal locking fixation screws, bio‐cortical fixation was not achievable for all screws. In these cases locking screws were used in the two proximal holes and one cortical screw inserted into the third hole for bio‐cortical fixation. No bone grafts were used.
Figure 1.
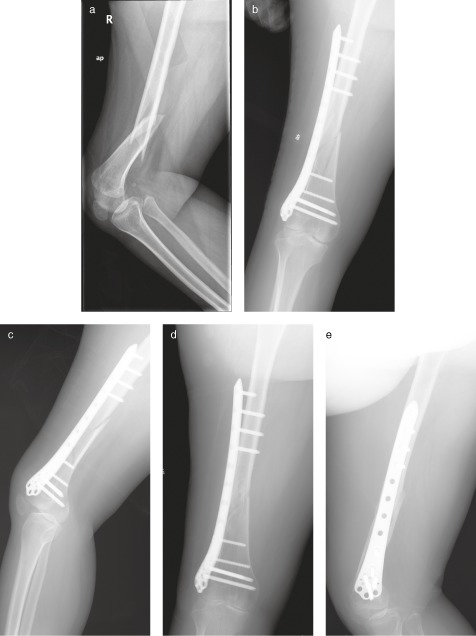
Distal femoral fracture in a 50‐year‐old woman with post‐polio syndrome treated with an LCP. (a) Radiograph showing a type 32‐B1 fracture and osteopenia. (b, c) Postoperative radiographs showing fixation with an LCP with four unused plate holes at the fracture site. (d, e) Radiographs 5 months after surgery showing fracture union.
On the second postoperative day, passive motion of the ankle and knee joints was started. Active exercises (straight leg raising and active knee flexion) were started 3 days after surgery.
Follow‐up and Evaluation
The patients were followed up 6 weeks, 3 months, 6 months, 1 year, and 2 years after surgery. At each follow‐up visit, anteroposterior and lateral radiographs of the distal femur were taken to evaluate bone callus and fracture union by the treating surgeon (JX) and an independent observer (WJW). Bony union was defined as callus bridging being present for three of four cortices on orthogonal radiographs25. Once bony union had been achieved, the functional outcome was assessed by measuring range of motion and determining the Hospital for Special Surgery (HSS) score of the knee joint (1 month after union)26. If bone union had not been confirmed by 12 weeks after surgery, further follow‐up was recommended (1 month intervals) until radiographs showed solid continuous callus formation. Bony union was defined as solid when cross trabeculation was visible on anteroposterior and lateral radiographs.
Results
The mean duration of surgery was 86 mins (range, 75–130 mins). Mean intraoperative blood loss was 120 mL (range, 50–200 mL); no patients required blood transfusion. Mean postoperative hospital stay was 6 days (range, 5–8 days). Knee flexion was >70° in 13 (68%) patients before discharge from the hospital. Callus bridging was visible in 12 (63%) patients 6 weeks after surgery. Bony union was confirmed three months after surgery in four fractures, four months in six fractures, five months in seven fractures, and six months in one fracture and seven months in another one fracture (mean, 4.4 months, Fig. 2). The callus was directly across the fracture in 15 fractures whereas external callus alone was noted with 4 fractures. At the time of bony union, the mean range of knee flexion was 105° (range, 90°–130°) and the mean HSS score 76 points (range, 60–93 points). There were no cases of nonunion, implant cutout or other complications.
Figure 2.
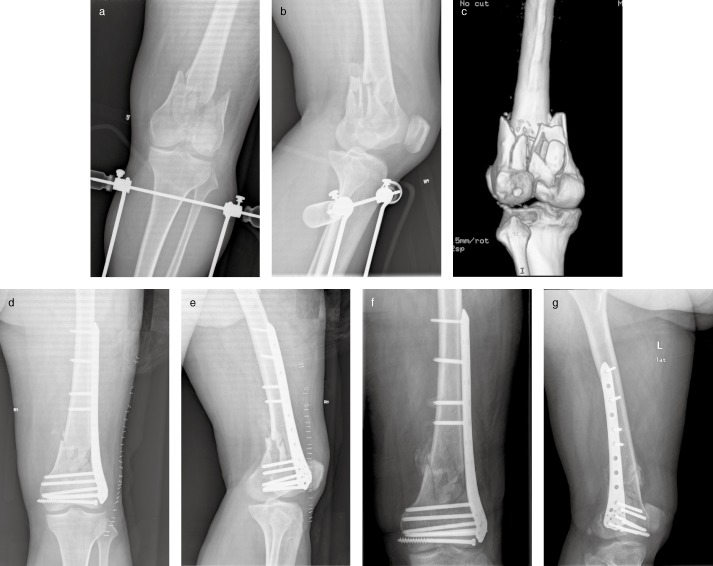
Type 33‐C1 fracture in patient with post‐polio syndrome. A 50‐year‐old man had from polio during childhood. Fracture of his distal femur occurred when he slipped and fell at home. (a, b) Anteroposterior and lateral radiographs showing a type 33‐C1 fracture. (c) Posterior view of three dimensional construction of the communicated fracture. (d, e) Postoperative radiographs of distal femur fracture fixed by LCP. (f, g) Radiographs 3 months after surgery showing bone callus on the medial side of the fracture.
Discussion
Our findings show that LCPs can provide stable fixation and good functional outcomes for distal femoral fractures in post‐polio patients.
The incidence of fracture in aging post‐polio patients ranges from 28% to 38%; they occur predominantly on the side of polio involvement2 , 10. The estimated cumulative incidence of any fracture after 40 years is 48%11. Similar to previous findings11, the commonest site of fracture in post‐polio patients in our center was the distal femur and most fractures occurred in the leg affected by polio. The high incidence of fracture in post‐polio patients may, in part, result from the high incidence of falls during activities of daily living, which may be as high as 64% within 1 year2 and 79%–82% over 5 years2, 10. As in other published series, falls caused most of our patients' fractures2 , 4 , 10.
Post‐polio patients have lower bone mineral density than subjects who have not had poliomyelitis7. Only 4% of post‐polio patients (mean age, 59 years) have normal bone density and 40% have osteopenia10. Furthermore, the bone mineral density in the limb affected by poliomyelitis (the weaker and shorter limb) is characteristically less than that in the other (stronger and longer limb)7 , 10, which may help explain the higher fracture risk in the limb affected by poliomyelitis.
The surgical treatment of distal femoral fractures of post‐polio patients is much more difficult than is fracture fixation in patients without a history of poliomyelitis. Although our patients had weakness in the lower limbs, especially the affected limb, we noted markedly increased tension during traction, probably because of tissue contractures26 , 27. Another difficulty was their thin cortical bone, making them at risk of further comminution from the reduction clamps. In such cases, intraoperative traction is helpful in improving alignment and reducing rotation. However, with comminuted fractures, in order to minimize soft tissue stripping and disruption of the blood supply the primary goal of reduction is functional alignment and not anatomic reduction. Therefore, longer operative times and greater intra‐operative blood loss than is usually expected in the general population occur22 , 23.
Various implants and treatment methods are available for reduction and fixation of distal femoral fractures. Blade plates were contraindicated in our cases because of the large incisions they involve and their requirement for direct compression of the plate on bone, which causes disruption of the blood supply at the fracture site. Intramedullary nails, especially those with multiple distal locking screws, do improve stability of distal femoral fractures and require minimal incisions28. However, these devices are relatively contraindicated for comminuted fractures such as intracondylar fractures (type 33‐C) or post‐polio patients with deformity and small femoral shafts and medullary cavities13, 14, 15. Furthermore, flexion contracture of the knee joint can impede entry of nails26 , 27 and the osteoporosis that is so often present in these patients7 , 10 may increase susceptibility to implant cutout28.
In recent years, LCPs have been increasingly used for treating metaphyseal comminuted fractures29. In contrast to conventional screw‐plate systems that depend on the bone‐plate interface for stability30, LCPs have been designed with a fixed‐angle construct, enabling placement of the plate without any contact with bone23 , 31, 32, 33. With associated insertion guides, these plates can be inserted and fixed by minimally invasive techniques, as in the present cases29. These characteristics of LCPs facilitate closed reduction of these fractures and preservation of the blood supply at the fracture site. LCPs have improved fixation strength, pull‐out strength of locking screws and purchase in osteoporotic bone23 , 29 , 31 , 32. Because of these advantages, LCPs were helpful in our patients with post‐polio syndrome.
In a previous study, surgical management of 13 femoral fractures in post‐polio patients with LCPs resulted in radiographic union in 12 fractures by 12 to 20 weeks after surgery and return to the same level of disability at the end of follow‐up as before occurrence of the fracture; only one patient had nonunion with a decreased disability score and daily walking time24. However, some of the patients in that previous study had proximal femoral fractures24, whereas in the present study, we used LCPs only for distal femoral fractures. In this hospital, intramedullary nails are used for proximal femoral fractures. The present results are comparable to those of the previous study and confirmed that LCPs result in satisfactory union and functional outcomes in post‐polio patients with distal femoral fractures24.
In the present cases, because most fracture reductions were functional and not anatomic, bone healing was achieved by callus formation and not by direct bony union. The theoretical possibility of nonunion with LCPs did not occur. Although LCPs provide excellent stability and the procedure is minimally invasive34, previous studies have found that these devices are too rigid to allow for micromotion at the fracture site in response to axial loading35 , 36. In a systematic review of distal femoral fractures (excluding periprosthetic fractures) treated with LCPs, complications included nonunion (0%–19%), delayed union (0%–15%) and implant failure (0%–20%)34. Although the hypovascularity of the bone and muscles characteristic of post‐polio patients potentially increases the risk of these complications, this did not occur in the present study. This could be attributable to the use of closed reduction and leaving three plate holes vacant at the fracture site. Both these measures avoid disruption of the blood supply and allow micromotion, which stimulates callus formation. Our findings suggest that preservation of blood supply is important when treating distal femoral fractures in post‐polio patients. Moreover, another concern is that poor bone stock quality caused by osteoporosis can result in poor implant anchorage in the distal femur, leading to screw cutout. In our cases, there were no screw malpositions or cutouts or implant failure. The angular fixation of screws with plate in LCPs used in these patients may explain the absence of these complications.
Limitations of the present study include the small sample size, which precluded separate analysis of the outcomes of intra‐ and extra‐articular fractures, and the absence of a control group. Furthermore, comparison of these results with previous cases that had been treated with bridge plates was not possible because suitable previous patients and their radiographs were not available.
Nevertheless, this study is helpful in confirming successful treatment of distal femoral fractures in post‐polio patients by closed reduction and internal fixation with LCPs. These fractures may be associated with difficulties in fracture reduction, risk of nonunion, bony deformity, small bones and osteopenia. However, satisfactory outcomes were achieved by avoiding disruption of blood supply, allowing micromotion by not using fixation screws at the fracture site and starting early postoperative rehabilitation.
Acknowledgements: This work was supported by the Starting Grant for Young Scientific and Technological Talents (Health Bureau of Nanjing, QYK10147).
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1. Trojan DA, Cashman NR. Post‐poliomyelitis syndrome. Muscle Nerve, 2005, 31: 6–19. [DOI] [PubMed] [Google Scholar]
- 2. Silver JK, Aiello DD. Polio survivors: falls and subsequent injuries. Am J Phys Med Rehabil, 2002, 81: 567–570. [DOI] [PubMed] [Google Scholar]
- 3. Silver JK, Aiello DD. What internists need to know about postpolio syndrome. Cleve Clin J Med, 2002, 69: 704–706, 709–712. [DOI] [PubMed] [Google Scholar]
- 4. Hill KD, Stinson AT. A pilot study of falls, fear of falling, activity levels and fall prevention actions in older people with polio. Aging Clin Exp Res, 2004, 16: 126–131. [DOI] [PubMed] [Google Scholar]
- 5. Habel M, Strong P. The late effects of poliomyelitis: nursing interventions for a unique patient population. Medsurg Nurs, 1996, 5: 77–84; quiz 85–76. [PubMed] [Google Scholar]
- 6. Windebank AJ, Litchy WJ, Daube JR, Kurland LT, Codd MB, Iverson R. Late effects of paralytic poliomyelitis in Olmsted County, Minnesota. Neurology, 1991, 41: 501–507. [DOI] [PubMed] [Google Scholar]
- 7. Chang KH, Lai CH, Chen SC, et al Femoral neck bone mineral density in ambulatory men with poliomyelitis. Osteoporos Int, 2011, 22: 195–200. [DOI] [PubMed] [Google Scholar]
- 8. Cosgrove JL, Alexander MA, Kitts EL, Swan BE, Klein MJ, Bauer RE. Late effects of poliomyelitis. Arch Phys Med Rehabil, 1987, 68: 4–7. [PubMed] [Google Scholar]
- 9. Pieterse AJ, Luttikhold TB, de Laat K, Bloem BR, van Engelen BG, Munneke M. Falls in patients with neuromuscular disorders. J Neurol Sci, 2006, 251: 87–90. [DOI] [PubMed] [Google Scholar]
- 10. Mohammad AF, Khan KA, Galvin L, Hardiman O, O'Connell PG. High incidence of osteoporosis and fractures in an aging post‐polio population. Eur Neurol, 2009, 62: 369–374. [DOI] [PubMed] [Google Scholar]
- 11. Goerss JB, Atkinson EJ, Windebank AJ, O'Fallon WM, Melton LJ 3rd. Fractures in an aging population of poliomyelitis survivors: a community‐based study in Olmsted County, Minnesota. Mayo Clin Proc, 1994, 69: 333–339. [DOI] [PubMed] [Google Scholar]
- 12. Wahnert D, Hoffmeier K, Frober R, Hofmann GO, Muckley T. Distal femur fractures of the elderly—different treatment options in a biomechanical comparison. Injury, 2011, 42: 655–659. [DOI] [PubMed] [Google Scholar]
- 13. Lloyd ME, Spector TD, Howard R. Osteoporosis in neurological disorders. J Neurol Neurosurg Psychiatry, 2000, 68: 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharrard WJ. Paralytic deformities of the lower limb. Int Orthop, 1984, 8: 147–154. [DOI] [PubMed] [Google Scholar]
- 15. Shim SS. Bone and joint circulation. Physiological basis for clinical practice. Yonsei Med J, 1986, 27: 91–99. [DOI] [PubMed] [Google Scholar]
- 16. Nayak RM, Koichade MR, Umre AN, Ingle MV. Minimally invasive plate osteosynthesis using a locking compression plate for distal femoral fractures. J Orthop Surg (Hong Kong), 2011, 19: 185–190. [DOI] [PubMed] [Google Scholar]
- 17. Kao FC, Tu YK, Su JY, Hsu KY, Wu CH, Chou MC. Treatment of distal femoral fracture by minimally invasive percutaneous plate osteosynthesis: comparison between the dynamic condylar screw and the less invasive stabilization system. J Trauma, 2009, 67: 719–726. [DOI] [PubMed] [Google Scholar]
- 18. Schütz M, Müller M, Krettek C, et al Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury, 2001, 32 (Suppl. 3): SC48–SC54. [DOI] [PubMed] [Google Scholar]
- 19. Dar GN, Tak SR, Kangoo KA, Halwai MA. Bridge plate osteosynthesis using dynamic condylar screw (DCS) or retrograde intramedullary supracondylar nail (RIMSN) in the treatment of distal femoral fractures: comparison of two methods in a prospective randomized study. Ulus Travma Acil Cerrahi Derg, 2009, 15: 148–153. [PubMed] [Google Scholar]
- 20. Handolin L, Pajarinen J, Lindahl J, Hirvensalo E. Retrograde intramedullary nailing in distal femoral fractures—results in a series of 46 consecutive operations. Injury, 2004, 35: 517–522. [DOI] [PubMed] [Google Scholar]
- 21. Dunlop DG, Brenkel IJ. The supracondylar intramedullary nail in elderly patients with distal femoral fractures. Injury, 1999, 30: 475–484. [DOI] [PubMed] [Google Scholar]
- 22. Fulkerson E, Egol KA, Kubiak EN, Liporace F, Kummer FJ, Koval KJ. Fixation of diaphyseal fractures with a segmental defect: a biomechanical comparison of locked and conventional plating techniques. J Trauma, 2006, 60: 830–835. [DOI] [PubMed] [Google Scholar]
- 23. Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury, 2003, 34 (Suppl. 2): B11–B19. [DOI] [PubMed] [Google Scholar]
- 24. El‐Sayed Khalil A. Locked plating for femoral fractures in polio patients. Arch Orthop Trauma Surg, 2010, 130: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 25. Dijkman BG, Kooistra BW, Pemberton J, Sprague S, Hanson BP, Bhandari M. Can orthopedic trials change practice? Acta Orthop, 2010, 81: 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zouari O, Gargouri A, Jenzri M, Hadinane R, Slimane N. Supracondylar femoral extension osteotomy for knee flexion contracture correction in poliomyelitic conditions. Rev Chir Orthop Reparatrice Appar Mot, 2001, 87: 361–366. [PubMed] [Google Scholar]
- 27. Asirvatham R, Rooney RJ, Watts HG. Proximal tibial extension medial rotation osteotomy to correct knee flexion contracture and lateral rotation deformity of tibia after polio. J Pediatr Orthop, 1991, 11: 646–651. [PubMed] [Google Scholar]
- 28. Wähnert D, Hoffmeier KL, von Oldenburg G, Fröber R, Hofmann GO, Mückley T. Internal fixation of type‐C distal femoral fractures in osteoporotic bone. J Bone Joint Surg Am, 2010, 92: 1442–1452. [DOI] [PubMed] [Google Scholar]
- 29. Ring D, Kloen P, Kadzielski J, Helfet D, Jupiter JB. Locking compression plates for osteoporotic nonunions of the diaphyseal humerus. Clin Orthop Relat Res, 2004, 425: 50–54. [DOI] [PubMed] [Google Scholar]
- 30. Higgins TF. Distal femoral fractures. J Knee Surg, 2007, 20: 56–66. [DOI] [PubMed] [Google Scholar]
- 31. Schutz M, Sudkamp NP. Revolution in plate osteosynthesis: new internal fixator systems. J Orthop Sci, 2003, 8: 252–258. [DOI] [PubMed] [Google Scholar]
- 32. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma, 2004, 18: 488–493. [DOI] [PubMed] [Google Scholar]
- 33. Wagner M, Frenk A, Frigg R. New concepts for bone fracture treatment and the Locking Compression Plate. Surg Technol Int, 2004, 12: 271–277. [PubMed] [Google Scholar]
- 34. Henderson CE, Kuhl LL, Fitzpatrick DC, Marsh JL. Locking plates for distal femur fractures: is there a problem with fracture healing? J Orthop Trauma, 2011, 25 (Suppl. 1): S8–14. [DOI] [PubMed] [Google Scholar]
- 35. Marti A, Fankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma, 2001, 15: 482–487. [DOI] [PubMed] [Google Scholar]
- 36. Stoffel K, Lorenz KU, Kuster MS. Biomechanical considerations in plate osteosynthesis: the effect of plate‐to‐bone compression with and without angular screw stability. J Orthop Trauma, 2007, 21: 362–368. [DOI] [PubMed] [Google Scholar]


