Abstract
Objective: To determine differences in paraspinal muscle injury between a modified minimally invasive approach (MMIA) and a traditional operative approach (TOPA) for one‐level instrumented posterior lumbar inter‐body fusion (PLIF).
Methods: From March 2006 to May 2008, a consecutive series of 91 patients who underwent a one‐level instrumented PLIF procedure using one of two different approaches (MMIA in 41 patients and TOPA in 50), and who were operated on by one group of surgeons at a single institution, was studied. The following data were compared between the two groups: surgical time, blood loss, and changes in postoperative serum concentration of creatinine kinase (CK). More than 1 year post operation, low back pain was evaluated by a visual analog scale (VAS) and the Oswestry disability index (ODI). Some patients were also evaluated by MRI to allow comparison of the preoperative and postoperative cross sectional area (CSA) and fat degeneration grades at the operative level.
Results: There was no statistically significant difference in surgical time, but blood loss, serum concentration of CK, and scores of the VAS and ODI were markedly less in the MMIA group compared with the TOPA group. In the TOPA group, the postoperative CSA of the multifidus muscles was significantly smaller than it was pre‐operatively. In contrast, there was no significant difference between the pre‐ and post‐operative CSA of the multifidus muscles in the MMIA group. There was more fatty infiltration postoperatively than preoperatively in both the TOPA and MMIA groups, the increase in fatty infiltration being greater in the TOPA than in the MMIA group.
Conclusion: Compared with TOPA, MMIA can significantly lessen paraspinal muscle injury, and reduce the incidence of low back pain.
Keywords: Lumbar vertebrae; Spinal fusion; Surgical procedures, minimally invasive
Introduction
The traditional open surgery procedure for posterior lumbar inter‐body fusion (PLIF) is still widely accepted for the management of a variety of spinal disorders which need spinal stabilization. However, the approach‐related morbidity due to iatrogenic muscle and other soft tissue injury has become an increasing concern for many surgeons. The long posterior midline incision, extensive stripping of muscles from the spinal processes and vertebral laminae, and subsequent prolonged wide retraction can result in ischemic necrosis and denervation changes in the paraspinal musculature 1 , 2 . Spinal process and ligament resection, which severely destroy the architecture of the spinal posterior column, combined with compromised physiology of the paraspinal muscles due to scarring and denervation, results in a decrease in trunk muscle strength 3 , 4 , 5 , late onset of spinal instability 6 , and severe back pain which is called “failed back surgery syndrome (FBSS)” 2 , 7 .
In order to reduce iatrogenic soft tissue injury, and lessen approach‐related morbidity, some minimally invasive techniques have been developed. Foley and Smith developed a tubular retractor system which was initially applied to the treatment of herniated lumbar discs and lateral recess stenosis in 1994 8 . With the appearance of some new tubular retractor systems, this method has been utilized for PLIF, transforaminal lumbar interbody fusion (TLIF) and posterolateral fusion (PLF) 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 . Preliminary results suggest that the minimally invasive approach is superior to the TOPA in terms of postoperative intramuscular pressure, blood loss during operation, postoperative back pain and paraspinal muscle edema. In particular, the posterior midline supporting musculoligamentous structures all remain relatively intact with this technique.
However, the performance of successful minimally invasive spinal fusion requires facing several technical challenges, including the limited visual field, understanding how two‐dimensional video images correspond to three‐dimensional anatomy, and the manual dexterity needed to operate through small working channels, all of which mean that mastering of this method by the spinal surgeon has a very steep learning curve 17 . In our clinical practice, we found that the visual field accessible with the extensive tubular retractor system was limited and invariable, making this technique hard to apply widely. Based on the above technique, we developed a modified minimally invasive approach (MMIA) which requires two small paramedian skin incisions lateral to the midline, making it possible to retract the paraspinal muscles with mini‐laminectomy retractors.
Materials and methods
Patients
From March 2006 to May 2008, 91 patients with low back pain were treated with PLIF procedures in our hospital. All of the patients met the following conditions: (i) no history of previous lumbar disease or surgery, (ii) severe low back and leg pain, and no improvement with conservative therapy for at least 6 months, (iii) one‐level PLIF. The patients were randomly divided into two groups according to odd or even admission numbers: the modified minimally invasive approach (MMIA), and the traditional open approach (TOPA). The patients' demographic characteristics and data concerning procedures are listed in Table 1. None of these data show any significant differences between the two groups (sex: χ2= 0.529, P= 0.467; age: Z=−0.268, P= 0.789; diagnosis: χ2= 0.125, P= 0.989; spinal level: χ2= 0.206, P= 0.902).
Table 1.
Demography of patients
| Item | All patients | 1 year postoperatively | MRI evaluation | P | |||
|---|---|---|---|---|---|---|---|
| MMIA | TOPA | MMIA | TOPA | MMIA | TOPA | ||
| No. of patients | 41 | 50 | 25 | 30 | 11 | 10 | – |
| Gender (M/F) | 22/19 | 23/27 | 11/14 | 12/18 | 6/5 | 6/4 | NS |
| Mean age (years) | 53.5 | 53.4 | 51.2 | 52.4 | 52.4 | 50.8 | NS |
| Preoperative diagnosis (No. of patients) | NS | ||||||
| Lumbar disc herniation | 10 | 13 | 5 | 7 | 4 | 4 | – |
| Spinal stenosis | 7 | 8 | 4 | 3 | 2 | 2 | – |
| Posterior element distraction | 4 | 4 | 1 | 1 | 0 | 0 | – |
| Spondylolisthesis | 20 | 25 | 15 | 19 | 5 | 4 | – |
| Grade I | 9 | 13 | 5 | 7 | 3 | 2 | – |
| Grade II | 11 | 12 | 10 | 12 | 2 | 2 | – |
| Level of fusion (No. of patients) | NS | ||||||
| L3,4 | 2 | 3 | 0 | 1 | 0 | 0 | – |
| L4,5 | 25 | 32 | 16 | 19 | 8 | 7 | – |
| L5S1 | 14 | 15 | 9 | 10 | 3 | 3 | – |
NS, not significant.
Surgical techniques
In the MMIA group, two 3 cm long paramedian skin incisions were made 2.5 cm from the midline. Access to the interlaminar space was obtained via blunt dissection of the natural cleavage plane between the multifidus muscle fascicles with a periosteum elevator or scalpel holder. The operating field was exposed by mini‐laminectomy retractors. Pedicle screw instrumentation was implanted first, and then bilateral hemi‐laminectomy and medial facetectomy were performed under direct visualization. Adequate decompression was achieved by cutting the laminae, and hypertrophied superior and inferior articular processes. The ligamentum flavum was resected and the nerve roots retracted medially. A complete discectomy was performed following exposure of the disc space. The endplates were then prepared with Tangent (Medtronic Sofamor Danek, Memphis, TN, USA) interbody instruments. The anterior disc space was packed with autologous bone graft, following which interbody cages were placed. Finally pedicle rod instrumentation was fixed, and interbody compression performed with screw‐rod instrumentation.
In the TOPA group, after one 12 cm long skin incision had been made in the midline, the paravertebral muscles were stripped from the bony structures according to conventional technique. The subsequent steps were as same as for MMIA.
The cages used in both groups were the same (Telamon PEEK cage, Medtronic Sofamor Danek,). Autologous morselized bone for € graft material was obtained from decompression procedures.
Clinical assessment
Data concerning operative and clinical parameters were collected for comparison. Operative measures included operation time, and intra‐operative blood loss. For clinical outcome assessment, the visual analog scale (VAS) was determined for back pain, along with the Oswestry disability index (ODI) evaluation, in which the section about sexual life was deleted for cultural reasons.
Evaluation of back muscle injury
The creatinine kinase (CK) concentration was measured on days 1, 3, 5, and 7 postoperatively. The CK concentration was determined with a Synchron Clinical System LX20 (Beckman Coulter, Fullerton, CA, USA).
MRI was performed on a 1.5 Tesla System (Siemens, Erlangen, Germany) preoperatively and at the final follow‐up, more than 1 year postoperatively. All images were obtained using a T2‐weighted fast spin echo pulse sequence, with matrix size 255 × 512, field of view 240 mm × 240 mm, bandwidth 120 Hz/Px, and echo factor 15. Slice thickness was 4 mm and the inter‐slice gap 1 mm. Patients were placed supine with a pillow positioned under the knees, ensuring that they were lying symmetrically with their weight evenly distributed across both sides. The experienced musculoskeletal radiologists who took the MRI were blinded to the operation method. They used anatomic markers, such as facet configuration, and locating lines on sagittal plane scans to select the most similar preoperative and follow‐up axial images of one spinal level for comparison.
The cross‐sectional area (CSA) of the multifidus muscles were measured bilaterally. Fatty infiltration of the multifidus muscle was visually graded using the standard criteria introduced by Goutallier et al.: Grade A, normal muscle; Grade B, fat tissue sparsely distributed between muscle fibers; Grade C, fat tissue almost equal to muscle fibers; Grade D, more fat tissue than muscle fibers 18 .
Statistical assessments
Student's t‐test was used to make comparisons between groups of CK concentration at every time point. The Wilcoxon signed ranks test was used for comparison between the two groups of the CSA of the multifidus muscles. In all analyses, a P‐value of <0.01 was considered to be significant.
Results
Clinical results
There was no statistical difference in surgical time between the two groups. However, intra‐operative blood loss in the MMIA group was significantly less than that in the TOPA group (P < 0.01) (Table 2).
Table 2.
Peri‐operative data and clinical outcome (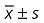 )
)
| Variable | MMIA Group | TOPA Group | t value | P value |
|---|---|---|---|---|
| Operative time (min) | 163 ± 38 | 151 ± 31 | 0.730 | 0.468 |
| Intra‐operative blood loss (ml) | 394.6 ± 226.3 | 776.3 ± 512.7 | −0.385 | <0.001 |
| VAS scores | ||||
| Pre‐operation | 8.1 ± 0.8 | 7.8 ± 1.3 | 0.991 | 0.326 |
| Post‐operation | 1.1 ± 0.4 | 3.1 ± 0.8 | −10.862 | <0.001 |
| Oswestry disability index (%) | ||||
| Pre‐operation | 88.6 ± 13.7 | 83.5 ± 10.1 | 0.871 | 0.388 |
| Post‐operation | 6.9 ± 2.1 | 24.3 ± 5.7 | −7.358 | <0.001 |
Twenty‐five patients in the MMIA group and 30 in the TOPA group were followed up for more than 1 year (Table 1). The mean follow‐up period was not different significantly between the two groups (14.2 months in the MMIA group, 15.1 months in the TOPA group). Again, there were no statistical differences in general data between the two groups (sex: χ2= 0.090 P= 0.765; age: Z=−0.448, P= 0.654; diagnosis: χ2= 0.496, P= 0.920; spinal level: χ2= 0.862, P= 0.650). There was no significant difference in the preoperative VAS or ODI scores between the two groups (P > 0.01), but at the last follow up, both were significantly lower in the MMIA than in the TOPA group (P < 0.01) (Table 2).
Evaluation of back muscle injury
All patients were available for measurement of the CK concentration. The mean CK concentration was greater in the TOPA than in the MMIA group on days 1, 3, and 5 postoperatively (P < 0.01, Table 3).
Table 3.
CK value (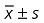 , IU/l)
, IU/l)
| Time | MMIA group | TOPA group | t value | P value |
|---|---|---|---|---|
| Pre‐operation | 78.46 ± 28.31 | 82.64 ± 36.91 | −0.278 | 0.782 |
| 1 day postoperatively | 347.89 ± 94.65 | 650.41 ± 231.62 | −6.732 | <0.001 |
| 3 days postoperatively | 182.27 ± 59.27 | 361.93 ± 129.46 | −5.824 | <0.001 |
| 5 days postoperatively | 94.6 ± 24.84 | 187.97 ± 60.85 | −4.296 | <0.001 |
| 7 days postoperatively | 69.56 ± 20.42 | 79.09 ± 29.13 | −0.680 | 0.499 |
At the last follow‐up, 11 patients in the MMIA and 10 in the TOPA group underwent MRI examination. There was also no statistical difference between the two groups in the general data of patients undergoing MRI examination (sex: χ2= 0.064 P= 0.801; age: Z=−0.247, P= 0.805; diagnosis: χ2= 0.064, P= 0.969; spinal level: χ2= 0.019, P= 0.890; Table 1). The longitudinal changes in CSA of the multifidus muscle are shown in Fig. 1. The results show a significant decrease in the CSA of the multifidus muscle in the TOPA group, being 1211.98 ± 256.58 mm2 and 703.95 ± 167.87 mm2 preoperatively and at the last follow‐up, respectively (Z=−3.920, P < 0.001). However, in the MMIA group there was no statistical difference between the preoperative value and that at the last follow‐up, the results being 1066.69 ± 175.21 mm2 and 975.24 ± 183.51 mm2, respectively (Z=−1.120, P= 0.263).
Figure 1.
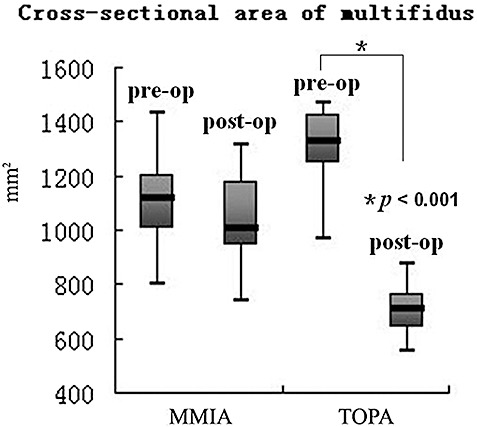
Box plot showing the longitudinal changes in CSA of the multifidus muscle in the MMIA and TOPA groups. Box plots show the median value (horizontal line in box), and the interquartile range (25%–75%) is represented by the box. Whiskers encompass the 5% to 95% range.
The grade of fatty infiltration in the multifidus muscle was evaluated bilaterally. In the MMIA group, fatty infiltration was grade A in 5, B in 13, and C in 4 cases preoperatively; and grade B in 8, C in 10, and D in 4 cases postoperatively (Fig. 2, Table 4). In the TOPA group, fatty infiltration was grade A in 6, and B in 14 cases preoperatively; and grade C in 6, and D in 14 cases postoperatively (Fig. 3, Table 4).
Figure 2.
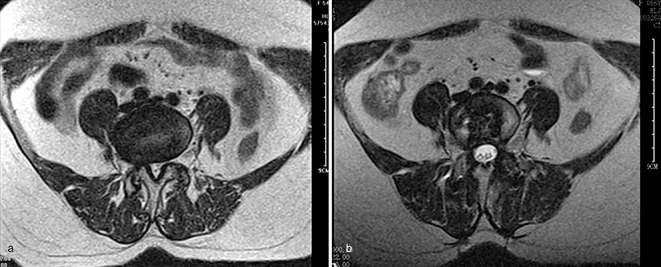
Female patient, 54 years old, L5S1 PLIF with the MMIA (a) pre‐operation, fatty infiltration Grade B; (b) 12 months postoperatively, fatty infiltration Grade B.
Table 4.
Fatty infiltration grade (number of cases)
| MMIA | TOPA | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| Pre‐operation | 5 | 13 | 4 | – | 6 | 14 | – | – |
| Post‐operation | – | 8 | 10 | 4 | – | – | 6 | 14 |
Figure 3.
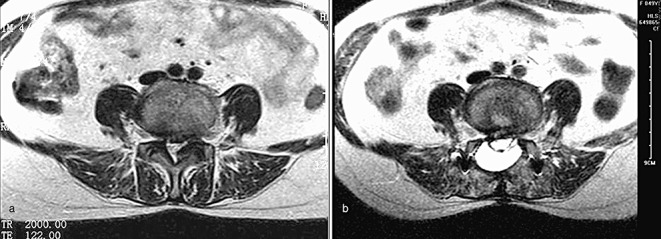
Female patient, 48 years old, L4,5 PLIF with the TOPA (a) pre‐operation, fatty infiltration Grade B; (b) 12 months postoperatively, fatty infiltration Grade D.
Discussion
Anatomical characteristics of the lumbar paraspinal muscles
The lumbar paraspinal muscles consist mainly of the multifidus, longissimus and iliocostalis muscles. The multifidus muscle has five fascicles, which arise from the spinous process and lamina, and attach to the mammillary process of the lumbar vertebra, accessory process, zygapophysial joint capsule, posterior superior iliac spine, and sacrum 19 . The multifidus contributes to the stability of the lumbar spine. The superficial fibers control spine orientation and the deep fibers control intervertebral shear and torsion 20 , 21 . The multifidus muscle is the most vulnerable to injury during posterior spinal surgery, as it is innervated only by the medial branch of the dorsal ramus of the lumbar nerve, and lacks the intersegmental nerve supply which the other paraspinal muscles have 22 .
Advantage of the MMIA
The MMIA is superior to the TOPA in terms of shorter incision, and less intra‐operative blood loss. In this study, the MMIA showed no significant difference from the TOPA in surgical time, but the incision in the MMIA group was only 3 cm, and the mean intra‐operative blood loss 394.60 ml, significantly less than that in the TOPA group (776.32 ml). In addition, nobody in the MMIA group needed transfusion, avoiding the related complications.
Unlike the TOPA, the MMIA relies on muscle‐sparing approaches with limited incisions, which decreases paraspinal muscle damage while still providing adequate access to the surgical target. The reasons for the reduced paraspinal muscle damage are as follows. Firstly, the supraspinal and interspinal ligaments remain intact. Secondly, blunt dissection of the natural cleavage plane between the multifidus muscle fascicles preserves the origin of the multifidus muscle, and postoperative scar healing among the muscle fascicles maintains the muscle strength as much as possible. Thirdly, two short incisions result in less muscle dissection and damage to the innervation of the multifidus. All these factors can decrease fat degeneration in the multifidus muscle and lessen the incidence of low back pain. However, for the TOPA, extensive detachment of muscles from the spinal processes and vertebral laminae, and subsequent lengthy retraction can result in ischemic necrosis and denervation of the paraspinal musculature. In the PLIF procedure, the paraspinal muscles usually need to be retracted laterally in order to place the pedicle screws in an inward direction, so the smaller median incision means more retraction injury. In contrast, the two paramedian incisions in MMIA over the site for placing the pedicle screws can facilitate their placement and create less muscle retraction injury. In addition, the ODI and VAS scores in the study were lower in the MMIA than in the TOPA group, which also indicates there is less injury with the MMIA.
In the PLIF procedure, the multifidus is affected more severely than other muscles. Muscle injury during spinal surgery can increase the serum concentration of CK, which is routinely used for muscle injury evaluation in the early postoperative stages. CK activity increases after surgery, reaching a maximum on day 1 postoperatively, and subsequently declining to the normal value by 7 days 23 . Our results showed that serum CK concentrations were significantly lower in the MMIA group than in the TOPA group on days 1, 3 and 5 postoperatively, which indicated that the muscle injury caused by the MMIA was less than that caused by the TOPA.
The long‐term effects of muscle injury were evaluated by assessing decrease in muscle CSA and deposition of fat and connective tissue on MRI. A previous study has reported muscle swelling due to edema can last for 10 months postoperatively 24 , indicating that chronic fatty infiltration changes should be evaluated more than 10 months postoperatively, in order to avoid the interference of edema. Suwa et al. studied postoperative paraspinal muscle atrophy with several different approaches for lumbar spinal surgery, and concluded that surgical trauma was one of the reasons for paraspinal muscle atrophy24. In our study, we found that the multifidus muscle had atrophied noticeably with considerable fatty infiltration in the TOPA group by more than 1 year postoperatively. However, in the MMIA group muscle atrophy and fatty infiltration were much less severe. We also found that greater fatty infiltration was invariably accompanied by a higher incidence of postoperative low back pain. Accordingly we thought that postoperative low back pain might be related to paraspinal muscle degeneration (fatty infiltration).
Future direction of minimally invasive lumbar spine surgery
The future of minimally invasive lumbar spine surgery appears encouraging. Maybe, in the future, more and more new minimally invasive techniques will be developed. We should know that the concept of “minimally invasive” means not only short incisions, but also less extensive soft tissue injury and optimal therapeutic results. Some blind techniques which seem “minimally invasive”, but actually create more injury to soft tissue and inferior clinical effects, should avoid. At present, preventing iatrogenic injury of paraspinal muscles is more important than treatment.
Conclusion
The MMIA can reduce multifidus muscle damage compared with the TOPA according to measurement of CK concentrations in the early postoperative period, and changes in CSA of the multifidus and fatty infiltration in the long‐term. In addition, the MMIA can reduce the incidence of back pain and functional disability.
Acknowledgments
This study was sponsored by the Zhejiang Provincial Program for the Cultivation of High‐level Innovative Health Talents, General Program for Society Developing of Science and Technology Department of Zhejiang Province (2009C33025), and Key Program for Society Developing of Science and Technology Department of Zhejiang Province (2009C03014‐1).
References
- 1. Taylor H, McGregor AH, Medhi‐Zadeh S, et al The impact of self‐retaining retractors on the paraspinal muscles during posterior spinal surgery. Spine, 2002, 27: 2758–2762. [DOI] [PubMed] [Google Scholar]
- 2. Sihvonen T, Herno A, Paljärvi L, et al Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine, 1993, 18: 575–581. [DOI] [PubMed] [Google Scholar]
- 3. Vasseljen O, Dahl HH, Mork PJ, et al Muscle activity onset in the lumbar multifidus muscle recorded simultaneously by ultrasound imaging and intramuscular electromyography. Clin Biomech (Bristol, Avon), 2006, 21: 905–913. [DOI] [PubMed] [Google Scholar]
- 4. Kim DY, Lee SH, Chung SK, et al Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine, 2005, 30: 123–129. [PubMed] [Google Scholar]
- 5. Mayer TG, Vanharanta H, Gatchel RJ, et al Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine, 1989, 14: 33–36. [DOI] [PubMed] [Google Scholar]
- 6. Quint U, Wilke HJ, Shirazi‐Adl A, et al Importance of the intersegmental trunk muscles for the stability of the lumbar spine. A biomechanical study in vitro . Spine, 1998, 23: 1937–1945. [DOI] [PubMed] [Google Scholar]
- 7. Onesti ST. Failed back syndrome. Neurologist, 2004, 10: 259–264. [DOI] [PubMed] [Google Scholar]
- 8. Foley KT, Smith MM. Microendoscopic discectomy. Tech Neurosurg, 1997, 3: 301–307. [Google Scholar]
- 9. Khoo LT, Palmer S, Laich DT, et al Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery, 2002, 51 (5 Suppl.): S166–S181. [PubMed] [Google Scholar]
- 10. Schwender JD, Holly LT, Rouben DP, et al Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech, 2005, 18 (Suppl.): S1–S6. [DOI] [PubMed] [Google Scholar]
- 11. Isaacs RE, Podichetty VK, Santiago P, et al Minimally invasive microendoscopy‐assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine, 2005, 3: 98–105. [DOI] [PubMed] [Google Scholar]
- 12. Mummaneni PV, Rodts GE Jr. The mini‐open transforaminal lumbar interbody fusion. Neurosurgery, 2005, 57 (4 Suppl.): S256–S261. [DOI] [PubMed] [Google Scholar]
- 13. Stevens KJ, Spenciner DB, Griffiths KL, et al Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech, 2006, 19: 77–86. [DOI] [PubMed] [Google Scholar]
- 14. Park Y, Ha JW. Comparison of one‐level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine, 2007, 32: 537–543. [DOI] [PubMed] [Google Scholar]
- 15. Fan SW, Fang XQ, Zhao X, et al Preliminary report of minimally invasive transforaminal lumbar interbody fusion assisted by X‐Tube system in the treatment of low back disorders (Chin). Zhonghua Gu Ke Za Zhi, 2007, 27: 81–85. [PubMed] [Google Scholar]
- 16. Fan SW, Fang XQ, Zhao X, et al Clinical value of minimally invasive posterior lumbar interbody fusion assisted by X‐Tube system in the treatment of low back disorders (Chin). Zhonghua Wai Ke Za Zhi, 2008, 46: 488–492. [PubMed] [Google Scholar]
- 17. Perez‐Cruet MJ, Fessler RG, Perin NI. Review: complications of minimally invasive spinal surgery. Neurosurgery, 2002, 51 (5 Suppl.): S26–S36. [PubMed] [Google Scholar]
- 18. Goutallier D, Postel JM, Bernageau J, et al Fatty muscle degeneration in cuff ruptures. Pre‐ and postoperative evaluation by CT scan. Clin Orthop Relat Res, 1994, 304: 78–83. [PubMed] [Google Scholar]
- 19. Macintosh JE, Bogduk N. Volvo award in basic science. The morphology of the lumbar erector spinae. Spine, 1987, 12: 658–668. [DOI] [PubMed] [Google Scholar]
- 20. Panjabi M, Abumi K, Duranceau J, et al Spinal stability and intersegmental muscle forces. A biomechanical model. Spine, 1989, 14: 194–200. [DOI] [PubMed] [Google Scholar]
- 21. Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clin Biomech, 1986, 1: 205–213. [DOI] [PubMed] [Google Scholar]
- 22. Shindo H. Anatomical study of the lumbar multifidus muscle and its innervation in human adults and fetuses. Nippon Ika Daigaku Zasshi, 1995, 62: 439–446. [DOI] [PubMed] [Google Scholar]
- 23. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 1: Histologic and histochemical analyses in rats. Spine, 1994, 19: 2590–2597. [DOI] [PubMed] [Google Scholar]
- 24. Suwa H, Hanakita J, Ohshita N, et al Postoperative changes in paraspinal muscle thickness after various lumbar back surgery procedures. Neurol Med Chir (Tokyo), 2000, 40: 151–155. [DOI] [PubMed] [Google Scholar]


