Abstract
Thoracic ossification of the ligamentum flavum (TOLF) is the most common cause for thoracic spinal stenosis. TOLF is usually complicated by thoracic disc herniation, ossification of the posterior longitudinal ligament and degenerative spinal diseases such as cervical spondylosis and lumbar spinal stenosis, and the ossification also usually has a discontinuous or continuous multi‐segment distribution. The resultant superposition of several symptoms makes the clinical manifestations complex. Currently, the diagnosis of TOLF depends mainly on the patient's symptoms, physical examination and thoracic CT and MRI examinations. Identification of the location of TOLF depends more on the doctor's subjective judgement. Diagnostic problems are related to the specific region and level of surgical decompression: if the extent of decompression is insufficient, the treatment is inadequate, resulting in residual symptoms. Obversely, unnecessary trauma and a various complications will occur if the decompression is too extensive. Hence, the clinical features and process of diagnosis, especially the means of identifying the location, still require further improvement. It is necessary to establish a simple and accurate means of identifying the segment of TOLF that is responsible for the neurologic deficit: a number of spinal surgeons have been working hard on this. This article will provided an overview of the clinical features of TOLF and the related problems of clinical identification of the location of the segment causing the neurological deficit. The relationship between the imaging manifestations and clinical characteristics still need to be explored with the aim of establishing a simple and precise method for determining precisely whether TOLF is related to spinal cord injury or not, thus reducing surgical trauma and achieving an optimal prognosis.
Keywords: Clinical features, Identification of location, Thoracic ossification of ligamentum flavum
Introduction
The term thoracic spinal stenosis (TSS) refers to a group of clinical syndromes caused by the compression of the thoracic spinal cord that as a result of one or several pathological factors such as degenerative ligamentous hypertrophy and even ossification, disc herniation, formation of osteophytes at the posterior edges of vertebrae and facet joint hypertrophy1. Thoracic ossification of the ligamentum flavum (TOLF) is the most common cause of TSS1, 2, 3. Thoracic myelopathy caused by thoracic degeneration that requires surgery is reportedly attributable to TOLF in 51% of cases4.
Since Yamaguchi et al. first reported thoracic myelopathy caused by TOLF in 19605, this condition has received attention from scholars of every country; however, the pathogenesis remains unclear. The anatomic structure of the thoracic spine is relatively stable because it is protected by the thoracic cage. The incidence of thoracic myelopathy caused by TSS is considerably lower than that of myelopathy caused by cervical or lumbar spinal stenosis6. Understanding of thoracic myelopathy is therefore far less clear than that of diseases of the cervical and lumbar vertebrae. TOLF is commonly recognized to be a form of pathological heterotopic ossification with an occult onset. Because the blood supply to the thoracic spinal cord is poor, spinal stenosis significantly compromises it, resulting in severe spinal cord dysfunction. The risk of TOLF surgery is correspondingly considerable higher than that of cervical and lumbar surgery. There are several surgical approaches for the various types of ossification7, 8, 9, 10. Great differences in the effective rate and rate of excellent outcomes have been reported for surgeries performed in different orthopaedic centers. Most of the excellent rate reported were less than 75%11, 12, 13, 14, 15, 16. One reason for these poor outcomes is that it remains difficult to accurately identify the location of symptom‐producing TOLF.
Because TOLF is usually complicated by thoracic disc herniation (TDH), ossification of the posterior longitudinal ligament (OPLL) and degenerative spinal diseases such as cervical spondylosis and lumbar spinal stenosis, ossification usually presents with a discontinuous or continuous multi‐segment distribution. The superposition of several symptoms makes the clinical manifestations complex. Moreover, the relationship between the spinal cord and thoracic vertebral canal is also complex. Lesions in the lower thoracic vertebrae or thoracolumbar segment can even present with mixed damage of both upper and lower motor neurons. Therefore, TSS is often misdiagnosed, delaying appropriate treatment17.
At present, the diagnosis of TOLF mainly depends on patient's symptoms, physical examination and thoracic CT and MRI examinations. Because there is no established objective index similar to that for the diagnosis of developmental cervical spinal stenosis, identification of the location depends largely on the physician's subjective judgement. This results in a lack of objectivity when planning surgery: either wrong or insufficient decompression results in poor postoperative outcomes, whereas overly extensive surgery can lead to various complications.
Hence, the clinical features, diagnostic approach and, in particular, the means of identifying the location diagnosis still require further investigation. This article provided an overview of the clinical features and classification of TOLF and the related problems of clinical identification of location.
Epidemiology and Predilection Site
It was previously believed that TOLF was a disease that occurred infrequently, mainly in East Asia, especially in Japan and Korea. In Japan, surgery for TOLF treatment accounts for about 0.09% of all spinal surgeries and only 0.006% of all residents of Japan require surgery for TOLF4. However, in recent years, TOLF has been increasingly reported in other countries18, 19, 20, 21, 22, 23, particularly China24, 25, 26, 27, 28. Epidemiological investigations in China have found that TOLF is quite common in adults. In a study in which 1736 healthy volunteers from south China were examined by CT and MRI, Guo et al. found an incidence of TOLF of 3.8%; these subjects had no obvious clinical symptoms27. Lang et al. retrospectively analyzed CT examinations of 993 patients with chest symptoms28, 63.9% of whom were diagnosed with TOLF after reconstruction of their thoracic vertebrae; however, these patients had no significant spinal stenosis. Thus, it seems that only a small proportion of patients with TOLF have clinical symptoms caused by compression of the spinal cord. Thus, the key to accurate diagnosis is to identify the degree and type of ossification that may cause spinal cord injury.
Various studies have shown that ossification of the ligamentum flavum (OLF) mostly develops in the lower thoracic segment27, 28, 29, possibly because protection by the bony thorax is weaker in the lower thoracic than in the upper thoracic spine.
Clinical Features
The clinical manifestations of TOLF are relatively complex. Patients usually have sensory and motor dysfunction below the involved segment. In the early stages, patients present with chest and back pain and numbness in the lower limbs. As the disease progresses, zonesthesia in the chest and spastic paralysis in the lower limbs can develop. Patients may have sphincter dysfunction and sensory dysfunction in the saddle region when the conus medullaris is involved. The following symptoms indicate a high probability of TOLF: paresthesiae such as diffuse numbness and pain in one or both lower limbs; motion abnormality such as weakness, heaviness and instability walking in one or both lower limbs; spinal cord‐derived claudication; difficulty urinating and urinary incontinence.
On physical examination, TOLF patients typically have evidence of upper motor neuron‐type damage in the lower limbs, such as hypoesthesiae or complete loss of sensation, increased muscle tension, decreased muscle strength, hyperactive tendon and ankle reflexes and positive pathological reflexes. When the OLF locate in the T10‐12 segment, the situation may be more complex. The clinical manifestations may include evidence of mixed damage of both upper and lower motor neuron type (such as decreased or even absent sensation in both lower limbs)11, 30. Because it is easy to misdiagnose TOLF as lumbar spinal stenosis, careful clinical differentiation is required. When patients also have cervical spondylotic myelopathy, the diagnosis of TOLF may be missed and inappropriate treatment given because both of these conditions can cause dysfunction of the lower limbs. The following physical signs make the diagnosis of TOLF probable: upper motor neuron type dysfunction in the lower limbs with normal function of the upper limbs; and mixed upper and lower motor neurons type dysfunction in the lower limbs.
Imaging Features and Related Classification
Radiographs
The main radiographic feature of TOLF is a hook‐ or beak‐like radio‐opacity in the intervertebral foramina in a lateral X‐ray film of the thoracic vertebrae. According to its morphology, TOLF can be classified as acanthomatous, beak‐like, nodular and linear types. The acanthomatous type is the commonest13. However, interference from the shoulder and liver make it hard to make an early diagnosis of TOLF on an X‐ray film. Moreover, the radiographic type displayed in a lateral X‐ray film of the thoracic vertebrae does not precisely correlate with the pathological type of ossification. Thus, plain radiographs cannot be used to assess the maturity and degree of ossification or the severity of compression of the spinal cord31.
CT Scanning
At present, CT and MRI are the main imaging techniques for diagnosing TOLF clinically; they are usually combined for this purpose.
CT scans can display the location and morphology of TOLF, degree of invasion of the spinal canal and extent of dural ossification. TOLF appears as a high‐density linear shadow along the laminae or articular capsule in cross‐sectional CT images. Sagittal reconstruction CT scans usually show formation of bone bridges. Ossification and hypertrophy of the ligamentum flavum can be differentiated by CT scan. Performing axial CT scanning layer by layer ensures that the layer with the severest ossification will be identified; however, CT scans sometimes miss multi‐segmental lesions. Several authors have proposed classifications based on TOLF morphology in axial CT views12, 31, 32. The Sato classification is the most commonly used (Fig. 1). It classifies TOLF morphology in cross‐sectional CT images as one of five types; namely lateral, extended, enlarged, fused and nodular types1, 4. Although CT classification is mainly used to evaluate the postoperative prognosis of patients with TOLF, there is still controversy about whether this is accurate26, 29, 33, 34, 35, 36, 37. The location of OLF and degree of invasion of the spinal canal vary according to the different Sato types of ossification. There has been insufficient research on the relationship between Sato type and degree of spinal cord injury. If the degree of injury could be assessed by location and type of ossification, identification of location of symptom‐related pathology would be improved.
Figure 1.
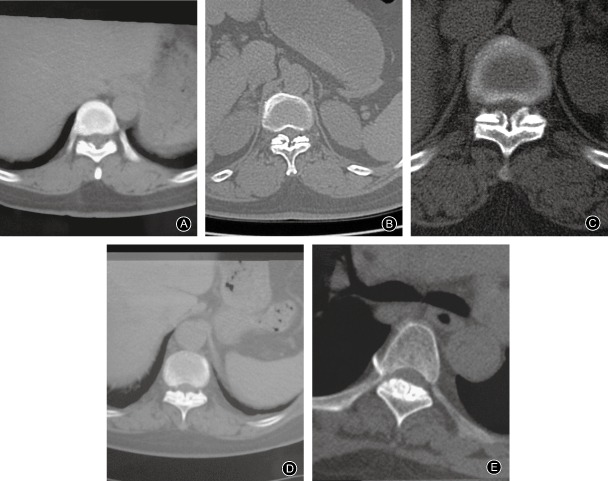
CT images showing Sato classification. (A) Lateral type; (B) Extended type; (C) Enlarged type; (D) Fused type; (E) Tuberous type.
MRI
Morphology on MRI images is not as well‐established as that on CT images. However, MRI can identify the involved segment(s) and changes in spinal cord signals, thus identifying multi‐segmental lesions that have been missed by CT38, 39. However, because axial MRI scans have a definite layer thickness and cannot provide layer by layer images, the layer with the severest ossification may be missed. TOLF presents with low signal intensity in T1‐ and T2‐weighted images and appears as a ball‐ or beak‐like shape protruding into the spinal canal. TOLF‐related spinal cord compression may present with high signal intensity. In acute spinal cord injury, high signal intensity of the spinal cord is mostly considered to denote edema, necrosis or hemorrhage; whereas in chronic spinal cord injury it generally indicates demyelinating lesions and formation of tiny cavities within the spinal cord40.
The degree of spinal canal occupation is graded on axial T2 WI images according to the shape and size of the OLF as follows: Grade I, TOLF exists, and may be touching but not compressing the epidural sac; Grade II, the epidural sac is compressed and deformed, but the TOLF is not in contact with the spinal cord; Grade III, the subdural space is partially occluded and the TOLF is in contact with the spinal cord, but the spinal cord has not yet been deformed; and Grade IV, the spinal cord is obviously compressed and deformed (Fig. 2)41. TOLF is considered asymptomatic if it is only compressing the dural sac but is not yet touching the spinal cord (Grades I and II): conservative treatment and follow‐up is appropriate for these cases. When TOLF has resulted in obvious spinal cord compression and deformation (Grade IV) and the patients has neurologic deficits, decompression surgery should be considered. When the spinal cord has not yet been deformed (Grade III), a few patients have mild neurological signs, whereas most such patients are asymptomatic. Assessing whether or not the condition is symptomatic is rather subjective.
Figure 2.
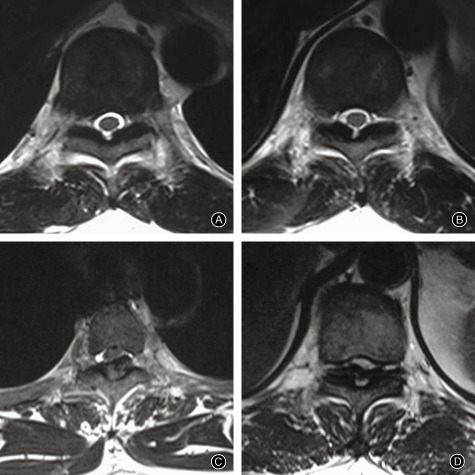
The degree of spinal canal occupation as graded on axial T2WI. MRI images. (A) Grade I; (B) Grade II, (C) Grade III, (D) Grade IV.
Based on the number and distribution of segments involved, TOLF is divided into three types: local, discontinuous and continuous32. Guo et al. assessed 66 asymptomatic volunteers with TOLF on MRI scans and found 45 cases of the local type and 21 with multi‐segmental ossification, including 11 with the continuous and 10 the discontinuous type27. Chen et al. retrospectively analyzed 82 TOLF patients who had undergone surgery15. Only 11.1% of these patients had the local type of ossification, whereas continuous and discontinuous types accounted for 63.0% and 35.4% of patients, respectively. It thus seems that the distribution of TOLF types differs greatly between asymptomatic patients and those with definite spinal cord injury that requires surgical intervention. Different types of ossification may be present in various locations of the spine in the same patient. Thus the question arises: do local, discontinuous and continuous types on MRI images reflect developmental stages or specific types of TOLF? More clinical research is required to determine the answer to this question.
Electroneurophysiology
At present, electromyography and electroneurophysiology are widely used for diagnosis, differential diagnosis, intraoperative monitoring and research into prognosis of spinal diseases. Somatosensory evoked potential and motor evoked potential (MEP) are mainly used to assess damage to cervical nerve roots, spinal cord and lumbosacral nerve roots, whereas anal sphincter electromyography is usually used for checking damage to the conus medullaris. Electrophysiological methods are seldom used in thoracic spinal cord injury. MEP of the spinal cord provides an objective measure of motor conduction in the spinal cord. Theoretically, assessment of MEP in multiple segments could assist in identifying the segment responsible for compression of the spinal cord in multi‐segment TOLF. MEP has been utilized to examine the dorsal extensors42, 43, 44, 45, 46, 47, abdominal internal oblique muscles48, 49 and intercostal muscles49, 50. Electromyographic responses are triggered by stimulating the cerebral cortex. However, because many factors interfere with the findings of MEP, it has limited localization value and is therefore mainly utilized for qualitative diagnosis and intraoperative monitoring.
Questions concerning Diagnosis and Progression
In the clinic, TSS can be rapidly diagnosed and thoracic OPLL and TDH excluded by a combination of detailed medical history, physical examination and MRI scan. The addition of an axial plain CT scan of the thoracic vertebrae and sagittal reconstruction enables diagnosis of TOLF and clearly displays the morphology and density of ossification (Fig. 3).
Figure 3.
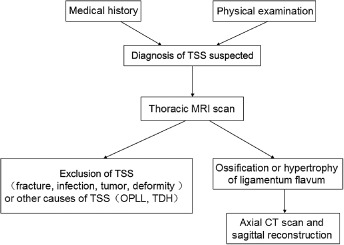
Flow chart showing process of diagnosis of thoracic ossification of ligamentum flavum.
Multi‐level involvement is a novel characteristic of TOLF2. This feature complicates clinical manifestations and leads to difficulties in clinical diagnosis and treatment. Local type TOLF reportedly accounts for 11.0% of cases, discontinuous type 53.6% and continuous type 35.4%15. In patients with multi‐segmental distribution of TOLF, the degree of spinal canal occupation varies between affected segments and spinal cord damage does not necessarily occur in every affected segment; it is therefore difficult to determine which segment(s) are involved in causing spinal deficits. Accurate identification of the segment(s) of OLF responsible for spinal cord damage enables minimization of the range of decompression and achievement of acceptable surgical outcomes, thus decreasing the extent of surgical invasion. Another significant feature of TOLF is the combination of thoracic spinal cord ventral compression and spinal degenerative diseases. Naohisa et al. retrospectively analyzed 45 TOLF cases and found that 35.6% of them were complicated by cervical spondylotic myelopathy (CSM) or lumbar disk herniation13. Chen et al. retrospectively analyzed 82 TOLF patients who had undergone surgery and found that in 48.8% of them TOLF was complicated by CSM and cervical OPLL. TOLF typically results in upper motor neuron damage15; however, it can cause mixed upper and lower motor neuron damage or a wide range of motor neuron damage if located in the thoracolumbar region and causing conus medullaris injury. It is thus easily confused with lumbar canal stenosis. Because cervical myelopathy and TOLF can both lead to lower limb dysfunction, it is difficult to determine the major cause of symptoms when both these conditions occur simultaneously and the symptoms are mainly in the lower limbs. These factors are all related to the region and level of surgical decompression. For example, when the lesions are relatively concentrated and limited and suspicious segments are adjacent to the definitely responsible segment, they can be removed together. However, discontinuous distribution is more common in TOLF. Removal of all lesions in the pursuit of a thorough treatment may increase the surgical risk, and excessive destruction of the posterior column structure of the spine may result in kyphosis deformity in the future.
Ono et al. classified ligamentous ossification into mature and immature types according to the pathological characteristics51. Based on comparison of imaging and pathological characteristics of TOLF, Zhou and Dang concluded that uniform ossification on CT and ossification without signals on MRI corresponds to mature‐type ossification according to pathologic classification, whereas nonuniform ossification on CT and ossification with a low signal intensity or isointensity on MRI corresponds to immature‐type ossification (Fig. 4)52. Ossification of the mature type belongs to the stationary type clinically. Provided the compression is not serious, close follow‐up is appropriate. Ossification of the immature type tends to be progressive and should therefore be resected preventatively. Zhao et al. followed up TOLF patients who did not undergo resection and verified the above conclusions32.
Figure 4.
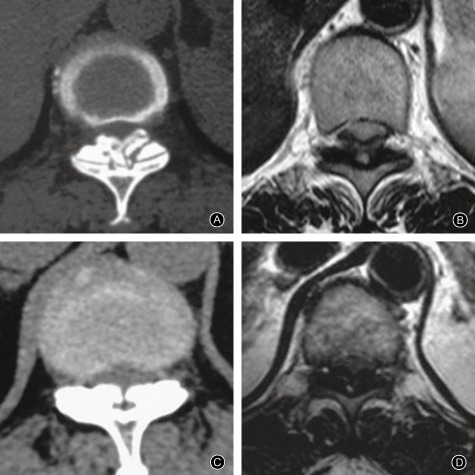
MRI and CT manifestations of mature type and immature type TOLF. (A) Nonuniform ossification on CT, with irregular and ground‐glass‐like ossification on ventral side (B) Low signal intensity or isointensity compared with spinal cord signal on MRI (C) Uniform ossification on CT, with regular ossification on ventral side (D) Ossification without signal on MRI.
Ogawa et al. performed 10 second stepping tests on 25 patients with simple compression of the thoracic spinal cord who were to undergo surgery53. They also evaluated Japanese Orthopedic Association (JOA) scores for thoracic myelopathy and lower limb motor function. Steps performed in 10 seconds correlated significantly with total JOA scores and scores for lower limb motor function both pre‐ and post‐operatively. Therefore, the stepping test can be used to evaluate the severity of thoracic spinal cord compression. However, this method does not help with identifying the location of thoracic spinal cord injury.
Sun and Chen analyzed the clinical manifestations and radiological representation of 35 cases of myelopathy resulting from OLF associated with CSM54. They concluded that the possibility of associated OLF should be considered in patients with CSM in whom the component ratio of the upper extremities in the JOA score was higher than 36%. This can be used as a screening method to prevent missing the diagnosis of OLF and to determine whether OLF is contributing to spinal cord damage when combined with cervical myelopathy. Its disadvantages are that it is rather subjective, relatively inaccurate and cannot be used to determine the segment responsible for the neurologic deficits.
Electrophysiological examination may have supplementary diagnostic value for cervical and lumbar nerve damage; however, it is less effective in localizing the segment responsible for thoracic myelopathy. Nakanishi et al. set up a control group and found that the ratio of the central conduction time of the abductor digiti minimi to that of the abductor pollicis muscle was helpful in differentiating between thoracic and cervical spinal cord compression55. When this ratio is less than or equal to 0.52, the odds ratio of diagnosing thoracic spinal cord compression is 68.4. Baba et al. used spinal cord evoked potentials to locate the segment(s) responsible for thoracic canal stenosis and classified them as follows (Fig. 5): Type I, normal or decreased amplitude less than 30% of the control; Type II, amplitude decrement of between 30% to 50%; Type III, amplitude decrement of more than 50% or disappearance of the NI wave; and Type IV, positive waves56. A 50% amplitude decrease is the absolute critical point for diagnosing compression injury (Type III and Type IV). However, this method is mainly employed for thoracic longitudinal ligament ossification and invasive manipulation near the spinal cord is very risky. What's more, spinal cord evoked potential is easily influenced by many factors such as age and anesthetics.
Figure 5.
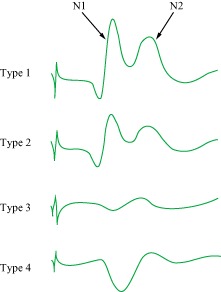
Types of spinal cord evoked potentials. N1: initial negative spike; N2: slow negative complex.
Currently, MRI is the major imaging diagnostic method for determining the segment responsible for OLF‐induced myelopathy. In clinical practice, OLF is considered asymptomatic if it is only compressing the dural sac but is not touching the spinal cord. Conservative treatment and follow‐up is appropriate for these cases; however, when OLF is causing spinal cord compression and deformation, surgery should be considered. Although MRI does provide important information on dural sac and spinal cord compression, the interpretation depends on the experience and subjective judgment of the physician, particularly in patients with moderate compression (Grade III cases).
Use of MRI and CT to measure the residual spinal canal area and sagittal diameter of the ossification segment was reported in a study of prognosis of OLF‐induced myelopathy10. These researchers found that the preoperative canal grade (anteroposterior canal diameter of stenosed level/normal canal diameter ×100) correlated with the preoperative JOA score; however, they did not perform further analyses to ascertain the critical value and were thus unable to make precise clinical diagnoses.
An advantage of CT imaging over MRI lies in the ability to perform axial scanning without intervals, thus avoiding missing the most compressed segment. What's more, CT imaging clearly shows bone structures, allowing for quantitative measurement of the bony canal. Liu et al. measured the residual spinal canal area based on CT scans and calculated the critical value of OLF‐induced thoracic myelopathy57. They concluded that when the residual area is <80%, the sensitivity of diagnosis of OLF‐induced thoracic myelopathy is 93%; the specificity and diagnostic coincidence rates were 95.5% and 93.8%, respectively (Fig. 6). Although the residual spinal canal area seems to be an accurate method of diagnosis, it has several disadvantages. First, measurement of the residual spinal canal does not determine the location of the ossification; for example, bilateral and unilateral ossification would result in significantly different degrees of compression despite having the same residual spinal canal area. Second, the residual spinal canal can only be measured electronically with the aid of a picture archiving and communication systems or some kind of software and is difficult to utilize in some regions in china. In contrast, measurement of the canal diameter can be performed directly on CT images.
Figure 6.
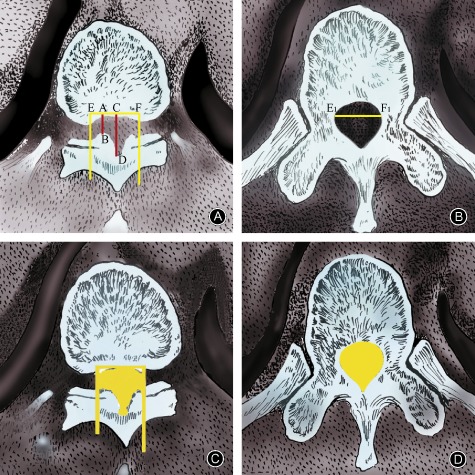
Measurement of the residual spinal canal area based on CT scans. (A) AB and CD are the sagittal diameter and developing sagittal diameter,respectively, of the ossification segment. Spinal canal transverse diameter is calculated at the maximally stenosed level (E1 to F1). A vertical line extending through the endpoints of the transverse diameter (E and F) determines the boundary of the spinal canal. (B) The widest distance between two pedicles as viewed on a CT scan image of a transverse section through the pedicle of the same vertebra is measured as the spinal canal transverse diameter (E1 to F1). (C) Photoshop is used to measure the spinal canal area of the stenosed level. (D) A normal spinal canal area is measured by using the transverse section through the pedicle of the same vertebra.
At present, identification of the location of the lesion responsible for neurological deficit in patients with distribution complicated by cervical spondylosis and with multi‐segmental TOLF is accomplished based on the following principles:
Diagnosis and treatment of TSS complicated by cervical spondylosis requires a close combination of clinical manifestations and imaging findings. Scores for spinal cord function can help in the differential diagnosis54.
Thoracic spinal cord compression should be considered the main responsible lesion in patients with mild upper limb and severe lower limb symptoms; decompression of the thoracic spinal cord is the first choice of treatment. Patients with severe symptoms in both upper and lower limbs should first undergo decompression of the cervical spinal cord and later decompression of the thoracic spinal cord in a second stage procedure. The interval between the two operations should not exceed 3 months. Patients with good general condition and tolerance can undergo both decompression operations in the same procedure. Decompression of all narrow segments in the first stage is highly recommended, especially in patients with cervical spondylosis and thoracic spinal cord stenosis in the upper levels9, 16, 58.
Asto identification of the location of the responsible segments in TOLF, the degree of invasion of the spinal canal is currently the most reliable clinical method. A previous method for measuring the area of spinal canal is troublesome and impractical. A study by the Third Hospital of Peking University retrospectively analyzed the clinical data of 44 TOLF patients who required surgery and 44 asymptomatic TOLF patients (control group)59. The residual ratios of the spinal area and anteroposterior diameter of the spinal canal were measured on CT images and compared. When the residual ratio of the paramedian sagittal diameter was less than 60%, the sensitivity of diagnosing spinal cord injury caused by TOLF was 95.5% and the specificity 95.5% (Fig. 7). This provides a reference for precise identification of the location of symptom‐producing TOLF.
Figure 7.
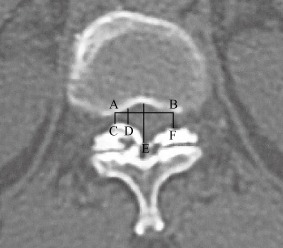
Measurement of the paramedian sagittal diameter based on CT scans. Spinal canal transverse diameter is calculated at the maximally stenosed level (AB). A vertical line extending through the endpoints of the transverse diameter (C and F) determines the boundary of spinal canal (AC and BF). The anteroposterior spinal canal diameter is measured at three sites: the midline of the canal (point E), the boundary of the canal (point C), and midpoint between the midline and boundary (point D). The normal anteroposterior canal diameter is calculated as the average diameter of the adjacent non‐stenosed segments.
Although previous research has helped in identifying the location of responsible TOLF lesions, it has not provided means of precisely achieving this because of the low accuracy, limited practicability and cumbersomeness of these procedures; these disadvantages have affected postoperative efficacy and resulted in various complications. However, the residual ratio of paramedian sagittal diameter reported by our hospital does reflect the degree of spinal cord injury accurately59. This measurement method is easy and practical therefore it may become a supplementary method for topical diagnosis of symptom‐producing TOLF. However, its accuracy still requires further clinical verification.
Summary
In conclusion, because of combinations of various lesions and multi‐segment stenosis, the clinical manifestations of TOLF are complex, resulting in a high possibility of misdiagnosis and improper treatment, or missed diagnosis and treatment. There is still no efficient method of identifying the responsible lesion or segment in patients with extensive TOLF or TOLF complicated by OPLL, TDH, cervical spondylosis or lumbar spinal stenosis. Hence, the relationship between the imaging manifestations and clinical characteristics still need to be explored with the aim of establishing a simple and precise method for determining precisely whether TOLF is related to spinal cord injury or not, thus reducing surgical trauma and achieving an optimal prognosis.
Disclosure: The submitted manuscript does not contain information regarding medical equipment. This work is not supported by any foundation and does not directly or indirectly have any formal relationships with business groups.
References
- 1. Sato T, Kokubun S, Tanaka Y, Ishii Y. Thoracic myelopathy in the Japanese: epidemiological and clinical observations on the cases in Miyagi Prefecture. Tohoku J Exp Med, 1998, 184: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Aizawa T, Sato T, Tanaka Y, et al Thoracic myelopathy in Japan: epidemiological retrospective study in Miyagi Prefecture during 15 years. Tohoku J Exp Med, 2006, 210: 199–208. [DOI] [PubMed] [Google Scholar]
- 3. Hou X, Sun C, Liu X, et al Clinical features of thoracic spinal stenosis‐associated myelopathy: a retrospective analysis of 427 cases. J Spinal Disord Tech, 2014, doi: 10.1097/BSD.0000000000000081 [DOI] [PubMed] [Google Scholar]
- 4. Aizawa T, Sato T, Sasaki H, Kusakabe T, Morozumi N, Kokubun S. Thoracic myelopathy caused by ossification of the ligamentum flavum: clinical features and surgical results in the Japanese population. J Neurosurg Spine, 2006, 5: 514–519. [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi MTS, Fujita S. A case of ossification of the ligamentum flavum with spinal cord tumor symptoms. Seikeigeka, 1960, 11: 951–956. (in Japanese) [Google Scholar]
- 6. Amato V, Giannachi L, Irace C, Corona C. Thoracic spinal stenosis and myelopathy: report of two rare cases and review of the literature. J Neurosurg Sci, 2012, 56: 373–378. [PubMed] [Google Scholar]
- 7. Zhao HJ, Xue Y, Li JP, et al Pathological unit and the octagonal en bloc resection of thoracic ossification ligamentum flavum. Zhonghua Gu Ke Za Zhi, 2010, 30: 1053–1058 (in Chinese). [Google Scholar]
- 8. Hao DJ, He BR, Xu ZW, Guo H, Liu TJ, Wang XD. Laminar thinned‐ segmented decompression for treatment of thoracic ossification of ligamentum flavum with myelopathy. Zhonghua Gu Ke Za Zhi, 2010, 30: 1030–1034 (in Chinese). [Google Scholar]
- 9. Sun CG, Chen ZQ, Liu XG, et al Selection of the surgical methods for the thoracic ossification of ligamentum flavum combined with cervical spondylotic myelopathy. Zhonghua Gu Ke Za Zhi, 2010, 30: 1087–1090 (in Chinese). [Google Scholar]
- 10. Sanghvi AV, Chhabra HS, Mascarenhas AA, Mittal VK, Sangondimath GM. Thoracic myelopathy due to ossification of ligamentum flavum: a retrospective analysis of predictors of surgical outcome and factors affecting preoperative neurological status. Eur Spine J, 2011, 20: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park BC, Min WK, Oh CW, et al Surgical outcome of thoracic myelopathy secondary to ossification of ligamentum flavum. Joint Bone Spine, 2007, 74: 600–605. [DOI] [PubMed] [Google Scholar]
- 12. Kuh SU, Kim YS, Cho YE, et al Contributing factors affecting the prognosis surgical outcome for thoracic OLF. Eur Spine J, 2006, 15: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyakoshi N, Shimada Y, Suzuki T, et al Factors related to long‐term outcome after decompressive surgery for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg, 2003, 99: 251–256. [DOI] [PubMed] [Google Scholar]
- 14. Shiokawa K, Hanakita J, Suwa H, Saiki M, Oda M, Kajiwara M. Clinical analysis and prognostic study of ossified ligamentum flavum of the thoracic spine. J Neurosurg, 2001, 94: 221–226. [DOI] [PubMed] [Google Scholar]
- 15. Chen ZQ, Sun CG, Dang GT, Liu ZJ, Guo ZQ, Qi Q. The surgical outcome of thoracic myelopathy due to ossification of the ligamentum flavum. Zhongguo Ji Zhu Ji Sui Za Zhi, 2006, 16: 485–488 (in Chinese). [Google Scholar]
- 16. Sun CG, Chen ZQ, Liu ZJ, et al Long term outcome after the decompressive surgery for thoracic myelopathy due to the ossification of the ligamentum flavum. Zhonghua Wai Ke Za Zhi, 2012, 50: 426–429 (in Chinese). [PubMed] [Google Scholar]
- 17. Epstein NE. Patients with “lumbar stenosis” and unrecognized distal thoracic cord compression. Spinal Surg, 2007, 21: 101–105. [Google Scholar]
- 18. Al‐Orainy IA, Kolawole T. Ossification of the ligament flavum. Eur J Radiol, 1998, 29: 76–82. [DOI] [PubMed] [Google Scholar]
- 19. Hamouda KB, Jemel H, Haouet S, Khaldi M. Thoracic myelopathy caused by ossification of the ligamentum flavum: a report of 18 cases. J Neurosurg, 2003, 99: 157–161. [DOI] [PubMed] [Google Scholar]
- 20. Jayakumar PN, Devi BI, Bhat DI, Das BS. Thoracic cord compression due to ossified hypertrophied ligamentum flavum. Neurol India, 2002, 50: 286–289. [PubMed] [Google Scholar]
- 21. Trivedi P, Behari S, Paul L, Das BS. Thoracic myelopathy secondary to ossified ligamentum flavum. Acta Neurochir (Wien), 2001, 143: 775–782. [DOI] [PubMed] [Google Scholar]
- 22. Pascal‐Moussellard H, Cabre P, Smadja D, Catonné Y. Symptomatic ossification of the ligamentum flavum: a clinical series from the French Antilles. Spine, 2005, 30: E400–E405. [DOI] [PubMed] [Google Scholar]
- 23. Toledo JA, Van Isseldyk F, Re M, Garrote M. Ossification of the ligamentum flavum as cause of thoracic cord compression: case report of a Latin American man and review of the literature. Surg Neurol Int, 2013, 4: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Z, Luo ZJ, Hu HM, Wu QT, Li XK, Du JJ. Diagnosis and one stage surgical treatment of lower thoracic ossification of ligamentum flavum combined with lumbar spinal stenosis. Zhonghua Gu Ke Za Zhi, 2010, 30: 1096–1100. (in Chinese). [Google Scholar]
- 25. Gao R, Yuan W, Yang L, Shi G, Jia L. Clinical features and surgical outcomes of patients with thoracic myelopathy caused by multilevel ossification of the ligamentum flavum. Spine J, 2013, 13: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 26. He S, Hussain N, Li S, Hou T. Clinical and prognostic analysis of ossified ligamentum flavum in a Chinese population. J Neurosurg Spine, 2005, 3: 348–354. [DOI] [PubMed] [Google Scholar]
- 27. Guo JJ, Luk KD, Karppinen J, Yang H, Cheung KM. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty‐six magnetic resonance imaging scans. Spine, 2010, 35: 51–56. [DOI] [PubMed] [Google Scholar]
- 28. Lang N, Yuan HS, Wang HL, et al Epidemiological survey of ossification of the ligamentum flavum in thoracic spine: CT imaging observation of 993 cases. Eur Spine J, 2013, 22: 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hur H, Lee JK, Lee JH, Kim JH, Kim SH. Thoracic myelopathy caused by ossification of the ligamentum flavum. J Korean Neurosurg Soc, 2009, 46: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen XQ, Yang HL, Wang GL, et al Surgery for thoracic myelopathy caused by ossification of the ligamentum flavum. J Clin Neurosci, 2009, 16: 1316–1320. [DOI] [PubMed] [Google Scholar]
- 31. Kudo S, Ono M, Russell WJ. Ossification of thoracic ligamenta flava. AJR Am J Roentgenol, 1983, 141: 117–121. [DOI] [PubMed] [Google Scholar]
- 32. Zhao JM, Dang GT. Imaging diagnosis of ossification of the ligamentum flavum in the thoracic spine. Zhongguo Ji Zhu Ji Sui Za Zhi, 2004, 14: 278–282 (in Chinese). [Google Scholar]
- 33. Okada K, Oka S, Tohge K, Ono K, Yonenobu K, Hosoya T. Thoracic myelopathy caused by ossification of the ligamentum flavum. Clinicopathologic study and surgical treatment. Spine (Phila Pa 1976), 1991, 16: 280–287. [DOI] [PubMed] [Google Scholar]
- 34. Kim JS, Jung B, Arbatti N, Lee SH. Surgical experience of unilateral laminectomy for bilateral decompression(ULBD) of ossified ligamentum flavum in the thoracic spine. Minim Invasive Neurosurg, 2009, 52: 74–78. [DOI] [PubMed] [Google Scholar]
- 35. Ando K, Imagama S, Ito Z, et al Predictive factors for a poor surgical outcome with thoracic ossification of the ligamentum flavum by multivariate analysis: a multicenter study. Spine (Phila Pa 1976), 2013, 38: E748–E754. [DOI] [PubMed] [Google Scholar]
- 36. Kang KC, Lee CS, Shin SK, Park SJ, Chung CH, Chung SS. Ossification of the ligamentum flavum of the thoracic spine in the Korean population: clinical article. J Neurosurg Spine, 2011, 14: 513–519. [DOI] [PubMed] [Google Scholar]
- 37. Takei H, Hayashi M, Ito Y, Hashimoto J, Sagae M, Goto F. Clinical evaluations of the surgical treatment for ossification of the ligamentum flavum. Rinsho Seikeigeka, 1997, 32: 1359–1365 (in Japanese). [Google Scholar]
- 38. Fong SY, Wong HK. Thoracic myelopathy secondary to ligamentum flavum ossification. Ann Acad Med Singapore, 2004, 33: 340–346. [PubMed] [Google Scholar]
- 39. Parekh HC, Gurusinghe NT, Perera SS, Prabhu SS. Ossification of the ligamentum flavum in a Caucasian: case report. Br J Neurosurg, 1993, 7: 687–690. [DOI] [PubMed] [Google Scholar]
- 40. Liao CC, Chen TY, Jung SM, Chen LR. Surgical experience with symptomatic thoracic ossification of the ligamentum flavum. J Neurosurg Spine, 2005, 2: 34–39. [DOI] [PubMed] [Google Scholar]
- 41. Dang GT. Thoracic ossification of ligamentum flavum and thoracic spinal stenosis. The Eighth National Orthopaedic New Progress and New Technologies, Hang Zhou 2005, 9–11.
- 42. Cariga P, Catley M, Sahal A, Savic G, Ellaway PH, Davey NJ. Corticospinal activation of paraspinal muscles above and below complete thoracic spinal cord injury in man. Abstr Soc Neurosci, 2001, 27: 304–308. [Google Scholar]
- 43. Cariga P, Catley M, Nowicky AV, Savic G, Ellaway PH, Davey NJ. Segmental recording of cortical motor evoked potentials from thoracic paravertebral myotomes in complete spinal cord injury. Spine (Phila Pa 1976), 2002, 27: 1438–1443. [DOI] [PubMed] [Google Scholar]
- 44. Ertekin C, Uludag B, On A, et al Motor‐evoked potentials from various levels of paravertebral muscles in normal subjects and in patients with focal lesions of the spinal cord. Spine (Phila Pa 1976), 1998, 23: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 45. Davey NJ, Lisle RM, Loxton‐Edwards B, Nowicky AV, McGregor AH. Activation of back muscles during voluntary abduction of the contralateral arm in humans. Spine (Phila Pa 1976), 2002, 27: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 46. Myriknas SE, Beith ID, Harrison PJ. Stretch reflexes in the rectus abdominis muscle in man. Exp Physiol, 2000, 85: 445–450. [PubMed] [Google Scholar]
- 47. Strutton PH, Beith ID, Theodorou S, Catley M, McGregor AH, Davey NJ. Corticospinal activation of internal oblique muscles has a strong ipsilateral component and can be lateralised in man. Exp Brain Res, 2004, 158: 474–479. [DOI] [PubMed] [Google Scholar]
- 48. Misawa T, Ebara S, Kamimura M, Tateiwa Y, Kinoshita T, Takaoka K. Evaluation of thoracic myelopathy by transcranial magnetic stimulation. J Spinal Disord, 2001, 14: 439–444. [DOI] [PubMed] [Google Scholar]
- 49. Ellaway PH, Anand P, Bergstrom EM, et al Towards improved clinical and physiological assessments of recovery in spinal cord injury: a clinical initiative. Spinal Cord, 2004, 42: 325–337. [DOI] [PubMed] [Google Scholar]
- 50. Theodorou S, Catley M, Strutton PH, Davey NJ. Examination of intercostal muscle facilitation evoked by transcranial magnetic stimulation (TMS) in man. J Physiol, 2003, 547: C144. [Google Scholar]
- 51. Ono K, Yonenobu K, Miyamoto S, Okada K. Pathology of ossification of the posterior longitudinal ligament and ligamentum flavum. Clin Orthop Relat Res, 1999, 359: 18–26. [DOI] [PubMed] [Google Scholar]
- 52. Zhou F, Dang GT. The matched control study between medical imaging and pathologic findings in ossification of the ligamentum flavum of the thoracic spine. Zhonghua Gu Ke Za Zhi, 2004, 24: 346–349 (in Chinese). [Google Scholar]
- 53. Ogawa Y, Yukawa Y, Morita D, Ito K, Machino M, Kato F. 10‐second step test for quantitative evaluation of the severity of thoracic compressive myelopathy. Spine (Phila Pa 1976), 2013, 38: 1405–1408. [DOI] [PubMed] [Google Scholar]
- 54. Sun CG, Chen ZQ. Clinical diagnosis of myelopathy resulted from thoracic ossification of the ligamentum flavum associated with cervical spondylosis. Ji Zhu Wai Ke Za Zhi, 2007, 5: 18–21 (in Chinese). [Google Scholar]
- 55. Nakanishi K, Tanaka N, Sasaki H, et al Assessment of central motor conduction time in the diagnosis of compressive thoracic myelopathy. Spine (Phila Pa 1976), 2010, 35: E1593–E1598. [DOI] [PubMed] [Google Scholar]
- 56. Baba H, Tomita K, Kawahara N, Kikuchi Y, Imura S. Spinal cord evoked potentials in thoracic myelopathy with multisegmental vertebral involvement. Spine (Phila Pa 1976), 1992, 17: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 57. Liu N, Chen ZQ, Qi Q, Guo ZQ. Correlation between spinal canal encroachment and neurologic deficits in thoracic ossification of ligamentum flavum. Zhonghua Gu Ke Za Zhi, 2007, 27: 481–484 (in Chinese). [Google Scholar]
- 58. Sun CG, Chen ZQ. Treatment of thoracic spine stenosis complicated with cervical spine myelopathy. Zhongguo Ji Ju Ji Sui Za Zhi, 2014, 24: 671–672 (in Chinese). [Google Scholar]
- 59. Feng FB, Sun CG, Chen ZQ, et al Surgical outcome and associated factors of “cap uncovering” en‐bloc removal of the spinal canal's posterior wall surgery for single‐level thoracic ossification of ligamentum flavum. Zhongguo Ji Ju Ji Sui Za Zhi, 2014, 24: 585–592 (in Chinese). [Google Scholar]


